Abstract
Primary infection with Toxoplasma gondii stimulates production of high levels of IL-12 and IFN-γ by cells of the innate immune system. These two cytokines are central to resistance to T. gondii. Signaling through the Toll-like receptor (TLR) adaptor protein MyD88 is indispensible for activating early innate immune responses. Recent studies have established that TLR11 plays a dominant role in sensing T. gondii. At the same time, TLR11 is represented in humans only by a pseudogene, and the major question of how innate and adaptive immune responses occur in the absence of TLR11 remains unanswered. In this article similarities and differences in sensors and effector molecules that determine host resistance to the parasite in humans and mice are discussed.
MyD88-IL-12-IFN-γ axis in host defense against Toxoplasma gondii
Toxoplasma gondii is an intracellular protozoan parasite that infects at least one-third of the world’s population. A majority of the seropositive humans display largely asymptomatic infection, with only a minor fraction of immunocompetent adults and children demonstrating non-specific clinical symptoms, predominantly limited to lymphadenopathy. In striking contrast, toxoplasmosis is a life-threatening disease in immunocompromised individuals, frequently manifesting in development of brain abscesses and encephalitis, indicating that an intact immune system is absolutely essential for controlling the parasite [1–2]. Experimental data and clinical observations strongly suggest that the innate immune recognition systems play a major role in predetermining susceptibility to the parasitic infection. As mice have become the major tool for identification of factors regulating host resistance to the parasite, we first focus on murine recognition systems and then discuss the similarities and differences in sensors and effector molecules that determine host resistance to this parasite in humans and mice.
Identification of IFN-γ as the major regulator of cell-mediated immunity to T. gondii in both mouse and human cells was a key discovery that served as a primer for enormous progress towards identification of major recognition and effector mechanisms essential for host resistance to T. gondii [3–4]. Lack of IFN-γ during initiation of immune responses to the parasite or blocking IFN-γ during chronic toxoplasmosis results in rapid mortality of infected animals, caused by uncontrolled parasite replication [4–7].
Studies during the last two decades have revealed that IL-12 plays a major role in regulation of IFN-γ production by NK and T cells (reviewed in [8–9]). In the absence of IL-12, mice are very susceptible to T. gondii, as blocking IL-12 during acute and chronic phases of infection results in uncontrolled parasite replication [10–13]. IL-12 can be produced by multiple innate immune cells, including dendritic cells (DC), macrophages, neutrophils, and monocytes [8, 14–17]. Each of these cell types plays an important role in host resistance. However, DC are crucial innate immune sensing cells responsible for the initial IL-12 responses and coordination of different cell type activation during acute responses to the parasite [18].
As discussed below, multiple mechanisms have been proposed to be involved in induction of IL-12 by DC. A groundbreaking observation revealed the TLR/IL-1R adaptor protein MyD88 as a major regulator of host defense to the parasite [19]. Because MyD88 can be activated by almost all TLRs, except TLR3 [20–21], major efforts were aimed at identifying TLRs responsible for the initial sensing of T. gondii.
TLR mediated recognition of T. gondii
TLR are a family of innate immune receptors involved in recognition of ‘pathogen-associated molecular patterns’ (PAMP), molecules that are essential for microorganisms and typically not expressed by host cells [22–24]. In the case of T. gondii, TLR11 is a major innate immune receptor that regulates IL-12 response to the parasite [25]. A defined ligand for TLR11 is an unconventional actin-binding protein profilin. Purified or recombinant T. gondii profilin elicits potent in vitro and in vivo IL-12 responses from DCs that are completely TLR11 and MyD88 dependent [25–26]. Formal evidence for profilin being the most robust parasitic protein capable of inducing IL-12 production was established by conditional inactivation of profilin in the parasite [26–27]. Both in vitro and in vivo studies demonstrate that abrogation of profilin expression completely diminishes IL-12 responses to the parasite by DC [26–27]. When combined with experiments involving TLR11-deficient animals or DC isolated from TLR11−/− or MyD88−/− mice, it can be concluded that T. gondii profilin is a major IL-12 inducing protein that elicits its immunological activity via TLR11-dependent activation of MyD88 [20]. Profilin fits to the bona fide definition of a PAMP [28–29] because it not only triggers TLR-dependent DC activation [25], but is also absolutely essential for the parasite biology and is a conserved motif across most apicomplexans [27]. Profilin-deficient T. gondii parasites are incapable of invading host cells and thus cannot survive without this molecule [27].
Despite major effects of TLR11 on induction of IL-12 responses to the parasite, the phenotype of infected TLR11−/− mice is distinct and less severe than that observed in MyD88−/− or IL-12−/− animals [12, 19–20, 30]. While in the absence of IL-12 (caused by IL-12p40 or IL-12p35 deficiency) or MyD88, mice are uniformly susceptible to parasitic infection, lack of TLR11 resulted in only partial mortality during the acute phase of the infection. TLR11−/− mice are also extremely vulnerable to the chronic phase of infection as measured by cyst burden in the brains of infected mice [25]. Nevertheless, results obtained with TLR11−/− mice indicate that additional factors should be able to trigger MyD88-dependent induction of IL-12 in response to the parasite in vivo. It was demonstrated that other TLRs, especially TLR2, can be activated in response to the parasite [31]. The defined TLR2 ligand, the parasitic GPI anchors, has been isolated and in addition to TLR2, T. gondii GPI can also activate TLR4 [32]. While in vitro experiments revealed that T. gondii GPI can trigger activation of both receptors, deficiency in TLR2 or TLR4 has little, if any, effect on IL-12 responses to the parasitic infection [19]. Instead, TLR2 seems to be involved in the regulation of TNF responses to the parasite while TLR4 plays no obvious role in regulation of cytokine production during experimental toxoplasmosis [19, 32–33].
A fundamental difference exists in the phenotypes of TLR11, TLR2, or TLR4-deficient mice infected with the parasite. TLR4−/− mice are practically indistinguishable from their wild type counterparts, indicating that this receptor displays no obvious function in host resistance to the parasite. Because TLR2 is involved in recognition of all pathogen groups including viruses, bacteria, and fungal pathogens [22], activation of TLR2 alone cannot elicit distinct parasite specific immune responses. Instead, this receptor may function as a danger-sensor, which can be activated by both microbial and self ligands. TLR2-deficient mice mount normal IL-12 responses to the parasite, but become susceptible to extremely high doses of infection [31]. Because a similar observation was made with other pathogens, where a function of TLR2 can be seen only with extremely high doses of pathogens [34], we hypothesize that enhanced susceptibility of TLR2 deficient mice to T. gondii is a result of compromised responses to both the microbial molecules and tissue damage caused by the infection. An alternate explanation stems from a hypothesis that a function of TLR2 is masked by the dominant effect of TLR11 on induction of IL-12 production by DC and other innate immune cells. This hypothesis can and needs to be addressed by careful analysis of TLR11- and TLR11xTLR2-doubly deficient animals. In either scenario, among identified innate sensors for T. gondii, TLR11 activation is unique in its ability to initiate a ‘parasite-specific‘ innate immune response. In contrast, because other TLRs, especially TLR2, are involved in recognition of multiple pathogens, their activation may coordinate, but would not be capable of inducing the highly specialized IL-12 dependent immune responses required for host defense against T. gondii.
In addition to TLRs, MyD88 is essential for signal transduction by IL-1R and IL-18R that, similar to TLRs, recruit adaptor proteins via their TIR-domains [35]. It is therefore possible that TLR-independent MyD88 activators play important roles in the regulation of host protection against T. gondii. Production of IL-1 and IL-18 requires a maturation step regulated by caspase-1 [36]. Because mice deficient in caspase-1 demonstrated no enhanced susceptibility to the parasitic infection, it was assumed that neither IL-1 nor IL-18 are important for immunity against T. gondii [37]. This conclusion is inconsistent, however, with the observations that IL-18 plays a crucial part in activation of NK cells, specifically, induction of IFN-γ production by these cells during acute toxoplasmosis [38]. Recent identification of mechanisms triggering maturation of IL-1 in the absence of caspase-1 may also be applicable to acute toxoplasmosis and thus explain a MyD88-dependent role for IL-18 in activation of NK cells during parasitic infection not seen in caspase-1 deficient mice [39].
Commensal gut bacteria drive indirect immune responses to T. gondii
The effects of TLR functions on the outcome of T. gondii infection are significantly influenced by the route of parasitic infection. For example, systemically (intraperitoneally) infected TLR9-deficient mice displayed no differences in the multiple parameters of host resistance to the parasite when compared with their wild type counterparts [20, 37]. Lack of TLR9 had no effects on the activation of innate or adaptive immune cells during acute and chronic phases of the infection. In contrast, TLR9 deficiency during the oral route of infection resulted in impaired DC maturation and migration to the draining lymph node, as well as reduced activation of CD8 and CD4 T cells [40–41]. As a consequence of impaired activation of both innate and adaptive arms of immunity against the parasite, TLR9−/− mice infected orally with T. gondii demonstrated enhanced susceptibility to the infection [40]. These results may imply that TLR9 is involved in sensing the parasite and is essential for coordinating both innate and adaptive immune responses against T. gondii. This interpretation is inconsistent with lack of detectable phenotype in TLR9−/− mice systemically infected with the parasite [37]. A similar discrepancy was observed in TLR2 or TLR4 deficient mice. Both TLR2 and TLR4 have profound roles in the regulation of immunity to the parasite during mucosal immune responses, but play limited roles during the systemic responses to the parasites [41].
Recent data revealed that it is commensal gut bacteria, rather than the parasite itself, responsible for the TLR9 as well as TLR4 dependent IL-12 production following T. gondii-mediated intestinal damage [41–43]. A role for TLR2 is more complex. This receptor can be directly activated by T. gondii and commensal bacteria in addition to host-derived activators for this receptor [32, 44–45]. The impact of commensal bacteria on immunity to T. gondii was revealed by analysis of germ-free mice lacking intestinal microbiota in addition to TLR2, TLR4, and TLR9 deficient mice infected systemically or orally with T. gondii [41]. While orally infected TLR2- and TLR4-deficient mice demonstrate impaired IL-12 and IFN-γ responses to the parasite, the same animals infected systemically with the parasite have no detectable defects in the induction of IL-12 production and Th1 responses against T. gondii. Similarly, TLR9 plays a significant role during oral but not systemic immune responses to the parasite. These results demonstrate that TLR2, TLR4, and TLR9, which systemically play a limited role in the regulation of IL-12 production in response to T. gondii, are indispensible regulators of IFN-γ mediated immunity during peroral infection. We suggest that indirect activation of these TLRs by commensal bacteria is crucial in controlling immunity to T. gondii [41, 46–47]. This conclusion is in strong agreement with work from other laboratories demonstrating that oral infection with the parasite results in expansion of gram-negative bacteria thus triggering activation of TLR4 [42, 48]. While most studies of the immune response aim to understand the ways in which infectious pathogens are directly sensed by specific receptors, these results suggest that mucosal immune response to T. gondii is indirect and requires activation of ‘bacterial’ TLRs and MyD88 in response to gut commensal bacteria. Importantly, the effects of commensal bacteria on immunity to other pathogens were recently discovered [49–50], suggesting that commensal dependent immunity is a common feature of immune defense against various groups of pathogens.
Cell-specific regulation of immune defense to T. gondii
Despite conflicting results regarding specific TLRs involved in recognition of T. gondii, there is a consensus that MyD88 is indispensible for host resistance. Previous studies demonstrated that MyD88 activation is essential for protection against T. gondii and for the generation of effective T cell responses [20]. Complete deficiency in MyD88 prevents induction of pro-inflammatory cytokines and as a result, MyD88−/− mice fail to establish strong Th1 responses to the parasite [19, 51]. An essential cell-intrinsic role for MyD88 in activation of neutrophils, macrophages, DC, and even T cells during immune response to the parasite have been described [8]. It was demonstrated that MyD88 in neutrophils, macrophages and DC is essential for IL-12 cytokine production, and TLR signaling in DC have an additional role for activation of T cell responses to the parasite [14, 19, 26, 52]. A previously unknown T cell intrinsic function for MyD88 was revealed during the chronic phase of parasitic infection [53].
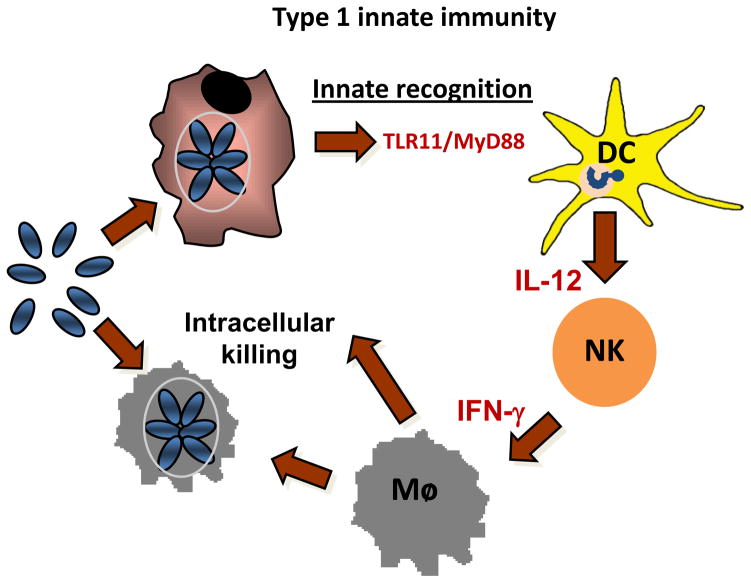
Analysis of tissue-specific MyD88-deficient mice unveiled that TLR signaling in DCs plays a critical role in the early restriction of T. gondii expansion prior to engagement of the T cell response [18]. Although MyD88 function in DCs is not required for recruitment of inflammatory cells into the peritoneum of T. gondii-infected mice, it was required for the vast majority of IL-12 production, which in turn was necessary for promoting IFN-γ production by NK cells and subsequent activation of the inflammatory monocytes in order to kill the parasites. Thus, the analysis of DC-specific MyD88-deficient mice revealed how innate cells cooperate in vivo to generate cell-mediated innate immunity to defend against a highly virulent intracellular pathogen [18]. These results illustrated in Figure 1 suggest that TLR activation in DC plays a central role in regulation of ‘type I innate immunity’, and that innate recognition predetermines host resistance versus susceptibility to the parasitic infection. Further studies are needed to clarify a role for MyD88 in neutrophils, macrophages, and monocytes in host resistance. It is especially important during analysis of mucosal responses to the parasite, since in addition to leukocytes, epithelial cells are involved in sensing both the parasite and commensal bacteria, and their role in regulation of immunity and immunopathology is unknown.
Figure 1. TLR activation in DC plays a central role in regulation of type I innate immunity.
Recent results demonstrated that the crucial function of TLR/MyD88 signaling in DCs in defense against T. gondii infection was to activate NK cells and inflammatory monocytes [18]. Although MyD88 function in DCs was not required for the recruitment of inflammatory cells into the peritoneum of T. gondii-infected mice, it was required for the vast majority of IL-12 production, which in turn was required for promoting IFN-γ production by NK cells and subsequent activation of the inflammatory monocytes and macrophages to allow them to kill the parasites [18]. This model demonstrates how innate cells cooperate in vivo to generate cell-mediated innate immunity to defend against a highly virulent intracellular pathogen.
MyD88-independent responses to T. gondii
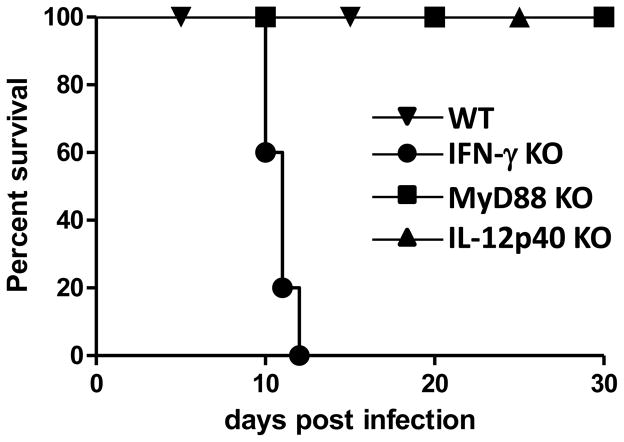
The major role for MyD88 in TLR-dependent host resistance to the parasite is well established, but it is important to emphasize the existence of immune recognition and protective immunity in the absence of MyD88. T. gondii-infected macrophages were revealed to be capable of inducing IL-12 independently of MyD88 [54]. Furthermore, despite uniform mortality of MyD88−/− mice, adaptive immunity can be generated in the absence of MyD88 with the induction of impaired and delayed, but still highly significant numbers of, IFN-γ producing CD4+ T cells [55]. These results demonstrated that while TLR/MyD88 axis plays a major role in sensing the parasite, it is not the singular method of parasite detection. Furthermore, immunization with an avirulent uracil auxotrophic parasite strain cps-1 protects MyD88−/− mice from subsequent challenge with the highly virulent T. gondii strain RH [55]. This work, for the first time, demonstrated that MyD88-independent immunity can be fully sufficient for establishing protective immune responses against the parasite. Experiments with the temperature sensitive T. gondii strain ts-4 revealed that while IFN-γ-deficient animals are highly susceptible to the infection, lack of MyD88 or IL-12p40 had no effects on host resistance to the parasite (Figure 2). Because host resistance to the ts-4 strain of T. gondii is mediated by CD8 T cells [56], these results imply that although coordinated activation of NK and CD4+ T cells is regulated by MyD88, the TLR-recognition system is dispensable for activation of CD8 T cells, at least in the case of the attenuated strains of T. gondii. It is essential to identify other recognition systems that may selectively regulate IFN-γ dependent host resistance to the parasite in the absence of MyD88. Elegantly designed experiments utilizing genetically modified T. gondii strains indisputably demonstrated that direct infection of MHC class I expressing cells is essential for induction of CD8+ T cell responses [57–58], but the nature of the cytosolic receptors regulating CD8+ T cell immunity is unclear at present.
Figure 2. MyD88-independent responses to T. gondii.
Effects of IL-12, MyD88, and IFN-γ deficiencies on survival of mice following ts-4 infection. WT, IL-12p40−/−, IFN-γ−/−, and MyD88−/− mice were infected intraperitoneally with 104 ts-4 tachyzoites and survival of mice was analyzed. Abbreviations: KO, knock out; WT, wild type.
Species specific responses to T. gondii
Mouse models have been invaluable in deciphering cellular and molecular mechanisms of induction of innate and adaptive immune responses to T. gondii. Despite the crucial importance of TLR11 and MyD88 in induction of IL-12, human TLR11 is a non-functional pseudogene [59], and the importance of MyD88 in human immune responses to T. gondii is unapparent [60]. Furthermore, while in mice the major function for IL-12 is to initiate IFN-γ dependent induction of multiple immune related GTPases [61], this effector system is largely non-functional in humans and is limited to expression of only one protein (compared to 21 IRG genes in the C57/BL6 mouse) [62]. In our view, lack of both major recognition and effector systems are linked events that predetermine chief differences between human and mouse immune systems involved in detection and elimination of T. gondii.
TLR11 is unusual in its recognition of T. gondii because it senses the pathogen based on detection of released microbial profilin, rather than by direct interaction with the live parasite [63]. This recognition system allows innate sensing of the parasite in non-infected cells, which we termed ‘recognition at a distance’ strategy. This means of recognition assures that innate sensor cells, in particular DC, can initiate immune responses without the major immunosuppressive effects observed in infected macrophages and DC.
As mice are a natural host for T. gondii, both parasite virulence factors and host defenses have likely co-evolved. It is well established that murine antigen presenting cells infected with the parasite are largely compromised in their ability to upregulate co-stimulatory molecules and secrete pro-inflammatory cytokines [64–68]. Mice appear to have developed spatially separated detection and effector functions to combat T. gondii. Non-infected DCs are capable of sensing parasite via TLR11 and producing IL-12 which promotes IFN-γ dependent killing mechanisms within infected cells. Thus, potential virulence factors aimed at suppression of TLR signaling would be ineffective in non-infected DC while secreted rhoptry proteins capable of inhibiting IRG effectors are only effective in naïve but not IFN-γ primed macrophages [3, 69]. Therefore, by recognizing the parasite at a distance, mouse innate immune cells confer a selective advantage over the parasite by avoiding or at least delaying the potent immunosuppressive effects of the parasite seen in infected cells. Humans, not being a principal host of T. gondii, are likely not under selective pressure to maintain such elaborate defenses. This possibility is at least partially supported by observations that in mice, T. gondii-strain associated virulence and host susceptibility are closely linked [70–73]. While there may be an association between disease occurrences and virulence of the parasite in humans, this correlation is less obvious [74].
The significance of TLR11 deficiency in humans is less clear given that infected individuals are capable of developing protective immunity upon infection. One possible benefit of TLR11 deficiency in humans stems from recent analysis of wild type and TLR11−/− mice infected orally with the parasite [41]. While wild type animals are extremely sensitive to the parasite as a result of TLR11-driven intestinal immunopathology, TLR11−/− mice are fully protected from parasitic infection as a result of gut commensal driven immunity against T. gondii. Remarkably, TLR11−/− mice infected orally with the parasite were not only able to control the pathogen, but were largely free from the intestinal pathology seen in wild type animals [41]. For this reason, we speculate that a balanced immune response in humans can be achieved in the absence of TLR11 via other recognition systems that are able to either sense the parasite directly or rely on other indirect stimuli, including commensal bacteria. TLR11 mediated recognition of the parasite at a distance being an essential component of innate immunity in mice, may be too ‘dangerous’ in other species, including humans, that are accidental hosts of T. gondii.
A search for human innate receptors based on analysis of genetic association between congenital toxoplasmosis in humans revealed several candidate genes that may be involved in responses to T. gondii. Among the candidates are TLR9, the purinergic receptor P2X7, and a member of the NOD-like receptor family NALP1 [75–78].
Genetic association of NALP1 with congenital toxoplasmosis is particularly intriguing because the Nalp1 gene is also located within the rat genome susceptibility/resistance region (Toxo1 region) [79]. Experimental silencing of NAPL1 expression enhances proliferation of T. gondii in NALP1-deficient human cells [78]. These results strongly indicate that NALP1 may function as an intracellular sensor for the parasite. At present the molecular events responsible for activation of NALP1 in the cytosol of the infected cells are not known, but the identification of NALP1 as a sensor for T. gondii is an important discovery that may shed light on intracellular recognition systems for the parasite.
Another molecule that can be considered as a ‘danger’ sensor, purinergic receptor P2X7, has also been associated with congenital and ocular toxoplasmosis [77]. Recent results revealed that a polymorphism at P2RX7 (a gene encoding the P2X7 receptor) influences susceptibility to T. gondii. Experiments with the ATP treated mouse or human macrophages also suggest that P2X7 regulates ROS-mediated killing of the parasite [76, 80]. In addition, P2X7 may enhance lysosomal fusion with the parasitophorous vacuole in the infected cells [80]. Similar to NALP1, activation of P2X7 should happen in conjunction with other innate immune receptors and their identification would be key to developing a better understanding of host–parasite interaction and generation of effective strategies for prevention and treatment of toxoplasmosis.
Future implications
Initial identification of MyD88 as a major regulator of resistance to T. gondii in mice precipitated many advances within the study of innate immunity to the pathogen. Both murine and human studies have contributed to identification of innate immune sensors that not only explain how mice and human develop immunity to the parasite but also revealed elements of parasite biology. These immunoparasitology approaches have uncovered a complex relationship between T. gondii and its hosts and shed light on fundamental aspects of host-parasite co-evolution. On a practical level, the identified molecules of interest will undoubtedly serve as a basis for design of vaccines targeting parasitic disease via selective activation of host immune system.
Acknowledgments
We are grateful to Donna Kirkland for the critical reading of the manuscript and apologize for not citing the valuable studies of many colleagues owing to space limitations. Work in F.Y.’s laboratory is supported by the National Institutes of Health (R01 AI085263).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasit. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan CF, et al. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative-metabolism and anti-microbial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki Y, et al. Interferon-gamma - the major mediator of resistance against Toxoplasma-gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli R, et al. Simultaneous depletion of CD4+ and CD8+ lymphocytes-t is required to reactivate chronic infection with Toxoplasma gondii. Journal of Immunology. 1992;149:175–180. [PubMed] [Google Scholar]
- 6.Suzuki Y, et al. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. Journal of Immunology. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 7.Scharton-Kersten TM, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. Journal of Immunology. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 8.Denkers EY, et al. Neutrophils, dendritic cells and Toxoplasma. Int J Parasit. 2004;34:411–421. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman LA, Hunter CA. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int Rev Immunol. 2002;21:373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, et al. Interleukin-12 is required for the T-lymphocyte-independent induction of interferon-gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter CA, et al. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16–20. [PMC free article] [PubMed] [Google Scholar]
- 12.Scharton-Kersten TM, et al. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman LA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. Journal of Immunology. 2004;173:1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 14.Mordue DG, Sibley LD. A novel population of Gr-1(+)-activated macrophages induced during acute toxoplasmosis. Journal of Leukocyte Biology. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 15.Sousa CRE, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bliss SK, et al. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. Journal of Immunology. 2000;165:4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 17.Pepper M, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. Journal of Immunology. 2008;180:6229–6236. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou B, et al. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A. 2011;4:278–283. doi: 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scanga CA, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. Journal of Immunology. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 20.Yarovinsky F. Toll-like receptors and their role in host resistance to Toxoplasma gondii. Immunol Lett. 2008;119:17–21. doi: 10.1016/j.imlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature Reviews Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 23.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Current Opinion in Immunology. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 24.Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annual Review of Immunology. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 25.Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 26.Yarovinsky F, et al. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4(+) T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Plattner F, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host & Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA. Approaching the asymptote - evolution and revolution in immunology. Cold Spring Harbor Symposia on Quantitative Biology. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 30.Yarovinsky F, et al. Recognition of Toxoplasma gondii by TLR11 Prevents Parasite-Induced Immunopathology. Journal of Immunology. 2008;181:8478–8484. doi: 10.4049/jimmunol.181.12.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mun HS, et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. International Immunology. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 32.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. Journal of Immunology. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 33.Debierre-Grockiego F, et al. Roles of glycosylphosphatidylinositols of Toxoplasma gondii - Induction of tumor necrosis factor-alpha production in macrophages. Journal of Biological Chemistry. 2003;278:32987–32993. doi: 10.1074/jbc.M304791200. [DOI] [PubMed] [Google Scholar]
- 34.Reiling N, et al. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. Journal of Immunology. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 35.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 37.Hitziger N, et al. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cellular Microbiology. 2005;7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 38.Cai GF, et al. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infection and Immunity. 2000;68:6932–6938. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer-Barber KD, et al. Cutting Edge: Caspase-1 Independent IL-1 beta Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling In Vivo. Journal of Immunology. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minns LA, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. Journal of Immunology. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 41.Benson A, et al. Gut Commensal Bacteria Direct a Protective Immune Response against Toxoplasma gondii. Cell Host & Microbe. 2009;6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimesaat MM, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of Immunology. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 44.Ju CH, et al. Early Response of Mucosal Epithelial Cells during Toxoplasma gondii Infection. Journal of Immunology. 2009;183:7420–7427. doi: 10.4049/jimmunol.0900640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? Journal of Leukocyte Biology. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 46.David R. INNATE IMMUNITY Help from ‘friendly’ bacteria. Nature Reviews Immunology. 2009;9:675–675. [Google Scholar]
- 47.Denkers EY. A Gut Feeling for Microbes: Getting It Going between a Parasite and Its Host. Cell Host & Microbe. 2009;6:104–106. doi: 10.1016/j.chom.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: A new model of inflammatory bowel disease? Journal of Infectious Diseases. 2002;185:S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 49.Lai YP, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nature Medicine. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature Medicine. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jankovic D, et al. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 52.Del Rio L, et al. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. Journal of Immunology. 2004;172:6954–6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- 53.LaRosa DF, et al. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci U S A. 2008;105:3855–3860. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim L, et al. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. Journal of Immunology. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 55.Sukhumavasi W, et al. TLR adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. Journal of Immunology. 2008;181:3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scharton-Kersten T, et al. Toxoplasma gondii: Evidence for interleukin-12-dependent and -independent pathways of interferon-gamma production induced by an attenuated parasite strain. Exp Parasitol. 1996;84:102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 57.Gubbels MJ, et al. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infection and Immunity. 2005;73:703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dzierszinski F, et al. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infection and Immunity. 2007;75:5200–5209. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roach JC, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor GA, et al. P47 GTPases: Regulators of immunity to intracellular pathogens. Nature Reviews Immunology. 2004;4:100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- 62.Hunn JP, et al. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mammalian genome. 2010;22:43–54. doi: 10.1007/s00335-010-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pifer R, et al. UNC93B1 is essential for TLR11 activation and IL-12 dependent host resistance to Toxoplasma gondii. Journal of Biological Chemistry. 2010;286:3307–14. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKee AS, et al. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. Journal of Immunology. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- 65.Bierly AL, et al. Dendritic Cells Expressing Plasmacytoid Marker PDCA-1 Are Trojan Horses during Toxoplasma gondii Infection. Journal of Immunology. 2008;181:8485–8491. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambert H, et al. The Toxoplasma gondii-Shuttling Function of Dendritic Cells Is Linked to the Parasite Genotype. Infection and Immunity. 2009;77:1679–1688. doi: 10.1128/IAI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tait ED, et al. Virulence of Toxoplasma gondii Is Associated with Distinct Dendritic Cell Responses and Reduced Numbers of Activated CD8(+) T Cells. Journal of Immunology. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butcher BA, et al. Cutting edge: IL-10-Independent STAT3 activation by toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. Journal of Immunology. 2005;174:3148–3152. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 69.Steinfeldt T, et al. Phosphorylation of Mouse Immunity-Related GTPase (IRG) Resistance Proteins Is an Evasion Strategy for Virulent Toxoplasma gondii. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saeij JPJ, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor S, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 72.Khan A, et al. Selection at a Single Locus Leads to Widespread Expansion of Toxoplasma gondii Lineages That Are Virulent in Mice. Plos Genetics. 2009:5. doi: 10.1371/journal.pgen.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grigg ME, et al. Success and virulence in toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–165. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- 74.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–442. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 75.Peixoto-Rangel AL, et al. Candidate gene analysis of ocular toxoplasmosis in Brazil: evidence for a role for toll-like receptor 9 (TLR9) Memorias Do Instituto Oswaldo Cruz. 2009;104:1187–1190. doi: 10.1590/s0074-02762009000800019. [DOI] [PubMed] [Google Scholar]
- 76.Lees MP, et al. P2X(7) Receptor-Mediated Killing of an Intracellular Parasite, Toxoplasma gondii, by Human and Murine Macrophages. Journal of Immunology. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jamieson SE, et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes and Immunity. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Witola WH, et al. NALP1 Influences Susceptibility to Human Congenital Toxoplasmosis, Proinflammatory Cytokine Response, and Fate of Toxoplasma gondii-Infected Monocytic Cells. Infect Immun. 2011;79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cavaillès P, et al. The rat Toxo1 locus directs toxoplasmosis outcome and controls parasite proliferation and spreading by macrophage-dependent mechanisms. Proc Natl Acad Sci U S A. 2006;103:744–749. doi: 10.1073/pnas.0506643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Correa G, et al. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes and Infection. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]