Abstract
Autophagy is a catabolic process for recycling of cellular contents in response to metabolic stress in malignant tumors. We explored efficacy of the synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) and the isoflavonoid apigenin (APG) in the serum-starved human malignant neuroblastoma cells. Combination of 0.5 μM 4-HPR and 50 μM APG synergistically decreased cell viability in the serum-starved neuroblastoma SH-SY5Y, SK-N-BE2, and IMR-32 cells. Acridine orange (AO) staining and LC3 II upregulation showed that serum-starvation for 12 and 24 h progressively increased formation of acidic vesicular organelles (AVO) and autophagy in SH-SY5Y cells. Further, AO stainning and flow cytometry showed blockage of formation of AVO and accumulation of auophagic population, respectively, following treatment of the serum-starved SH-SY5Y cells with combination of 0.5 μM 4-HPR and 50 μM APG. Combination therapy down regulated autophagy inducing proteins such as Beclin 1, LC3 II, TLR-4, and Myd88 while upregulated autophagy inhibitory p-Akt/mTOR singaling pathway. Consistent with the hypothesis that inhibition of autophagy could induce apoptosis, we noticed inhibition of autophagy and induction of apoptosis in the serum-starved SH-SY5Y cells with suppression of the survival factor NF-κB, upregulation of pro-apoptotic Bax, down regulation of anti-apoptotic Bcl-2, activation of caspase-3, and degradation of poly(ADP-ribose) polymerase (PARP) after combination therapy. Collectively, combination of 4-HPR and APG worked synergistically to suppress autophagy and promote apoptosis in human malignant neuroblastoma cells.
Keywords: apigenin, apoptosis, autophagy, N-(4-hydroxyphenyl) retinamide, neuroblastoma
Aautophagy or “self-eating” is a vital catabolic process characterized by massive degradation of cellular components such as cytoplasmic contents and internal organelles to maintain homeostasis in eukaryotic cells [1–3]. Autophagy is a short-term stress response mechanism in certain conditions [4,5]. It is characterized by appearance of double membrane cytoplasmic vesicles engulfing intracellular organelles termed as autophagosomes that mature into autophagolysosomes after fusion with lysosomes [6]. Among all markers of autophagy, microtubule-associated protein light chain 3 (MAP LC3), also simply known as LC3, serves as the most accurate marker protein for autophagic activity because of its crucial role in formation of autophagosomes. Cytosolic pro-form of LC3 is LC3 I that is converted to autophagosome membrane-bound LC3 II form for initiation of autophagy [7,8].
A previous study showed that serum-starved mammalian cells could activate autophagy as a cell survival mechanism; however, inhibition of autophagy triggered induction of apoptosis in those cells [9]. In nutrient-deprived conditions, cells undergo protein degradation via autophagic pathways and suppression of protein synthesis to maintain cellular sustainability during stress situations [10]. Excessive autophagy as a stress response can lead to induction of apoptosis. A better understanding of these two programmed cell death phenomena and their functional relationship is vital for exploring novel strategy as anti-cancer treatment [3,11].
Malignant neuroblastoma, an extracranial solid tumor in children, is responsible for 15% of childhood deaths in the United States [12]. There have been some advancement in understanding of neuroblastoma pathology but an effective treatment for most of the malignant neuroblastomas remains elusive. N-(4-Hydroxyphenyl) retinamide (4-HPR) is a synthetic retinoid that works with other therapeutic agents to increase apoptosis via activation of caspase cascades in various cancers [13,14]. Apigenin (APG), a well known flavonoid present in fruits and vegetables, induces cell death by activating extrinsic and intrinsic apoptotic pathways in many cancers, including neuroblastoma [15]. In this study, we found that serum-starvation induced autophagy in human malignant neuroblastoma cells but treatment with combination of 4-HPR and APG at sub-optimal doses resulted in suppression of autophagy and promotion of apoptosis.
We obtained human malignant neuroblastoma SH-SY5Y, SK-N-BE2, and IMR-32 cell lines from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in 1xRPMI 1640 medium (GIBCO, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS), 0.03% L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin, and maintained at 37°C with 5% CO2 in a fully-humidified incubator.
We used 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay for determining residual cell viability. The MTT assay was performed after treatments with 4-HPR, APG, and combination of these drugs at different doses in serum-starved neuroblastoma SH-SY5Y, SK-N-BE2, and IMR-32 cells. Approximately 104 cells were seeded into each well of a 96-well plate in serum-deprived medium for 24 h. The formazan formed from MTT was dissolved by adding isopropanol and optical density (OD) of final blue color was measured at 570 nm. Cell viability data were then analyzed using Compusyn software (ComboSyn, Paramus, NJ) to generate combination index (CI) and determine degree of synergism (CI < 1) of two drugs at specific doses [16,17]. Combination of drugs may show synergism (CI < 1), additive effect (CI = 1), or antagonism (CI >1). Here, 0.5 μM 4-HPR + 50 μM APG showed the best synergism (CI = 0.21) compared with other concentrations of these drugs (0.25 + 25 μM, 0.87; 0.25 + 50 μM, 0.41; 0.25 + 100 μM, 0.76; 0.5 + 25 μM, 0.42; 0.5 + 100 μM, 0.33; 1 + 25 μM, 0.33; 1 + 50 μM, 0.24; and 1 + 100 μM, 0.34) in SH-SY5Y cells.
We used acridine orange (AO) staining for detection and quantification of acidic vesicular organelles (AVO) in autophagic cells [18]. To detect formation of AVO in response to nutrient-starvation, SH-SY5Y cells were cultured in serum-free medium for 0, 12, and 24 h. Further, serum-starved cells were exposed to 4-HPR and APG alone and in combination for another 24 h. Cells were incubated with AO (1 μg/ml) for 15 min and examined under a fluorescence microscope for detection of AVO. We also performed fluorescence-activated cell sorting (FACS) analysis after AO staining of the cells. In AO stained cells, cytoplasm and nucleolus showed green fluorescence (500–550 nm, FL-1 channel) whereas AVO showed bright red fluorescence (650 nm, FL-3 channel) [18]. Intensity of red fluorescence is proportional to number of AVO in autophagic cells.
To detect morphological features of apoptosis, we performed Wright staining [17] using 24 h serum-starved SH-SY5Y cells after treatments for another 24 h. Briefly, cells were fixed with 95% ethanol, stained using Wright staining kit (Fisher Scientific, Kalamazoo, MI) and examined under light microscope.
We used flow cytometry for quantification of apoptosis. After treatments, cells were harvested by trypsinization, washed with cold phosphate-buffered saline (PBS), stained with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining kit (BD Biosciences, San Jose, CA), and incubated for 15 min. Cells were subjected to flow cytometry for determining amounts of apoptosis.
Protein samples were analyzed by Western blotting [17] to explore molecular mechanisms of inhibition of autophagy and induction of apoptosis. Primary antibodies for β-actin, Bax, Bcl-2, caspase-3, Myd88, NF-κB, PARP, and TLR-4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Primary antibodies for p-Akt, Beclin 1, LC3 I/II, and mTOR were procured from Cell Signaling Technology (Danvers, MA). Bots were washed, treated with ECL Reagent Plus (GE Healthcare BioSciences, Piscataway, NJ), and exposed to X-OMAT AR films (Eastman Kodak, Rochester, NY) for autoradiography.
Data from different experiments were analyzed using Minitab® 15 Statistical Software (Minitab, State College, PA). Results were expressed as mean ± standard error of mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA) followed by Fisher’s post-hoc test. Difference between two treatments was considered significant at * p≤0.05 or ** p≤0.001.
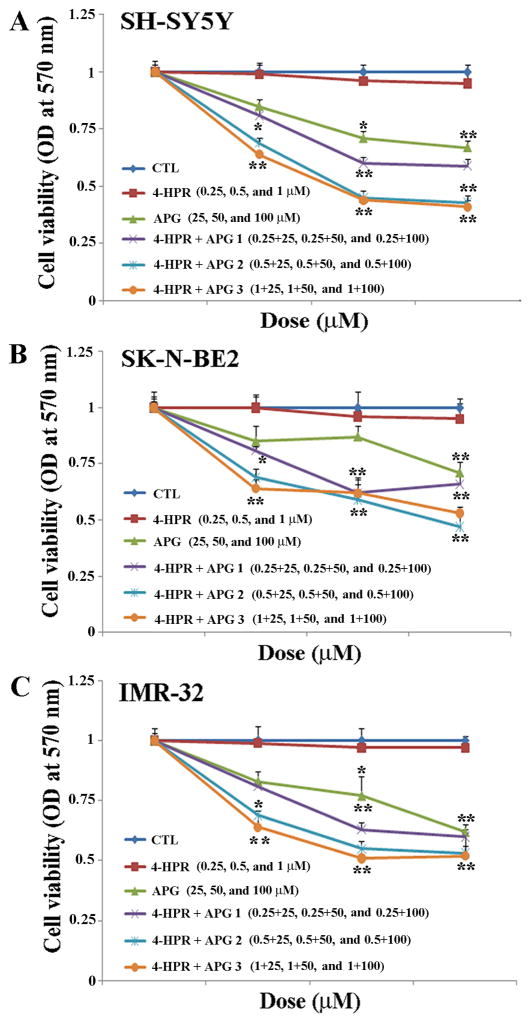
Combination of 4-HPR and APG synergistically reduced residual cell viability in the serum-starved neuroblastoma cells. Neuroblastoma SH-SY5Y, SK-N-BE2, and IMR-32 cells were grown in serum-starved media for 24 h and treated for another 24 h with 4-HPR and APG alone and in combination at different concentrations to determine the effects of drugs on residual cell viability (Fig. 1). We noticed gradual decreases in residual cell viability of all cell lines after treatments with increasing doses of 4-HPR and APG. Combination of 0.5 μM 4-HPR and 50 μM APG showed the best synergism (CI = 0.21) and more decrease in residual cell viability in SH-SY5Y cell line than that in SK-N-BE2 and IMR-32 cell lines. Therefore, we selected 0.5 μM 4-HPR or 50 μM APG as the single therapy while 0.5 μM 4-HPR + 50 μM APG as the combination therapy for maximum synergistic effect in SH-SY5Y cells in subsequent experiments.
Fig. 1.
The MTT assay for determination of reduction in residual cell viability in the serum-starved human malignant neuroblastoma cells after treatment with 4-HPR and APG at various doses. Serum-starved (24 h) neuroblastoma SH-SY5Y (A), SK-N-BE2 (B), and IMR-32 (C) cells were treated for another 24 h with different doses of 4-HPR (0.25, 0.5, and 1 μM) and APG (25, 50, and 100 μM) and their combinations. Based on CI value, as determined by Compusyn software, combination of 0.5 μM 4-HPR and 50 μM APG showed the best synergistic efficacy (CI = 0.21) for the highest reduction in residual cell viability in the serum-starved SH-SY5Y cells.
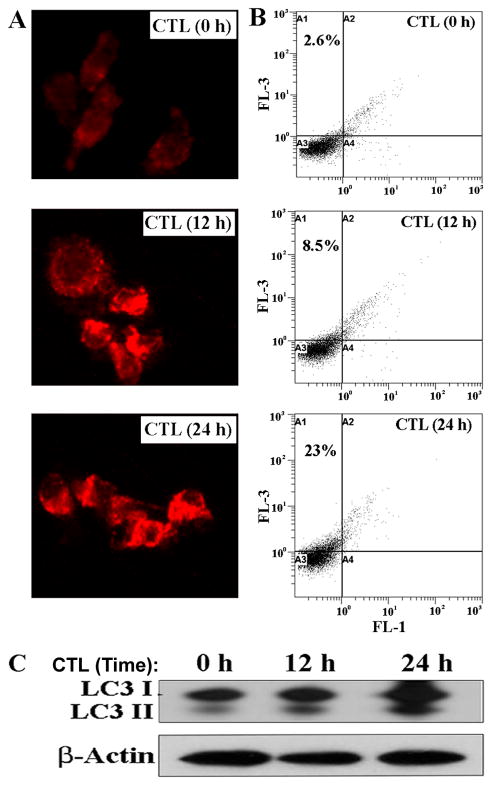
First, we tested whether serum-starvation induced autophagy in SH-SY5Y cells (Fig. 2). Following serum-starvation for 0, 12, and 24 h, SH-SY5Y cells were subjected to AO staining and fluorescence microscopy. AO staining showed progressive increase in formation of AVO in SH-SY5Y cells at 12 and 24 h time-points (Fig. 2A). To further confirm occurrence of autophagy, we performed FACS analysis using the AO stained cells. Results revealed the increases in bright red fluorescence (FL-3) at 12 and 24 h time-points following serum-starvation, indicating increased autophagic activity (Fig. 2B).
Fig. 2.
Determination of induction of autophagy in the serum-starved SH-SY5Y cells. (A) Serum-starvation of SH-SY5Y cells for 0, 12, and 24 h and then AO staining for detection of formation of acidic vesicular organelles (AVO) in autophagic cells. Microscopic images showed progressive increases in AVO in the cells under serum-starvation for 12 and 24 h. (B) Flow cytometric analysis of the serum-starved cells after staining with AO for quantification of AVO. Serum-starvation for 12 and 24 h progressively increased autophagic activity. (C) Western blotting for examination of expression of LC3 I/II.
Conversion of cytosolic LC3 I form to the autophagosome-associated LC3 II form is highly regarded as an indication of autophagy. Western blotting showed progressive increases in expression of LC3 II in SH-SY5Y cells undergoing serum-starvation for 12 and 24 h, thereby confirming the increase in autophagy in response to increase in time for serum-starvation (Fig. 2C). Thus, serum-starvation induced autophagy with upregulation of LC3 II. Expression of βactin was monitored to ensure equal loading of protein samples.
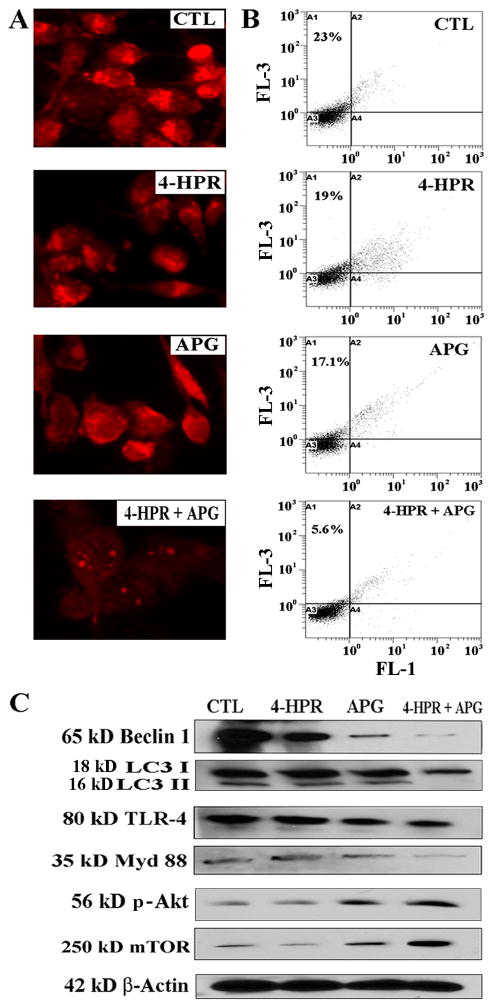
Next, we tested whether therapeutic treatments exerted any effect on autophagy induced by serum-starvation in SH-SY5Y cells (Fig. 3). The 24 h serum-starved cells were untreated (control) and treated with 4-HPR, APG, and 4-HPR + APG for another 24 h followed by AO staining. We noticed a dramatic decrease in formation of AVO in the cells after treatment with 4-HPR + APG while the control cells maintained formation of AVO (Fig. 3A). Further, we performed FACS analysis using AO stained cells from all treatments (Fig. 3B). Bright red fluorescence (FL-3) remained high in control cells (23%) but intensity of FL-3 dramatically decreased in 4-HPR + APG treated cells (5.6%), indicating dramatic inhibition of autophagy by combination therapy (Fig. 3B).
Fig. 3.
Inhibition of autophagy in serum-starved SH-SY5Y cells after treatment with combination of drugs. Serum-starvation (24 h) and then treatments for 24 h: control (CTL), 0.5 μM 4-HPR, 50 μM APG, and 0.5 μM 4-HPR + 50 μM APG. (A) Staining of cells with AO for detection of formation of AVO in autophagic cells. (B) Flow cytometric analysis of the AO stained cells from all treatments for detection and determination of autophagic cells. (C) Western blotting for levels of expression of Beclin 1, LC3 I/II, TLR-4, Myd88, p-Akt, and mTOR.
We have performed Western blotting to examine expression of some prominent proteins, which are known to modulate autophagy signaling pathways (Fig. 3C). Induction of autophagy is promoted by expression of Beclin 1, LC3 II, TLR-4, and Myd88. Levels of expression of Beclin 1 and LC3 II in the serum-starved SH-SY5Y cells were most clearly decreased after treatment with 4-HPR + APG. Likewise, levels of expression of TLR-4 and Myd88 were also most considerably decreased after treatment with 4-HPR + APG, suggesting inhibition of autophagy signaling. We also found that p-Akt/mTOR pathway, crucial for inhibition of autophagy signaling, was induced most prominently after treatment with 4-HPR + APG. Thus, combination therapy inhibited the autophagy inducing pathway but promoted the autophagy inhibitory pathway.
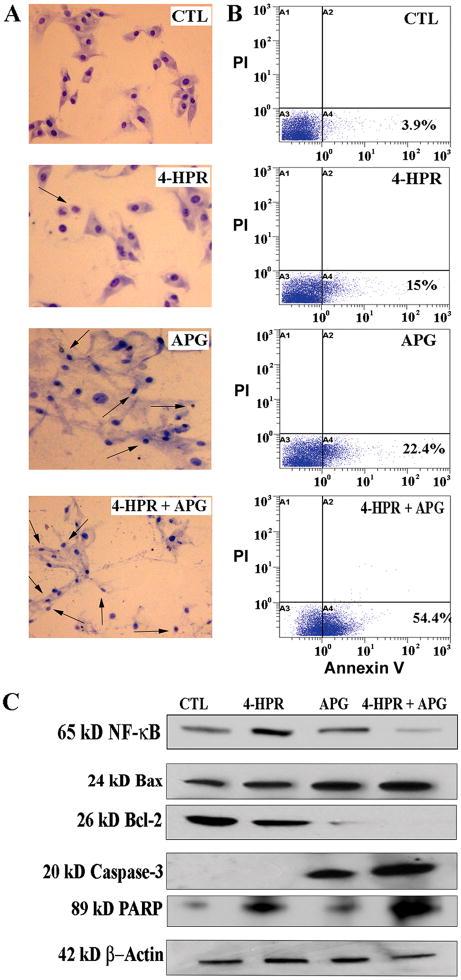
After determining suppression of autophagy in the serum-starved SH-SY5Y cells due to treatment with 4-HPR + APG, we assessed whether this combination therapy could induce apoptosis (Fig. 4) that would be a plausible mechanism for the decrease in cell viability. To detect the morphological features of apoptosis, we performed Wright staining following treatments and results showed dramatic increase in number cells with morphological features such as cell shrinkage, chromatin condensation, and membrane blebbing, indicating occurrence of apoptosis due to combination therapy (Fig. 4A). For estimation of apoptosis, we performed Annexin V-FITC/PI double labeling and flow cytometry revealing that combination therapy caused an astonishing increase (54.4%) in accumulation of cells in A4 quadrant (apoptotic cells) of the flow cytometric dot-plot; however, untreated (control) cells were not considerably accumulated in A4 quadrant (Fig. 4B).
Fig. 4.
Induction of apoptosis in the serum-starved SH-SY5Y cells after treatment with combination of drugs. Serum-starvation (24 h) and then treatments for 24 h: control (CTL), 0.5 μM 4-HPR, 50 μM APG, and 0.5 μM 4-HPR + 50 μM APG. (A) Wright staining to show morphological features of apoptosis in different treatment groups. Arrows indicated apoptotic cells. (B) Flow cytometric analyses of cells from all treatments after staining with Annexin V-FITC/PI. (C) Western blotting for levels of expression of Bax, Bcl-2, active caspase-3, and PARP fragment.
Suppression of the survival factor NF-κB can promote pro-apoptotic effects of the combination therapy. Also, alternations in levels of expression of anti-apoptotic and pro-apoptotic proteins of the Bcl-2 family can trigger activation of mitochondria-dependent caspase cascade for induction of apoptosis. We performed Western blotting to examine the expression of proteins of apoptotic pathway (Fig. 4). Combination therapy decreased the expression of NF-κB, suggesting a brake on the survival pathway in the serum-starved SH-SY5Y cells (Fig. 4C). Combination therapy most prominently increased expression of pro-apoptotic Bax and decreased expression of anti-apoptotic Bcl-2 (Fig. 4C). The alterations in levels of Bax and Bcl-2 could lead to an increase in Bax:Bcl-2 ratio for triggering activation of the mitochondria-dependent caspase cascade for apoptotic death. We noticed that combination therapy caused a huge increase in production of 20 kD caspase-3 active fragment along with cleavage of the DNA repair enzyme PARP to generate 89 kD PARP fragment (Fig. 4C) leading to apoptotic DNA fragmentation in the cells. Our estimation revealed significant increase in the formation of 89 kD PARP fragment after treatment with 4-HPR (69%), APG (34%), and combination therapy (135%) compared with untreated (control) cells. Therefore, combination therapy upregulated pro-apoptotic Bax, down regulated anti-apoptotic Bcl-2, and activated caspase-3 for induction of apoptosis in the serum-starved cells.
In this investigation, combination of 4-HPR and APG in a dose-dependent manner decreased residual cell viability in the serum-starved malignant neuroblastoma cells. Combination of 0.5 μM 4-HPR and 50 μM APG showed the best synergistic effect and caused more decrease in residual cell viability in SH-SY5Y cells than that in SK-N-BE2 and IMR-32 cells. Serum-starvation for 12 and 24 h resulted in progressive increase in autophagy in SH-SY5Y cells. Synergistic action of combination therapy significantly reduced residual cell viability in the serum-starved SH-SY5Y cells because of suppression of autophagy and induction of apoptosis.
Autophagy is induced in vital organs such as liver, kidney, and heart in response to 24 h nutrient-starvation [19]. Accordingly, we adopted 24 h serum-starvation in our experiments where we wanted to induce autophagy in malignant neuroblastoma cells. The serum-starved neuroblastoma cells when subjected to pharmacotherapy with 4-HPR + APG for 24 h demonstrated a significant decrease in autophagy. Several intracellular signaling pathways have been implicated in starvation-induced autophagy; among them p-Akt/mTOR is considered to play a prominent role in negative regulation of autophagy [20–22]. Our study showed increases in expression of p-Akt and mTOR in the serum-starved neuroblastoma cells after combination therapy.
Beclin 1 plays a crucial role in induction of autophagy by assisting formation of autophagosomes [23,24]. Also, conversion of cytosolic LC3 I form to autophagosome-bound LC3 II form is crucial for initiation of autophagy [7,8]. Treatment of the serum-starved SH-SY5Y cells with 4-HPR + APG resulted in decreases in expression of Beclin 1 and LC3 II, indicating decrease in autophagic activity. Deletion of the Beclin 1 harboring human chromosome 17q21 results in disruption of autophagy in breast carcinoma cells [25]. Toll-like receptors (TLR) induces formation of LC3 puncta, a hallmark of autophagic activity, in nutrient-starved macrophage [26]. TLR-4 in combination with its signaling adaptor Myd88 interacts with Beclin 1 and thus plays a critical role in inducing autophagy [27]. We found that treatment of the serum-starved neuroblastoma cells with 4-HPR + APG down regulated expression of both TLR-4 and Myd88, indicating inhibition of autophagy.
Inhibition of autophagy using chemotherapeutic agents is important for induction of apoptosis in cancer cells [28,29]. We noticed an increase in apoptosis after treatment of the serum-starved SH-SY5Y cells with 4-HPR + APG indicating that inhibition of autophagy triggered upregulation of pro-apoptotic factors for massive apoptotic death. Upregulation of NF-κB promotes survival and progression of a variety of human cancers [30]. We found suppression of NF-κB after combination therapy, suggesting blockade of survival pathway. Combination therapy upregulated pro-apoptotic Bax and down regulated anti-apoptotic Bcl-2, the alterations that could increase Bax:Bcl-2 ratio to trigger mitochondria-dependent caspase cascade, for ultimately increasing caspase-3 activation and activity for induction of apoptosis in the serum-starved SH-SY5Y cells. Previously, we reported that combination of retinoid and flavonoid induced apoptosis in human malignant neuroblastoma cells by upregulating Bax:Bcl-2 ratio and thus initiating the mitochondria-dependent caspase cascade [14,15].
In conclusion, serum-starvation induced autophagy as a survival mechanism in malignant neuroblastoma cells but serum-starved cells were forced to significant inhibition of autophagy and massive induction of apoptosis after treatment with combination of 4-HPR and APG.
Research Highlights.
N-(4-Hydroxyphenyl) retinamide (4-HPR) and apigenin (APG) worked synergistically.
4-HPR + APG synergistically reduced viability of serum-starved neuroblastoma cells.
Progressive serum-starvation increased autophagy in neuroblastoma cells.
4-HPR + APG blocked markers of autophagy in serum-starved neuroblastoma cells.
4-HPR + APG promoted markers of apoptosis in serum-starved neuroblastoma cells.
Acknowledgments
This work was supported in part by the R01 grants (NS-57811, NS-62327, and NS-65456) from the National Institutes of Health (Bethsda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, Dipaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;4:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beau I, Mehrpour M, Codogno P. Autophagosomes and human diseases. Int J Biochem Cell Biol. 2011;43:460–464. doi: 10.1016/j.biocel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 7.McLeland CB, Rodriguez J, Stern ST. Autophagy monitoring assay: qualitative analysis of MAP LC3-I to II conversion by immunoblot. Methods Mol Biol. 2011;697:199–206. doi: 10.1007/978-1-60327-198-1_21. [DOI] [PubMed] [Google Scholar]
- 8.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T, Nagashima K, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol. 2009;174:1705–1714. doi: 10.2353/ajpath.2009.080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 12.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 13.Karmakar S, Choudhury SR, Banik NL, Ray SK. Combination of N-(4-hydroxyphenyl) retinamide and genistein increased apoptosis in neuroblastoma SK-N-BE2 and SH-SY5Y xenografts. Neuroscience. 2009;163:286–295. doi: 10.1016/j.neuroscience.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janardhanan R, Banik NL, Ray SK. N-Myc down regulation induced differentiation, early cell cycle exit, and apoptosis in human malignant neuroblastoma cells having wild type or mutant p53. Biochem Pharmacol. 2009;78:1105–1114. doi: 10.1016/j.bcp.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmakar S, Davis KA, Choudhury SR, Deeconda A, Banik NL, Ray SK. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem Biophys Res Commun. 2009;388:705–710. doi: 10.1016/j.bbrc.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 17.Mohan N, Karmakar S, Choudhury SR, Banik NL, Ray SK. Bcl-2 inhibitor HA14-1 and genistein together adeptly down regulated survival factors and activated cysteine proteases for apoptosis in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Brain Res. 2009;1283:155–166. doi: 10.1016/j.brainres.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo PL, Hsu YL, Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 2006;5:3209–3221. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degtyarev M, De Mazière A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Huang F, Liu Y, Yang Y, Jiang Q, Xu C. Autophagy inhibition through PI3 K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB Rep. 2009;42:599–604. doi: 10.5483/bmbrep.2009.42.9.599. [DOI] [PubMed] [Google Scholar]
- 23.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of Beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 26.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longo L, Platini F, Scardino A, Alabiso O, Vasapollo G, Tessitore L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol Cancer Ther. 2008;7:2476–2485. doi: 10.1158/1535-7163.MCT-08-0361. [DOI] [PubMed] [Google Scholar]
- 29.Chen LH, Loong CC, Su TL, Lee YJ, Chu PM, Tsai ML, Tsai PH, Tu PH, Chi CW, HCSH Autophagy inhibition enhances apoptosis triggered by BO-1051, an N-mustard derivative, and involves the ATM signaling pathway. Biochem Pharmacol. 2011;81:594–605. doi: 10.1016/j.bcp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Basseres DS, Baldwin AS. Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]