Abstract
Polyethylene glycol (PEG)-surface modification can make nano-materials highly hydrophilic, reducing sequestration in the reticuloendothelial system. In this study, polyamidoamine (PAMAM) dendrimers bearing gadolinium chelates were PEGylated with different PEG-chain lengths and the effects on paramagnetic and pharmacokinetic properties were evaluated. Specifically, gadolinium chelate-bearing PAMAM dendrimers (G4 and G5) were conjugated with two different PEG chains (2k and 5k). Long PEG chains (5k) on the smaller (G4) dendrimer resulted in reduced relaxivity compared to unPEGylated dendrimer whereas short PEG (2k) and larger (G5) dendrimer produced comparable relaxivities to unPEGylated G4 dendrimer. The relaxivity of all PEGylated or lysine conjugated dendrimers increased at higher temperature, while that of intact G4 Gd-PAMAM-dendrimer decreased. All PEGylated dendrimers had minimal liver and kidney uptake and remained in circulation for at least 1 hour. Thus, surface-PEGylated Gd-PAMAM-Dendrimers showed decreased plasma clearance and prolonged retention in the blood pool. Shorter PEG, higher generation conjugates led higher relaxivity.
Keywords: MRI, dendrimer, polyethylene glycol (PEG), relaxivity, pharmacokinetics
Background
Dendrimers are unique synthetic polymeric macromolecules, which are very monodispersed and therefore, their pharmacokinetic properties can be precisely characterized. Dendrimers have the advantage of multiple surface functional groups that can be used to target or label the dendrimer for imaging and drug delivery. The interior space of dendrimers can be used to conjugate or encapsulate drugs [1–7]. For imaging, dendrimers can be labeled with a variety of imaging “beacons”. For instance, dendrimers conjugated to fluorescent dyes have been used for fluorescent imaging [3,6]. Dendrimers loaded with gold nanoparticles or conjugated to iodinated-compounds have been studied for X-ray computed tomography (CT) imaging [3,6,8–10]. Dendrimers containing chelated gadolinium can be used for magnetic resonance imaging (MRI) [3,6,10,11].
MRI applications of dendrimers are particularly appealing because the dendrimer can be labeled with multiple Gd-chelates creating a strongly paramagnetic macromolecule with higher relaxivities on a molar basis than conventional chelates, due to slower molecular tumbling rates of large macromolecules. Most FDA approved Gd-chelates, such as Gd(diethylene-triamine-pentaacetic acid (DTPA))(H2O)]2− (Magnevist, Berlex, Princeton, NJ), typically demonstrate relaxivities of 4–5 mM−1s−1 in clinically relevant magnetic fields ranging from 0.3-T to 7-T, [12,13]. Dendrimer-based MR contrast agents demonstrate enhanced relaxivity (20–30 mM−1s−1at magnetic fields ranging from 0.5-T to 1.5-T), while displaying reduced plasma clearance and prolonged retention in the blood pool [10,11]. This could have useful applications in the study of vessels for a variety of disease conditions (atherosclerosis, angiogenesis, inflammation, lymphatic imaging among others). Gd-chelate labeled Polyamidoamine (PAMAM) dendrimers are commonly proposed as imaging/drug delivery agents because they demonstrate a relatively neutral charge and excellent solubility. However, such agents commonly are excreted by the kidneys and/or the liver reducing plasma concentrations. Moreover, the size of the dendrimer, the composition of its interior and the surface charge also determine its overall biodistribution [10,11,14–16].
Polyethylene glycol (PEG) modification (PEGylation) of dendrimers could be a helpful addition, since PEGylation not only reduces the toxicity of the nanoparticles but also helps the molecule escape recognition by the reticuloendothelial system (RES) [17] resulting in reduced plasma clearance, which could be helpful for imaging and drug delivery applications [7,17,18]. Early results suggest that PEGylation could be a useful modification of the dendrimer [19,20], however, characterization of the length of PEGylation, size of dendrimer, the effects on relaxivity and clearance have not been systematically studied. Previous PEGylated Gd-dendrimers were prepared by conjugating both PEG and Gd-chelate to the terminal groups of the dendrimer [19,20]. PEG was attached to some termini, Gd-chelate was attached to others. However, it is possible that this preparation method interfered with the uniformity of the dendrimers. Kono et al designed a fully PEGylated dendrimer conjugating other compounds [21–23] that improved homogeneity. Thus, PEGylation may have the desirable features of reduced toxicity and improved retention in the circulation with decreased rates of non-specific renal and hepatic uptake. However, before this can be shown to be effective, the effects on relaxivity of dendrimer-based MRI contrast agents must be examined.
In this study, fully PEGylated PAMAM dendrimers loaded with Gd-DTPA were prepared. Lysine (Lys) was attached before the PEG modification for conjugation of Gd-DTPA to the side chain. As a control, unPEGylated acetylated lysine-conjugated PAMAM dendrimer was used. To investigate the influence of the dendrimer generation as well as the PEG chain length, the following compounds were synthesized: PEG2k-PAMAM(G4), PEG5k-PAMAM(G4) and PEG2k-PAMAM(G5) (Figure 1). 1H NMRD (nuclear magnetic relaxation dispersion) and temperature-dependent relaxivities were examined and in vivo dynamic MRI of mice injected with these agents was performed.
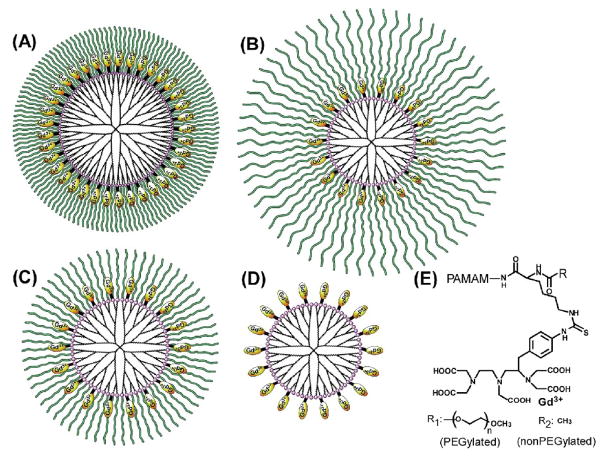
Figure 1.
PEGylated dendrimers conjugated with Gd-DTPA via lysine. (A) PEG2k-PAMAM(G5), (B) PEG5k-PAMAM(G4), (C) PEG2k-PAMAM(G4) and (D) Ac-PAMAM (G4). The chemical structure of the surface modification is shown in panel (E).
Methods
Materials
PEG2k-PAMAM(G4), PEG5k-PAMAM(G4), PEG2k-PAMAM(G5) and Ac-PAMAM(G4) loaded with Gd-DTPA were synthesized by previously reported methods [18]. Gd chelation was performed, as previously described [19,24,25]. The determination of Gd loading was performed by inductively coupled plasma optical emission spectroscopy (ICP-OES, SPS7800), according to the manufacturer’s instructions (SII NanoTechnology Inc., Chiba, Japan). The gel permeation chromatography (GPC) system (TSKgel G3000PW and G400PW; Tohso Corp., Japan) had differential refractive index detection (RI-930; Jasco Inc., Japan). Dendrimers were eluted with 10 mM phosphate buffer containing 0.2 M sodium sulfate (pH 7.4) at 0.5 ml·min−1 at 30°C. The polyethylene glycol with different molecular weight was used as a standard. And, the hydrodynamic diameters were estimated according to our previous report [26].
Relaxivity
1H NMRD was measured by a custom-designed variable field T1-T2 analyzer (Southwest Research Institute, San Antonio, TX), as previously described [25]. Briefly, the field strength was varied from 0.035 to 1.5-T (1.5–62 MHz) at 23°C. Solutions of PEGylated and nonPEGylated dendrimers with Gd-DTPA (PEG2k-PAMAM(G4), PEG5k-PAMAM(G4), PEG2k-PAMAM(G5) and Ac-PAMAM(G4)) (1 mMGd, PBS, pH 7.4) were prepared. Additional measurements were performed at 0.8-T field strength (35 MHz) by changing the temperature to 16, 23, and 37°C.
Animals
Dynamic contrast enhanced (DCE) MRI was performed, as described previously [25]. Mice (n=3 in each group) were chemically restrained with 2% isoflurane (Abbott Laboratories, NJ) and O2 was delivered using a Summit Anesthesia Solutions vaporizer (Bend, OR) at a flow rate of 0.8 L/min. Respiration rate was maintained at 25–30 respirations per min and monitored using a Biopac System MP150 (Biopac Inc., Goleta, CA). A tail vein cannula consisting of a 30-gauge needle attached to Tygon tubing (0.01 in i.d.) was then established. Prior to injection of the contrast agent, a T1 map was obtained by using a 3D-T1 fast field echo image (3D-T1FFE) sequence at two different flip angles (repetition time/echo time 15.1/1.98 ms; flip angles 5° and 24 °; water fat shift 2.508 pixels; acquisition matrix size 472 × 236 × 40; reconstruction matrix size 512 × 512 × 40; voxel resolution 170 × 170 × 600 μm; slice thickness 0.6 mm; two averages; scan time about 2 min). One hundred microliters (100 μL) of PEGylated and nonPEGylated Gd-dendrimers, (PEG2k-PAMAM(G4), PEG5k-PAMAM(G4), PEG2k-PAMAM(G5) and Ac-PAMAM(G4)) was injected (0.03 mmol Gd/kg) into the tail vein. The temperature of the mouse was maintained at 32°C using a Polyscience Model 210 heating recirculator with 3M Fluorinert Electronic Liquid FC-77 and body temperature was monitored using FOT-M fiberoptic sensors (Fiso Technologies Inc., San Jose, CA) and a UMI-8 multichannel instrument (Fiso Technologies Inc.). All DCE-MRI images were obtained at 3 T (Achieva, Philips Medical Systems, Best, Netherlands) with a 1-inch round receive-only modified saddle coil. A region of interest was placed on the kidney, the heart, the jugular vein and the muscle, and time-intensity curves were obtained. In addition, the single slice data was processed into 3D images with the maximum intensity projection (MIP) method. All animal experimental procedures were carried out in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), National Research Council, and approved by the local Animal Care and Use Committee.
Results
Preparation of PEGylated dendrimer-based MR contrast agents
Three kinds of PEGylated PAMAM dendrimers bearing Lys(Z), PEG2k-PAMAM(G5), PEG5k-PAMAM(G4) and PEG2k-PAMAM(G4), and a nonPEGylated dendrimer, PAMAM(G4) bearing acetylated lysine, Ac-PAMAM(G4), were synthesized. It was shown that essentially all amino groups of the PAMAM dendrimer were modified with Lys(Z) (or acetylated Lys(Z)) and PEG. Then, DTPA with p-isothiocyanatobenzyl group was attached to the ε amino group of lysine of these four dendrimers after the deprotection of the Z group, as described previously [18]. About 30 % of the amino groups were reacted with bifunctional DTPA [18]. Finally, gadolinium (III) was chelated to these dendrimers (Figure 1). The Gd content of these dendrimers were estimated by ICP-OES, indicating that Gd ions were chelated to essentially all DTPA molecules. These dendrimers were analyzed by GPC, and the data are displayed in Table 1. The theoretical molecular weights are also shown. The experimental values were much less than predicted. This is likely because the dendrimer is a spherical, highly dense molecule compared with the standard molecule, PEG [26]. The hydrodynamic diameters (Table 1) were estimated from the GPC data according to our previous report [26]. PEG2k-PAMAM(G5) and PEG5k-PAMAM(G4) were larger than PEG2k-PAMAM(G4) and the nonPEGylated Ac-PAMAM-G4 dendrimer.
Table 1.
Molecular weights and relaxivity of dendrimer-based MR contrast agents.
Relaxivity of the PEGylated dendrimers
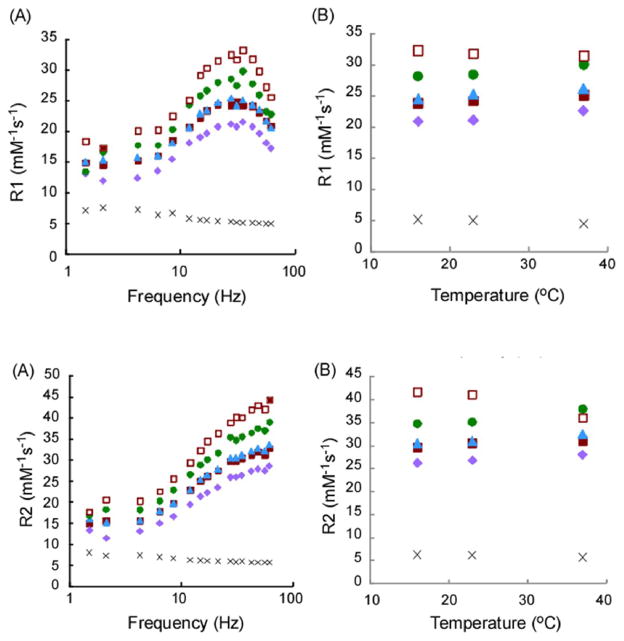
T1 relaxivity (Figure 2) shows frequency-dependent (Figure 2A) and temperature-dependent (Figure 2B) r1 values. Dendrimer relaxivity was ~6-fold higher than Gd-DTPA, which is consistent with the previous reports [11,19,25]. The highest and lowest relaxivities in our synthesized dendrimers at 23°C were observed in PEG2k-PAMAM(G5) (30 mM−1s−1, at 35MHz/0.8-T) and PEG5k-PAMAM(G4) (21 mM−1s−1, at 35MHz/0.8-T), respectively. The nonPEGylated Ac-PAMAM-G4 dendrimer and PEG2k-PAMAM(G4) exhibited the intermediate relaxivity (25 mM−1s−1, at 31MHz/0.7-T and 25 mM−1s−1, at 28MHz/0.7-T, respectively). Maximum r1 values of all samples were observed around 30 Hz. These profiles have been observed in other dendritic polymers of a similar size and a spherical shape [25,27–29]. Our PEGylated dendrimers exhibited increased r1 at 37°C compared with 16°C while non-PEGylated PAMAM G4 dendrimers showed lower r1 even with full conjugation with Gd-chelates [25]. T2 relaxivity demonstrated r2 values (Figure 3) with similar trends to r1, except for gradual increase in r2 with increasing frequency. It is likely that the water accessibility of PEGylated dendrimers was different from the nonPEGylated PAMAM dendrimer probably because accelerated motion of grafted PEG chains at high temperature facilitates access of water molecules to the Gd-chelate inside the PEG chains. The larger (G5) PEGylated PAMAM dendrimer also had enhanced relaxivity because of slower rotation of molecule.
Figure 2.
T1-Relaxivity of PEG2k-PAMAM(G5) (circle), PEG5k-PAMAM(G4) (diamond), PEG2k-PAMAM(G4) (triangle), Ac-PAMAM (G4) (closed square). Gd-DTPA (cross) and PAMAM (G4) (open square) (A) Frequency dependency and (B) Temperature dependency.
Figure 3.
T2-Relaxivity of PEG2k-PAMAM(G5) (circle), PEG5k-PAMAM(G4) (diamond), PEG2k-PAMAM(G4) (triangle), Ac-PAMAM (G4) (closed square). Gd-DTPA (cross) and PAMAM (G4) (open square). (A) Frequency dependency and (B) Temperature dependency.
MRI of mice injected with the PEGylated dendrimers
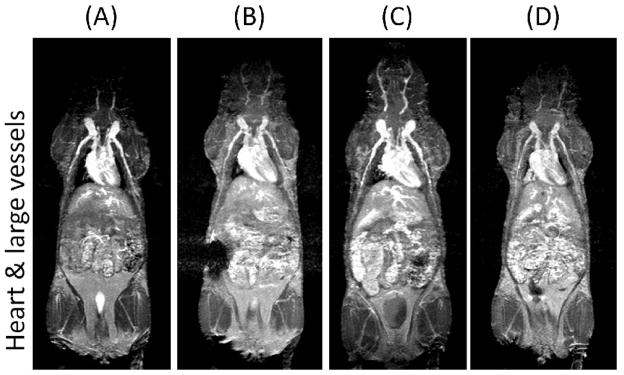
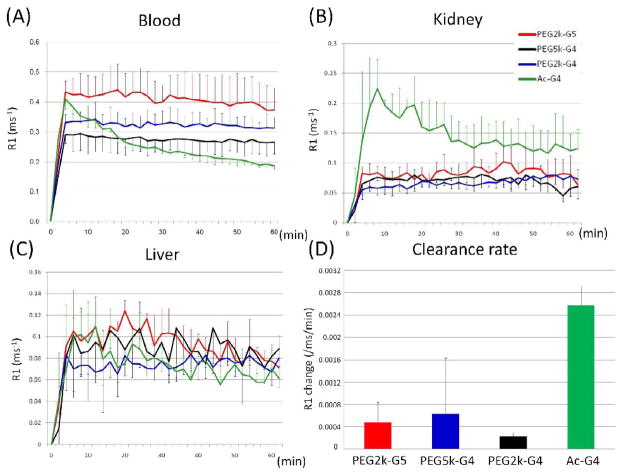
The compounds were injected into the tail vein of mice. MR images after injection with PEGylated and nonPEGylated dendrimers of the heart and kidneys of mice are shown in Figure 4. The PEGylated dendrimers enhanced the heart and blood pool and demonstrated prolonged retention in the circulation for at least 1 hour, however, the nonPEGylated dendrimer showed decreasing enhancement of the major vessels over the same time. Essentially no differences were observed among the three types of PEGylated dendrimers regarding their pharmacokinetics. In the case of the nonPEGylated dendrimer, on the other hand, the kidney gradually enhanced due to renal clearance. The MR intensity plots of the blood pool, kidneys and the liver validated imaging results, as shown in Figure 5. Neither the PEGylated nor the nonPEGylated dendrimers demonstrated visible enhancement in the liver. The clearance rate from the blood pool was calculated from Figure 5A and shown in Figure 5C. The clearance rate of all PEGylated dendrimers was clearly lower than the nonPEGylated dendrimer. In summary, the in vivo imaging data indicate that PEGylated dendrimers demonstrate decreased plasma clearance and minimal trapping by the RES. Despite a 2000-fold injected dose difference, this data was consistent with previous biodistribution results using indium-111 radiolabelled PEGylated dendrimers [18].
Figure 4.
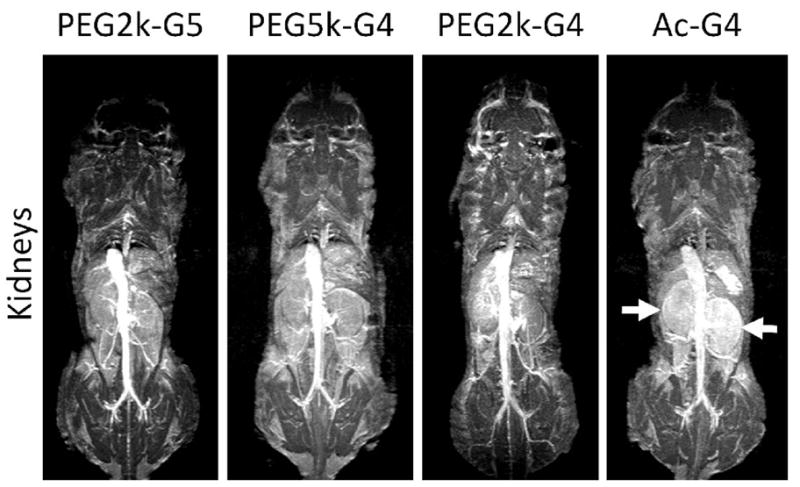
3D MR images of the heart and large vessels (top) and kidneys (bottom) of mice injected with (A) PEG2k-PAMAM (G5), (B) PEG5k-PAMAM (G4), (C) PEG2k-PAMAM (G4) and (D) Ac-PAMAM (G4) at 1h after injection. Only Ac-PAMAM (G4) shows decreasing intensity from the heart and vessels and enhancement in the kidney parenchyma (arrows).
Figure 5.
(A) Time-course of gadolinium concentration (mM) calculated from the T1 time in the jugular vein (A), kidney (B) and liver (C) of PEG2k-PAMAM(G5) (red), PEG5k-PAMAM(G4) (black), PEG2k-PAMAM(G4) (blue) and Ac-PAMAM (G4) (green). (D) Clearance rate (mMGd/min) from blood pool.
Discussion
In this study, we show that PEGylation of a Gd-labeled PAMAM dendrimer reduces the relaxivity, plasma clearance and alters susceptibility to temperature. While PEGylation reduces relaxivity by decreasing access to water, this effect can be recovered by using a larger dendrimer (G5 vs. G4) with inherently higher relaxivities due to slower molecular tumbling rates. The addition of PEG to a dendrimer prolongs retention in the vascular pool, a property that could be useful for vascular imaging in atherosclerosis, cancer and inflammatory disorders as well as for improving drug delivery.
The central advantage of dendrimer PEGylation is prolonged circulation times. In vivo biodistribution has previously demonstrated this advantage of PEGylated dendrimers [18], however, this study is different in that substantially higher doses of gadolinium chelated to dendrimer were injected in this study whereas indium-111 was previously injected at 1/2000th the dose. Despite of the huge differences in injected dose, the current MRI data also shows that the PEGylated dendrimers are retained in the blood, while the nonPEGylated dendrimer accumulated in the kidney, consistent with the previous report [18]. Previous reports suggested that in order to achieve stability in the blood pool, PAMAM dendrimers as large as G7 would be necessary, however, dendrimers of this size are more difficult to reliably synthesize and inevitably accumulate in the liver [11,24]. By PEGylating smaller dendrimers, which are more readily synthesized, blood pool retention can be achieved without the disadvantages of using a larger generation dendrimer [11,19,24]. Therefore, a fully PEGylated dendrimer is a useful platform for designing MRI contrast agents or other nano-sized drug carriers.
PEGylation has the disadvantage of reducing relaxivity. The relaxivity of Gd-chelate compounds depends on the water exchange rate as well as molecular mobility [25,27,28]. The PEGylation may interfere with water access, reducing relaxivity. This can be compensated for by using a slightly larger dendrimer with reduced molecular mobility and thus higher relaxivity. Our results indicate that the shorter PEG-2k chains produced only modest reductions in relaxivity. However, the longer PEG (5 kDa) clearly reduced both the r1 and r2 of the molecule. The molecular weight, diameter, and r1 relaxivity of these dendrimers are shown in Table 1. Even though PEG2k-PAMAM(G5) and PEG5k-PAMAM(G4) are similar in diameter, the T1 relaxivities were different indicating the substantial effect of PEGylation on relaxivity. Between the dendrimers with the same PEG length (PEG2k-PAMAM(G5) and PEG2k-PAMAM(G4)), the larger dendrimer exhibited the higher relaxivity. This indicates that both the dendrimer size and the PEG length affected the relaxivity. Thus, among the compounds tested PEG2k-PAMAM(G5) was the most effective contrast agent from the relaxivity point of view. Interestingly, while PEGylation did not strongly affect the 1H NMRD patterns compared to unPEGylated dendrimers [25,27–29], the temperature-dependent relaxivity was slightly different from unPEGylated G4 dendrimer which showed higher r1 at lower temperature. The nonPEGylated but lysine conjugated dendrimer also showed a similar temperature profile to the PEGylated dendrimers, which exhibited high r1 at higher temperature. In addition, our previous reports indicate that lysine-based dendri-grafts showed similar properties [25]. Lysine-based dendri-grafts have inner terminal amines and a loose interior structure, therefore, some of the chelates were not exposed on the surface, which is similar to PEG-conjugated dendrimer-agents but different from conventionally prepared agents. In addition, the conjugated lysine on the surface might be responsible for this behavior. The side chain of lysine is 4-aminobutyl, so that all of the conjugated chelates may not be on surface, but rather, inside the dendrimer or obscured by the PEG chain. As the chelate mobility increases with increasing temperature, water molecules would have greater accessibility to these “hidden” chelates resulting in higher relaxivities.
In conclusion, we have prepared and characterized the paramagnetic characteristics and in vivo pharmacokinetics of PEGylated PAMAM Gd-DTPA labeled dendrimers with different generations of dendrimers and PEG lengths. The relaxivities of these dendrimers were ~6-fold higher than Gd-DTPA chelates alone. The modification of short PEG chains of 2k to the PAMAM dendrimer minimally affected relaxivity, but longer PEG chains (5k) affected relaxivity more strongly. The PEGylated dendrimers were stably retained in the blood pool for at least 1 h, while nonPEGylated dendrimer were rapidly excreted through the kidney. Although PEGylated dendrimers were originally developed as drug delivery systems [21,26], they may also hold potential as stand-alone diagnostic agents or together as theranostic agents. These kinds of functional nanoparticles have become more important for achieving the personalized medicine.
Acknowledgments
All sources of support for research: This project was supported in part by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Improvement of Research Environment for Young Researchers (FY 2008-2012)). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
We thank Noriko Tano for her assistance of figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Svenson S, Tomalia DA. Dendrimers in biomedical applications-reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev. 2008;60:1037–55. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem Rev. 2009;109:49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]
- 5.Medina SH, El-Sayed MEH. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 2009;109:3141–57. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 6.Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110:1857–1959. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- 7.Gajbhiye V, Kuma V, Tekade K, Jain NK. Pharmaceutical and biomedical potential of PEGylated dendrimers. Cur Pharm Des. 2007;13:415–29. [Google Scholar]
- 8.Guo R, Wang H, Peng C, Shen M, Pan M, Cao X, et al. X-ray Attenuation Property of Dendrimer-Entrapped Gold Nanoparticles. J Phys Chem C. 2010;114:50–56. [Google Scholar]
- 9.Kojima C, Umeda Y, Ogawa M, Harada A, Magata Y, Kono K. X-ray computed tomography contrast agents prepared by seeded growth of gold nanoparticle in PEGylated dendrimer. Nanotechnology. 2010;21:245104. doi: 10.1088/0957-4484/21/24/245104. [DOI] [PubMed] [Google Scholar]
- 10.Longmire M, Choyke PL, Kobayashi H. Dendrimer-based contrast agents for molecular imaging. Current Topics in Medicinal Chemistry. 2008;8:1180–6. doi: 10.2174/156802608785849021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Aime S, Botta M, Fasano M, Terreno E. Prototropic and Water-Exchange Processes in Aqueous Solutions of Gd(III) Chelates. Acc Chem Res. 1999;32:941–949. [Google Scholar]
- 13.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H, Kawamoto S, Jo SK, Sato N, Saga T, Hiraga A, et al. Renal tubular damage detected by dynamic micro-MRI with a dendrimer-based magnetic resonance contrast agent. Kidney Int. 2002;61:1980–1985. doi: 10.1046/j.1523-1755.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Saga T, Kawamoto S, Sato N, Hiraga A, Ishimori T, et al. Dynamic micro-magnetic resonance imaging of liver micrometastasis in mice with a novel liver macromolecular magnetic resonance contrast agent DAB-Am64-(1B4M-Gd)64. Cancer Res. 2001;61:4966–4970. [PubMed] [Google Scholar]
- 16.Sena LM, Fishman SJ, Jenkins KJ, Xu H, Brechbiel MW, Regino CA, et al. Magnetic resonance lymphangiography with a nano-sized gadolinium-labeled dendrimer in small and large animal models. Nanomedicine (Lond) 2010;5:1183–91. doi: 10.2217/nnm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwald RB, Conover CD, Choe YH. Poly(ethylene glycol) conjugated drugs and prodrugs: a comprehensive review. Crit Rev Ther Drug Carrier Sys. 2000;17:101–61. [PubMed] [Google Scholar]
- 18.Kojima C, Regino C, Umeda Y, Kobayashi H, Kono K. Influence of dendrimer generation and polyethylene glycol length on the biodistribution of pegylated dendrimers. Int J Pharm. 2010;383:293–6. doi: 10.1016/j.ijpharm.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, et al. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn Reson Med. 2001;46:781–8. doi: 10.1002/mrm.1257. [DOI] [PubMed] [Google Scholar]
- 20.Margerum LD, Campion BK, Koo M, Shargill N, Lai JJ, Marumoto A, et al. Gadolinium(III) D03A macrocycles and polyethylene glycol coupled to dendrimers. Effect of molecular weight on physical and biological properties of macromolecular magnetic resonance imaging contrast agents. J alloys and compounds. 1997;249:185–190. [Google Scholar]
- 21.Kono K, Kojima C, Hayashi N, Nishisaka E, Kiura K, Watarai S, et al. Preparation and cytotoxic activity of poly(ethylene glycol)-modified poly(amidoamine) dendrimers bearing adriamycin. Biomaterials. 2008;29:1664–75. doi: 10.1016/j.biomaterials.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Haba Y, Harada A, Takagishi T, Kono K. Synthesis of biocompatible dendrimers with a peripheral network formed by linking of polymerizable groups. Polymer. 2005;46:1813–20. [Google Scholar]
- 23.Kono K, Fukui T, Takagishi T, Sakurai S, Kojima C. Preparation of poly(ethylene glycol)-modified poly(amidoamine) dendrimers with a shell of hydrophobic amino acid residues and their function as a nanocontainer. Polymer. 2008;49:2832–2838. [Google Scholar]
- 24.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Konishi J, et al. Micro-MR angiography of normal and intratumoral vessels in mice using dedicated intravascular MR contrast agents with high generation of polyamidoamine dendrimer core: reference to pharmacokinetic properties of dendrimer-based MR contrast agents. J Magn Reson Imaging. 2001;14:705–13. doi: 10.1002/jmri.10025. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa M, Regino CA, Marcelino B, Williams M, Kosaka N, Bryant LH, Jr, et al. New Nano-sized Biocompatible MR Contrast Agents Based on Lysine-Dendri-Graft Macromolecules. Bioconjugate Chem. 2010;21:955–60. doi: 10.1021/bc9005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjugate Chem. 2000;11:910–7. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 27.Rudovsky J, Botta M, Hermann P, Hardcastle KI, Lukes I, Aime S. PAMAM dendrimeric conjugates with a Gd-DOTA phosphinate derivative and their adducts with polyaminoacids: the interplay of global motion, internal rotation, and fast water exchange. Bioconjugate Chem. 2006;17:975–87. doi: 10.1021/bc060149l. [DOI] [PubMed] [Google Scholar]
- 28.Jászberényi Z, Moriggi L, Schmidt P, Weidensteiner C, Kneuer R, Merbach AE, et al. Physicochemical and MRI characterization of Gd3+-loaded polyamidoamine and hyperbranched dendrimers. J Biol Inorg Chem. 2007;12:406–20. doi: 10.1007/s00775-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 29.Nwe K, Bryant LH, Brechbiel MW., Jr Poly(amidoamine) Dendrimer Based MRI Contrast Agents Exhibiting Enhanced Relaxivities Derived via Metal Preligation Techniques Bioconjugate Chem. 2010;21:1014–17. doi: 10.1021/bc1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]