Abstract
Objective
Mild forms of HIV-associated neurocognitive disorders (HAND) remain prevalent in the era of combination antiretroviral therapy (cART). Although elevated LPS and immune activation are implicated in HAND pathogenesis, relationships of LPS and inflammatory markers to mild forms of HAND or impairment in specific cognitive domains are unknown. To examine these relationships, we compared plasma soluble CD14 (sCD14), CCL2, and LPS levels to neurocognitive test scores in a cART era cohort.
Methods
We analyzed plasma from HIV+ subjects (n=97) with nadir CD4 counts <300 and high frequency of HCV co-infection and illicit drug use for relationships between sCD14, CCL2, and LPS levels and neurocognitive test scores.
Results
Plasma sCD14 levels were higher in subjects with test scores indicating global impairment (p=0.007), particularly in attention and learning domains (p=0.015 and p=0.03, respectively), regardless of HAND diagnosis. Plasma sCD14 levels correlated inversely with global, attention, and learning T scores (p=0.036, 0.047, and 0.007, respectively), and yielded higher AUROC values for predicting impaired scores than single-marker models based on plasma or CSF viral load or CD4 count (AUROC 0.71, 0.81, and 0.71, respectively), and in four-marker models based on plasma sCD14 and three conventional markers compared to the three-marker models.
Conclusions
Plasma sCD14 is a biomarker associated with impaired neurocognitive testing in attention and learning domains in HIV-infected individuals with advanced disease, suggesting involvement of cortical and limbic pathways by inflammatory processes in the cART era. Plasma sCD14 is a potential biomarker to monitor HAND progression and therapeutic responses.
Keywords: HIV, AIDS, HIV-associated neurocognitive disorders, HCV, biomarkers, drug abuse
Introduction
Widespread use of combination antiretroviral therapy (cART) has decreased the prevalence of HIV-associated dementia (HAD),1,2 but the prevalence of milder forms of HIV-associated neurocognitive disorders (HAND) has remained stable or increased among both treated and untreated individuals.1,3–5 Prior to cART, low CD4 T cell counts and/or high plasma or CSF viral load (VL) were associated with HAD,6,7 but these markers are no longer associated with HAND.7 These findings suggest that cART has changed the pathophysiology of HAND.
HIV enters the CNS via trafficking of activated monocytes and lymphocytes across the blood brain barrier.8 Chronic immune activation has been implicated in HAND pathogenesis, but other mechanisms contribute as well.6 Biomarkers of monocyte activation (soluble CD14 and CCL2) and microbial translocation have been used to examine relationships between chronic inflammation, gut disease, and HAND in the cART era.9–12 Elevated plasma levels of soluble CD14 (sCD14), CCL2, and bacterial lipopolysaccharide (LPS), a marker of microbial translocation from a damaged gut, were previously associated with HIV infection, immune activation, and HAD.12–16
In a previous study, we found that elevated plasma LPS and sCD14 were associated with HAD and minor cognitive-motor disorder (MCMD) in HIV+ patients with advanced disease, but not with asymptomatic neurocognitive impairment (ANI),12 a less severe form of HAND that is increasingly prevalent (25–30% of HIV+ patients) in the cART era.1 Here, we examined relationships of plasma LPS, sCD14, and CCL2 to HAND diagnoses of varying severity and demographically normalized global and domain-specific neurocognitive test scores (T scores) in a cohort of HIV+ subjects with nadir CD4 counts <300. The majority of subjects had detectable plasma HIV RNA (> 400 copies/ml) despite treatment with cART. Because the cohort had a high frequency of illicit drug use and HCV co-infection, we also evaluated how these comorbidities modulate relationships between these biomarkers and neurocognitive outcomes. Since HAND was associated with deficits in speed of information processing, attention, learning, and motor function in pre-cART17 and post-cART18 cohorts, we hypothesized that any differences between impaired neurocognitive domains in the present study and prior cohorts might provide insight into neuroanatomical pathways currently affected by HAND and their relationship to these biomarkers.
Methods
Subjects
HIV-infected patients with nadir CD4 counts <300 cells/μL (n=97) were from four sites in the National NeuroAIDS Tissue Consortium (NNTC) (n=85) or from CHARTER, a six-center, observational cohort study (n=12). All patients provided written informed consent for use of their blood samples under local institutional IRB approval. The cohort had a high prevalence of current opiate and/or cocaine abuse, and most subjects had detectable plasma HIV RNA (61%) despite being on antiretroviral therapy at the time of testing (74%). HAND diagnoses were determined using American Academy of Neurology diagnostic criteria19 based on formal neurocognitive testing and neurological evaluation; when patients lacked other known causes for NCI, diagnoses included HAD, MCMD, ANI, and no neurocognitive impairment (no NCI). Neuropsychological impairment due to other causes (NPI-O) was diagnosed where factors in addition to primary HIV could contribute to neurocognitive impairment (Table 1). Current substance abuse was determined by the Psychiatric Research Interview for Substance Mental Disorders (PRISM)20 or Composite International Diagnostic Interview (CIDI)21 and urine toxicology at time of blood draw. Patients with severe psychiatric diagnoses that might confound neurological evaluation, a confounding neurological disorder (e.g., active CNS opportunistic infection or brain neoplasm), or active bacterial or opportunistic infection were excluded. CD4 T cell counts were performed at time of neurocognitive testing for 80 subjects and within 6 months of testing for 6 subjects. Eleven had CD4 counts >6 months from the time of neurocognitive testing; these values were excluded from analyses. Plasma and CSF HIV RNA were determined at the time of neurocognitive testing and log10 transformed for statistical analysis. “Undetectable” plasma VL values were assigned a log10 value of 2.6, reflecting the sensitivity cutoff of the assay most widely used during these assessments; values below this cutoff reflect lower assay sensitivity (25 or 50 copies) for some sites.
Table 1.
Demographic and clinical characteristics of HIV subjects in the study cohort (n=97).
| Age, years | |
|---|---|
| Mean ± SD | 47.1 ± 8.0 |
| Median (range) | 46 (32–69) |
| Gender | |
| Male (%) | 74 (76) |
| Female (%) | 23 (24) |
| Race | |
| African American (%) | 43 (44) |
| Caucasian (%) | 33 (34) |
| Hispanic (%) | 13 (13) |
| Other (%) | 8 (8) |
| Plasma HIV RNA, copies/mL | |
| Mean ± SD | 83,868 ± 162,893 |
| Median (range) | 14,924 (undetectable-843,720) |
| >400 copies/mL (%) | 59 (61) |
| <400 copies/mL (%) | 35 (36) |
| Unknown (%) | 3 (3) |
| CD4 T cell count, cells/μL | |
| Mean ± SD | 138 ± 139 |
| Median (range) | 87 (1–581) |
| Nadir CD4 T cell count, cells/μL | |
| Mean ± SD | 63 ± 65 |
| Median (range) | 40 (1–267) |
| cART | |
| Yes | 72 |
| No | 20 |
| Unknown | 5 |
| HAND diagnosis | |
| No NCI | 20 |
| ANI | 16 |
| MCMD | 20 |
| HAD | 11 |
| NPI-O* | 25 |
| Unknown | 5 |
| HCV co-infection | |
| Negative | 38 |
| Positive | 37 |
| Unknown | 22 |
| Substance abuse | |
| Opiates | 35 |
| Cocaine | 22 |
| None | 38 |
| Unknown | 2 |
| Neurocognitive test scores, mean ± SD | |
| Global T | 40.3 ± 9.0 |
| Motor T | 38.3 ± 13.8 |
| SIP T | 42.7 ± 10.1 |
| Attention T | 43.1 ± 9.8 |
| Learning T | 37.5 ± 12.7 |
| Memory T | 37.7 ± 12.1 |
| Fluency T | 45.5 ± 12.1 |
| Executive Function T | 42.3 ± 9.1 |
Abbreviations used: HAND=HIV-associated neurocognitive disorders; No NCI= no neurocognitive impairment; ANI=asymptomatic neurocognitive impairment; MCMD=minor cognitive-motor disorder; HAD=HIV-associated dementia; NPI-O= Neuropsychological impairment due to other causes.
Neuropsychological impairment due to other causes (NPI-O) was diagnosed in patients with factors in addition to primary HIV that could contribute to neurocognitive impairment (past traumatic head injury (n=5), remote cerebral vascular accident (n=4), low pre-morbid function or learning disability (n=4), remote CNS toxoplasmosis (n=3), visual loss (n=1), chronic hepatic cirrhosis (n=1), old frontal lesion (n=1), remote neurosyphilis (n=1), chronic obstructive pulmonary disease (n=1), and unknown NPI-O diagnosis (n=4)).
Plasma sCD14, CCL2, and LPS
Plasma sCD14 and CCL2 levels were quantified by ELISA (R&D Systems). Plasma LPS levels were quantified as described.12
Neurocognitive testing
Subjects were administered a comprehensive test battery designed to assess seven categories of neurocognitive function: motor skills, speed of information processing (SIP), attention (working memory), learning (memory encoding), memory (memory recall), language fluency, and executive function as described.22 Test batteries were identical for all CHARTER and NNTC sites. Performance on each test generated a raw score, which was converted to a demographically corrected T score. T scores were then grouped by cognitive domain, and a global T score was generated from the individual T scores. T scores correlate negatively with severity of neurocognitive impairment, with values below 40 signifying impairment.
Statistical analysis
Medians were compared by Mann-Whitney U test. Correlations between continuous variables were analyzed by Spearman correlation. Of primary interest was to explore possible associations between biomarkers and T scores and to examine whether plasma sCD14 was more strongly associated with T scores than other markers examined. Thus, p-values were not adjusted for multiple comparisons. Associations of biomarkers to T scores were examined for predictive value by generating area under receiver operating characteristic (AUROC) curves calculated to measure the ability of each single marker to predict neurocognitive impairment. AUROC was also calculated for a three-marker model (current and nadir CD4 count, plasma VL) and four-marker model (current and nadir CD4 count, plasma VL, plasma sCD14). All analyses were performed using GraphPad Prism and R ver. 2.9, R-project (www.cran.org).
Results
T scores reflect severity of categorical HAND diagnoses in the study cohort
The cohort consisted of 97 HIV+ patients with nadir CD4 count <300 (Table 1). Subjects were predominantly male (76%) with mean age of 47 years and relatively high plasma VL (median 14,924 copies/mL; range, undetectable - 843,720) and low CD4 counts (median 87 cells/μL, range, 1–581) compared to other current cohorts, and high frequency of drug abuse (59%) and HCV co-infection (38%). 72 subjects were on cART (74%), 59 (61%) had detectable plasma VL (>400 HIV RNA copies/mL), and 20 (21%) were not on cART; for 5 (5%) of the subjects, the use of cART was unknown.
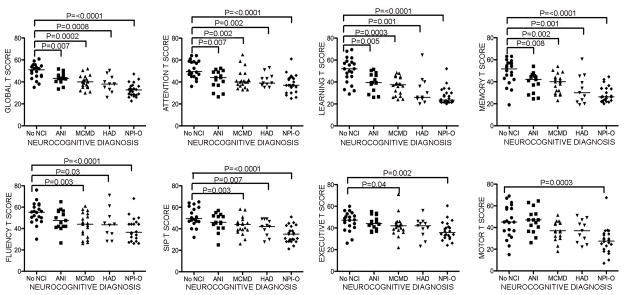
As samples were obtained from multiple sites within 2 consortia, we first evaluated categorical cognitive diagnoses within the cohort by comparing global T scores grouped by HAND diagnosis to those of subjects with no NCI. The mean global T score for the study cohort was 40.3; mean T scores for the seven domains of neurocognitive function assessed by formal testing ranged from 37.5 to 45.5 (Table 1). Global T scores were significantly lower for more severe HAND diagnoses when compared to subjects with milder HAND diagnoses or no NCI (Figure 1). We also evaluated T scores by domain and found strong associations between lower attention, learning, and memory T scores and all HAND diagnoses (Figure 1). Fluency, SIP, and executive function T scores were lower in the groups with symptomatic (MCMD, HAD, NPI-O), but not asymptomatic (ANI) HAND diagnoses. Thus, impairment in attention, learning, and memory are associated with ANI, MCMD, and HAD, whereas impairment in fluency, SIP, and executive function were associated only with symptomatic HAND diagnoses.
Figure 1. Subjects with more severe HAND diagnoses have lower global T scores.
Neurocognitive test scores were compared to each HAND diagnosis in HIV+ subjects. No NCI is followed in severity by ANI, in which subjects demonstrate neurocognitive impairment but have no daily functioning deficits, which in turn is followed in severity by MCMD, where mild neurocognitive impairment is combined with daily functioning deficits, and then HAD, in which patients evidence moderate to severe neurocognitive impairment and daily functioning impairments. Global T scores were lower with diagnoses of increased severity of neurocognitive impairment as compared to no NCI (top left panel). Domain T scores for attention, learning, and memory were significantly lower for all diagnoses of impairment, whereas T scores for fluency, SIP, executive function, and motor were generally associated only with more severe forms of neurocognitive impairment (i.e., HAD and MCMD) and NPI-O. (SIP, speed of information processing; NCI, neurocognitive impairment; ANI, asymptomatic neurocognitive impairment; MCMD, minor cognitive-motor disorder; HAD, HIV-associated dementia; NPI-O, other neuropsychiatric impairment). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test; significant differences (p<0.05) are indicated.
Higher plasma sCD14 levels are associated with global T scores indicating neurocognitive impairment
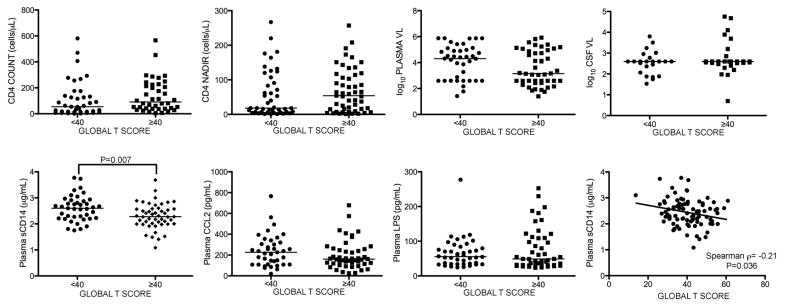
To examine the relationship of plasma sCD14, CCL2, and LPS across the spectrum of HAND diagnoses, we compared biomarker levels to global T scores by grouping subjects dichotomously as impaired or unimpaired. Plasma sCD14 levels were significantly higher in the impaired group (global T scores <40) (Figure 2; p=0.0067), and correlated negatively with global T (Figure 2; p=0.036). The correlation between CD4 count and global T trended toward significance (p=0.086), while plasma LPS, CCL2, and plasma and CSF VL were not associated with a significant difference in global T. Among virologically suppressed subjects with plasma VL <400 copies/mL (n=35, median CD4 175 cells/μL, range 6 – 581), no difference in plasma sCD14 levels was detected between the impaired and unimpaired groups (p=0.76). When NPI-O subjects (n=68) were excluded, there was still a trend toward significantly higher sCD14 levels in the impaired group (p=0.06). Thus, higher plasma sCD14 levels were associated with impaired global T scores, current CD4 counts trended toward a significant association, and plasma and CSF VL, LPS, and CCL2 were not associated with impaired global T scores.
Figure 2. Higher plasma sCD14 levels are associated with global T scores indicating neurocognitive impairment.
Subjects were grouped by global T <40 or ≥40 to compare biomarker levels. Only plasma sCD14 levels were significantly different between subjects with versus without impaired global T scores (second row, first panel), and correlated negatively with global T scores (second row, last panel). (VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test, and significance among continuous variables was calculated using Spearman rho correlation; significant differences (p<0.05) are indicated.
Higher plasma sCD14 levels are associated with impaired test performance in attention and learning domains
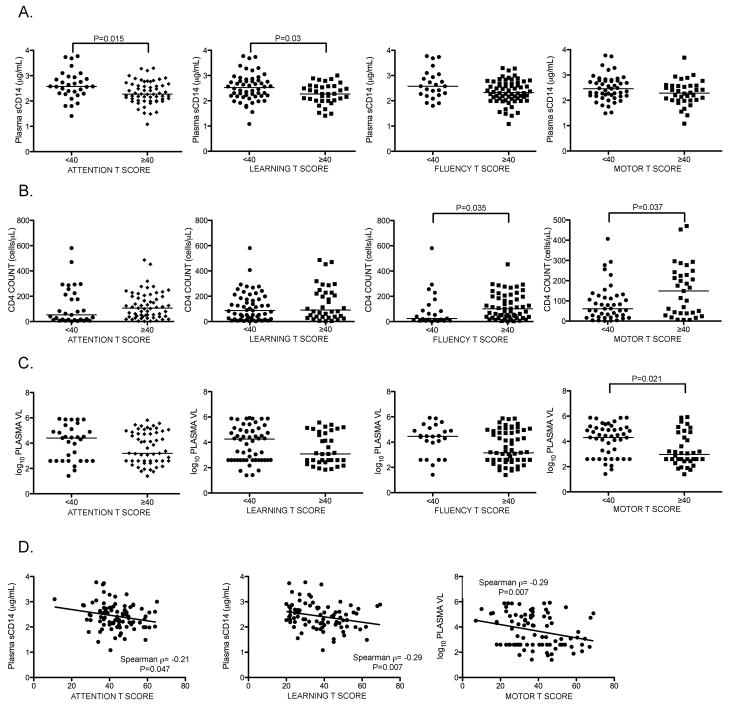
Plasma sCD14 levels were significantly lower and CD4 counts trended toward lower levels in subjects with global T <40 (Figure 2). To determine which domain T scores were associated with these differences, subjects were grouped according to domain score cutoffs <40 or ≥40 and levels of these markers were compared for each domain. Analysis was also performed for plasma VL. Plasma sCD14 levels were higher in subjects with impaired attention and learning T scores (Figure 3A; p=0.015 and p=0.03, respectively), and Spearman correlation was also significant (Figure 3D; p=0.047 and p=0.007, respectively). Analysis of CD4 count and plasma VL by domain T score showed lower CD4 counts in subjects with impaired fluency and motor T scores (Figure 3B; p=0.035 and p=0.037, respectively) and higher plasma VL in subjects with impaired motor T (Figure 3C; p=0.021). Spearman correlation of plasma VL to motor T was significant (Figure 3D; p=0.0068). Therefore, subjects with impaired attention and learning T scores had significantly elevated plasma sCD14 levels, those with impaired motor T scores had higher plasma VL and lower CD4 counts, and those with impaired fluency T scores had lower CD4 counts than those with unimpaired scores in these domains.
Figure 3. Higher plasma sCD14 levels are associated with impaired test performance in attention and learning domains.
Plasma levels of sCD14, CD4 count, and plasma VL were compared to domain T scores in subjects grouped by T scores <40 or ≥40. A. Plasma sCD14 levels were elevated in subjects with attention and learning T scores <40, B. Lower CD4 counts in subjects with fluency and motor T scores <40. C. Higher plasma VL in subjects with motor T scores <40. D. Plasma sCD14 correlated negatively with attention and learning T scores, and plasma VL correlated negatively with motor T scores. (VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test, and correlations between continuous variables were analyzed using Spearman rho correlation; significant differences (p<0.05) are indicated.
Plasma sCD14 levels are associated with impaired attention, learning, and global T scores in univariate and multivariate models
We used ROC curves to examine the ability of plasma sCD14 to predict impaired neurocognitive test scores (global, attention, and learning T scores) compared to conventional disease markers (current and nadir CD4 count, plasma VL, CSF VL). First, ROC curves were generated for all cases in which current and nadir CD4 count, plasma and CSF VL, and sCD14 levels were all available (n=43); these subjects all had current CD4 counts <300. Plasma sCD14 yielded higher AUROC values in single-marker models for predicting impaired global T, attention T, and learning T scores (below 40) (AUROC=0.71, 0.81, and 0.71, respectively) than current CD4 count AUROC=0.65, 0.74, and 0.61, respectively), nadir CD4 count (AUROC=0.61, 0.72, and 0.61, respectively), plasma VL (AUROC=0.55, 0.56, and 0.56, respectively), or CSF VL (AUROC=0.52, 0.62, and 0.53, respectively). Four-marker models based on plasma sCD14 in combination with current CD4 count, nadir CD4 count, and plasma VL yielded higher AUROC values for predicting impaired global T, attention T, and learning T scores (below 40) compared to the three-marker models (AUROC 0.73, 0.87, and 0.71 vs. 0.67, 0.76, and 0.63, respectively). The effect sizes for plasma sCD14 as defined by Cohen’s d for predicting impaired global, attention, and learning T scores (below 40) were 0.69, 1.3, and 0.8, respectively. The cutoff value for plasma sCD14 levels that maximized accuracy for predicting global T-score < 40 was 2.6 μg/ml (specificity and sensitivity 0.78 and 0.60, respectively, determined by maximizing the Youden Index). In subjects with plasma VL>400 copies/mL (n=35), which included 23 subjects on cART, AUROC values in single-marker models for plasma sCD14 were higher than those yielded by current or nadir CD4 count and CSF or plasma VL for predicting impaired global and learning T scores (below 40) (AUROC=0.70 and 0.71, respectively, vs. AUROC=0.61 and 0.48, respectively, for current CD4; AUROC 0.63 and 0.61, respectively, for nadir CD4; AUROC 0.61 and 0.67, respectively, for CSF VL; AUROC 0.61 and 0.51, respectively, for plasma VL). A four-marker model based on plasma sCD14 levels in combination with three conventional markers (current CD4, nadir CD4, and plasma VL) increased AUROC values for predicting impaired global and learning T scores (below 40) compared to the three-marker model (AUROC 0.73 and 0.76 vs. 0.66 and 0.58, respectively). Therefore, plasma sCD14 is a biomarker associated with neurocognitive impairment in HIV+ subjects with CD4 count <300 and plasma VL >400 copies/mL, and sCD14 yields higher AUROC values for predicting impaired neurocognitive test scores compared to conventional HIV disease markers in univariate and multivariate models.
Plasma sCD14 levels do not distinguish between subjects stratified by HAND clinical diagnosis
Because global and domain T scores were strongly associated with HAND clinical diagnoses and plasma sCD14 levels were associated with impaired global T scores, we examined plasma sCD14 levels in subjects grouped by HAND clinical diagnoses. Despite the association between elevated plasma sCD14 level and impaired global, attention, and learning T scores, no differences in sCD14 levels were found between those grouped by HAND diagnosis and no NCI (see Figure, Supplemental Digital Content 1). Furthermore, plasma sCD14 levels compared between no NCI and any HAND diagnosis excluding NPI-O also showed no difference. Likewise, plasma and CSF VL, current and nadir CD4 count, and plasma CCL2 and LPS levels did not differ by HAND diagnosis, with rare exception. Thus, plasma sCD14 levels did not distinguish between subgroups stratified by HAND clinical diagnoses compared to those with no NCI.
HIV+ opiate users demonstrate impaired learning, memory, motor, and SIP T scores compared to non-users but no difference in plasma sCD14 levels
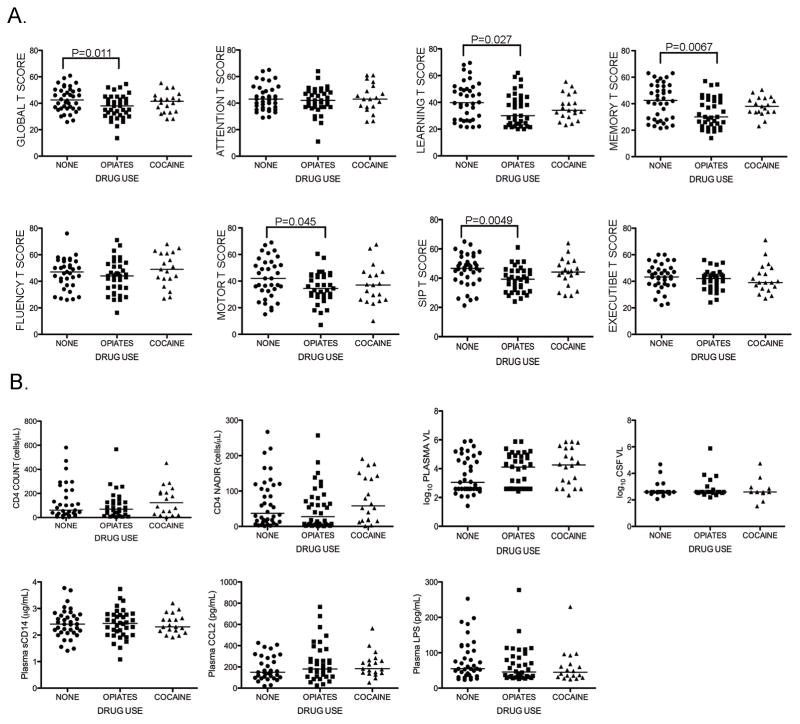
Illicit drug use modulates the immune system and may increase risk of developing HAND.23,24 To assess whether specific patterns of illicit drug use modify the relationship between plasma sCD14 levels and neurocognitive performance, we compared biomarkers and T scores of opiate or cocaine users to those of non-users. This analysis demonstrated lower global T scores (Figure 4A) associated with opiate compared to no current drug use (p=0.011). Opiate users had lower T scores for learning, memory, motor, and SIP domains (p=0.027, p=0.0067, p=0.045, p=0.0049, respectively). There was no difference in global or domain T scores between opiate users and cocaine users (Figure 4A), or in biomarker levels between opiate or cocaine users and non-users (Figure 4B). We also found no difference in plasma sCD14 levels between non-users with impaired vs. unimpaired global T scores. Thus, HIV+ opiate users had lower global T scores, along with lower learning, memory, motor and SIP domain T scores, but no difference in plasma sCD14 levels compared to non-users.
Figure 4. HIV+ opiate users demonstrate impairment in global T scores and learning, memory, motor, and SIP domain T scores but no difference in plasma sCD14 levels compared to non-users.
Global and domain T scores, as well as plasma biomarker levels and HIV disease markers, were compared between subjects with no current drug use, current opiate use (including 18 subjects using both opiates and cocaine), and current cocaine use. Subjects were categorized as opiate or cocaine users if they endorsed syndromic abuse and/or urine toxicology was positive for opiates (including methadone) or cocaine (n=35 and 22, respectively). Subjects with negative toxicology screening for opiates or cocaine and without PRISM or CIDI diagnosis of substance abuse were categorized as non-users (“None”, n=38). A. Opiate users had lower global T scores than cocaine users or non-users, and lower learning, memory, motor and SIP T scores than non-users. B. Current and nadir CD4 count, plasma VL, CSF VL, and plasma sCD14, CCL2, and LPS levels did not differ between groups. (SIP, speed of information processing; VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test; significant differences (p<0.05) are indicated.
HCV co-infection is associated with high plasma LPS but no difference in global T scores or plasma sCD14 levels
HCV co-infection has been associated with immune activation, elevated plasma LPS levels, and increased frequency of neurocognitive impairment in AIDS.12,25–27 Therefore, we examined whether HCV serostatus modified the relationship between biomarkers and neurocognitive test performance. Plasma LPS were higher in HCV+ compared to HCV- subjects (Figure, Supplemental Digital Content 2; p=0.0097). HCV+ subjects also had significantly higher current or nadir CD4 counts and lower plasma VL than HCV- subjects. Plasma sCD14 levels and global T scores between groups defined by HCV serostatus showed no difference (Figure, Supplemental Digital Content 2).
Discussion
In a cohort of HIV+ subjects with nadir CD4 counts <300, we demonstrated elevated plasma sCD14, but not CCL2 or LPS, in subjects with neurocognitive test scores indicating global impairment compared to unimpaired subjects. Furthermore, plasma sCD14 level yielded higher AUROC values for predicting impaired neurocognitive testing than plasma or CSF VL in subjects with CD4 counts <300 cells/μL or plasma VL >400 copies/mL in univariate and multivariate models. Plasma sCD14 levels were higher in subjects with impaired testing in attention and learning domains and correlated inversely with global, attention, and learning T scores, suggesting these domains are the main drivers of impairment. These findings indicate that chronic inflammation, in particular monocyte activation, is associated with dysfunction of cortical and limbic pathways in HIV-infected individuals in the cART era, and argue in support of a shift in neuropathology underlying HAND in the cART era from the subcortical process characteristic of pre-CART HAND toward a mixed cortical and subcortical process.1,28–31 The explanation for the association between sCD14, indicative of monocyte activation, and dysfunction of cortical and limbic pathways is unknown, but one possible reason is the preferential recruitment of circulating activated monocytes to these brain regions. Indeed, chemokines that attract monocytes are highly expressed in the cortex and limbic system.32 Thus, therapeutic strategies to reduce chronic inflammation in HIV infection may be beneficial for prevention or treatment of HAND.
By using continuous descriptors of neurocognitive status (T scores) and evaluating relationships between plasma sCD14 and neurocognitive impairment by specific domain, our findings extend those of our previous study12 and those of Ryan, et al.14. Ryan and colleagues found elevated plasma sCD14 in HIV+ subjects with impaired neurocognitive testing, but did not compare plasma sCD14 levels to impairment by specific domain. Because our previous study showed elevated plasma sCD14 levels in MCMD and HAD, we designed the current study to evaluate milder forms of HAND. The current cohort included more subjects with ANI (8% vs. 17%) and fewer subjects with HAD (28% vs. 11%) compared to the previous cohort12, and plasma sCD14 levels were compared to global and domain-specific T scores. As expected, T scores were more sensitive indicators of neurocognitive impairment than categorical diagnoses. Importantly, we found that using continuous descriptors rather than categorical clinical diagnoses was important for demonstrating an association between plasma sCD14 levels and impaired neurocognitive function.
Although monocyte activation continues to play a role in HAND pathogenesis in the cART era,34–36 additional mechanisms are likely to contribute as well.33 Indeed, for virologically suppressed subjects on cART (plasma VL <400 copies/mL), we found no difference in plasma sCD14 levels between those with impaired vs. unimpaired global T scores. However, our study was underpowered for this analysis, given the sample size for virologically suppressed subjects (n=35). HAND pathogenesis in the cART era is likely to be multifactorial, with other processes distinct from monocyte activation contributing to development of neurocognitive impairment in both viremic and aviremic subjects.
Previously, we found higher levels of plasma LPS and CCL2 in HAD,12 while others reported neurocognitive impairment associated with low nadir CD4.37,38 We did not find these associations in the present study, however, most likely reflecting cohort differences. Subjects in the present study had nadir CD4 <300, making this relationship difficult to assess. Compared to the previous cohort12, the current cohort included more subjects not currently on cART (12% vs. 21%, respectively), more with ANI, and fewer with HAD. Despite these cohort differences, however, sCD14 levels were associated with neurocognitive impairment in both studies. Together, these findings imply that the monocyte response to activating stimuli (indicated by sCD14 levels), rather than elevated LPS or other activating stimuli per se, is related more closely to underlying mechanisms involved in cART-era HAND in subjects with advanced disease.
Studies of neurocognitive function in HIV infection and illicit drug use have reported mixed results, some suggesting more severe cognitive impairment in HIV+ subjects associated with drug use and others reporting no association.39–41 We found evidence that opiate abuse adversely affects test performance on learning, memory, motor, and SIP tasks in HIV+ individuals, but no association between plasma sCD14 levels and drug use. Thus, illicit drug use is not likely to account for the association between elevated sCD14 levels and impaired global, attention, and learning T scores in the study cohort.
HCV co-infection has been associated with increased risk of impaired neurocognitive function in patients with advanced HIV disease.27,42,43 In well-controlled HIV, however, Clifford, et al did not find any differences in neurocognitive function associated with HCV co-infection.44 Our subgroup analysis is consistent with this finding, as we found no differences in global cognitive function associated with HCV co-infection; HIV infection was better controlled in the HCV+ subgroup, based on higher CD4 counts and lower VL. Thus, the impact of HCV co-infection on neurocognition may be attenuated when HIV is well controlled.
Our study has several limitations including its cross-sectional design and small sample size, which may have limited the power to detect some associations between biomarkers and neurocognitive test scores, particularly in subgroup analyses. Also, the narrow selection criteria used to define the study cohort (CD4 nadir <300 cells/μL) limits our findings to those with advanced HIV disease. As such, we cannot reach any conclusion regarding the predictive ability of sCD14 compared with CD4 nadir. We included NPI-O subjects because many of them likely exhibit neurocognitive effects attributable to HIV. Furthermore, there was marked site-to-site variation in assigning a diagnosis of NPI-O, and HIV patients frequently have complex histories with more than one risk factor for cognitive impairment, making it difficult to ascertain relative contributions of HIV versus co-morbid conditions in contributing to cognitive impairment. No adjustment was made for multiple comparisons, as there was no a priori hypothesis regarding specific cognitive domains that would be associated with elevated levels of inflammation markers or LPS. Finally, the study cohort was from two larger cohorts — NNTC, which specifically recruits individuals with advanced disease, and CHARTER — to represent a diverse population of HIV-infected individuals with a broad range of viral loads. The results cannot be generalized to all populations of HIV-infected patients because the cohort had a high prevalence of co-morbid drug use and HCV co-infection, 26% were not on cART at the time of testing, and only 36% had undetectable VL.
Overall, our study provides evidence that inflammation continues to contribute to HAND pathogenesis in the cART era. Plasma sCD14, a marker of monocyte activation, is associated with impaired attention and learning test performance in patients with nadir CD4 counts <300 who are not well-controlled on cART. Plasma sCD14 was not clearly linked to HAND diagnoses, suggesting that additional mechanisms contribute to these clinical diagnoses. Together, these findings point toward neuroanatomical pathways involved in cART era HAND, and suggest that plasma sCD14 is a potential biomarker that may be useful to monitor HAND progression and therapeutic responses.
Acknowledgments
Funding
This work was supported by NIH DA26322 and MH083588 to D.G. and an MSINAD Scholar Grant (funded through R25MH080663) to J.L.L. Core facilities were supported by the Harvard Center for AIDS Research and DFCI/Harvard Center for Cancer Research grants. This publication was made possible from NIH funding through the NIMH and NINDS Institutes supporting sites in the NNTC by the following grants: Manhattan HIV Brain Bank: U01MH083501, R24MH59724 Texas NeuroAIDS Research Center U01MH083507, R24 NS45491 National Neurological AIDS Bank 5U01MH083500, NS 38841 California NeuroAIDS Tissue Network U01MH083506, R24MH59745 Statistics and Data Coordinating Center U01MH083545, N01MH32002. CNS HIV Antiretroviral Therapy Effects Research (CHARTER) was supported by N01MH22005. The funders, NNTC, and CHARTER had no role in study design, data analysis, or preparation and decision to submit the publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH, NNTC, or CHARTER.
We thank NNTC and CHARTER sites for providing plasma samples and clinical data for AIDS patients. We also acknowledge support from the Harvard Center for AIDS Research Biostatistics Core and the Mount Sinai Institute for NeuroAIDS Disparities.
Footnotes
The study authors report no disclosures or competing interests.
Data presented in part at Conference on Retroviruses and Opportunistic Infections (CROI), 27 February 2011 – 2 March 2011, Boston, MA.
References
- 1.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010 Jun;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 2.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008 Jun-Jul;16(2):94–98. [PubMed] [Google Scholar]
- 3.Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008 Feb;63(2):213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 4.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 2003 Apr;13(2):195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002 Dec;8( Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 6.McArthur JC, McDermott MP, McClernon D, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004 Nov;61(11):1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 7.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001 Jan 23;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005 Jan;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 9.Sevigny JJ, Albert SM, McDermott MP, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol. 2007;64(1):97–102. doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Brew BJ, Letendre SL. Biomarkers of HIV related central nervous system disease. Int Rev Psychiatry. 2008 Feb;20(1):73–88. doi: 10.1080/09540260701878082. [DOI] [PubMed] [Google Scholar]
- 11.Bandaru VV, McArthur JC, Sacktor N, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007 May 1;68(18):1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998 Sep 15;92(6):2084–2092. [PubMed] [Google Scholar]
- 14.Ryan LA, Zheng J, Brester M, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001 Sep 15;184(6):699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 15.Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 17.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 18.Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008 Feb;20(1):33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 19.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996 Sep;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 21.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988 Dec;45(12):1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 22.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004 Sep;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 23.Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006 Jun;1(2):182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009 Jun;19(2):215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letendre S, Paulino AD, Rockenstein E, et al. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007 Aug 1;196(3):361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- 26.Morgello S. The nervous system and hepatitis C virus. Semin Liver Dis. 2005 Feb;25(1):118–121. doi: 10.1055/s-2005-864787. [DOI] [PubMed] [Google Scholar]
- 27.Parsons TD, Tucker KA, Hall CD, et al. Neurocognitive functioning and HAART in HIV and hepatitis C virus co-infection. AIDS. 2006 Aug 1;20(12):1591–1595. doi: 10.1097/01.aids.0000238404.16121.47. [DOI] [PubMed] [Google Scholar]
- 28.Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008 Feb;20(1):15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 29.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005 Jun;64(6):529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 31.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005 Dec;78(6):1204–1209. doi: 10.1189/jlb.0405222. [DOI] [PubMed] [Google Scholar]
- 33.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010 Apr;16(2):115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 34.Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004 Dec;45(6):549–559. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 35.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003 Apr;9(2):205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 36.Gartner S. HIV infection and dementia. Science. 2000 Jan 28;287(5453):602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008 Oct;24(10):1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- 38.Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol. 2006 Oct;12(5):387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- 39.Egan V, Brettle RP, Goodwin GM. The Edinburgh cohort of HIV-positive drug users: pattern of cognitive impairment in relation to progression of disease. Br J Psychiatry. 1992 Oct;161:522–531. doi: 10.1192/bjp.161.4.522. [DOI] [PubMed] [Google Scholar]
- 40.Selnes OA, McArthur JC, Royal W, 3rd, et al. HIV-1 infection and intravenous drug use: longitudinal neuropsychological evaluation of asymptomatic subjects. Neurology. 1992 Oct;42(10):1924–1930. doi: 10.1212/wnl.42.10.1924. [DOI] [PubMed] [Google Scholar]
- 41.Royal W, 3rd, Updike M, Selnes OA, et al. HIV-1 infection and nervous system abnormalities among a cohort of intravenous drug users. Neurology. 1991 Dec;41(12):1905–1910. doi: 10.1212/wnl.41.12.1905. [DOI] [PubMed] [Google Scholar]
- 42.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004 Mar 23;62(6):957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis. 2008;27(2):11–17. doi: 10.1300/j069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifford DB, Smurzynski M, Park LS, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology. 2009 Jul 28;73(4):309–314. doi: 10.1212/WNL.0b013e3181af7a10. [DOI] [PMC free article] [PubMed] [Google Scholar]