Abstract
Invariant Natural Killer T (iNKT) cells expressing an invariant Vα14 TCR recognize self and foreign lipid antigens when presented by the non-classical MHCI homolog CD1d. While the majority of known iNKT cell antigens is characterized by the presence of a single α-linked sugar, mammalian self-antigens are β-linked glycosphingolipids, posing the interesting question of how the semi-invariant TCR can bind to such structurally distinct ligands. Here we show that the mouse iNKT TCR recognizes the complex β-linked antigen isoglobotrihexosylceramide (iGb3, 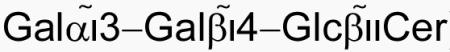 ) by forcing the proximal β-linked sugar of the trisaccharide headgroup to adopt the typical binding orientation of α-linked glycolipids. The squashed iGb3 orientation is stabilized by several interactions between the trisaccharide and CD1d residues. Finally, the formation of novel contacts between the proximal and second sugar of iGb3 and CDR2α residues of the TCR suggests an expanded recognition logic that can possibly distinguish foreign antigens from self-antigens.
) by forcing the proximal β-linked sugar of the trisaccharide headgroup to adopt the typical binding orientation of α-linked glycolipids. The squashed iGb3 orientation is stabilized by several interactions between the trisaccharide and CD1d residues. Finally, the formation of novel contacts between the proximal and second sugar of iGb3 and CDR2α residues of the TCR suggests an expanded recognition logic that can possibly distinguish foreign antigens from self-antigens.
Introduction
Invariant Natural Killer T (iNKT) cells constitute a T lymphocyte population that is able to modulate the functions of several different cell types such as NK cells, B cells and T cells, therefore bridging the innate and adaptive immune response to self and foreign Ags. Critical to this role is the ability of iNKT cells to rapidly secrete, upon stimulation, large amounts of both Th1 and Th2 cytokines, such as IFN-γ, TGF-β, IL-4 and IL-13 (1). iNKT cells express a semi-invariant αβ T cell receptor (TCR, Vαi4Jα18-Vβ8.2/7/2 in mouse, Vα24Jα18-Vβ11 in human) that recognize glycolipid Ags presented by the non-classical MHC class I homolog CD1d (2). Although mouse and human CD1d only share 60% sequence identity in the α1-α2 superdomain which presents the lipid Ag (3), the structures of human and mouse iNKT TCR in complex with CD1d and the potent sphingolipid Ag α–galactosylceramide (αGalCer) demonstrated evolutionary conserved CD1d Ag presenting and TCR docking strategies that differ from classical TCR-peptide-MHC interactions (4, 5). Despite the progress made in characterizing novel iNKT ligands, the nature of the self Ags involved in the positive selection and development of NKT cells remains elusive (6). Interestingly, while the vast majority of known iNKT Ags are characterized by the presence of an α-linked glycosidic residue, mammals cannot synthetize α-linked glycolipids but instead express β-linked antigens (6). One of the most well-characterized β-linked Ags is isoglobotrihexosylceramide (iGb3) in which the ceramide backbone is β-linked to a trisaccharide (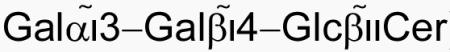 ) with the terminal sugar being important for antigenicity (7). Although the role of iGb3 as a self-Ag is controversial (6), it represents to date the most complex iNKT ligand able to activate both mouse and human cells. The size and connectivity of the polar moiety of this Ag therefore pose interesting structural questions involving the specificity and mode of recognition of β-linked glycolipids by the iNKT TCR. The crystal structure of the mouse CD1d-iGb3-iNKT TCR complex described here illustrates how the iNKT TCR is able to recognize complex β-linked glycolipids by flattening the trihexosyl sugar of iGb3 between the CD1d and TCR surface to allow for the conserved TCR binding mode on CD1d that has previously been observed for the α-linked Ags (4, 5, 8-11), therefore explaining how this conserved TCR can recognize a range of highly diverse Ags.
) with the terminal sugar being important for antigenicity (7). Although the role of iGb3 as a self-Ag is controversial (6), it represents to date the most complex iNKT ligand able to activate both mouse and human cells. The size and connectivity of the polar moiety of this Ag therefore pose interesting structural questions involving the specificity and mode of recognition of β-linked glycolipids by the iNKT TCR. The crystal structure of the mouse CD1d-iGb3-iNKT TCR complex described here illustrates how the iNKT TCR is able to recognize complex β-linked glycolipids by flattening the trihexosyl sugar of iGb3 between the CD1d and TCR surface to allow for the conserved TCR binding mode on CD1d that has previously been observed for the α-linked Ags (4, 5, 8-11), therefore explaining how this conserved TCR can recognize a range of highly diverse Ags.
Materials and methods
Lipids
β-lactosylceramide (β-LacCer) and β-glucosylceramide (β-GlcCer) were obtained from Avanti Polar Lipids, Inc. iGb3 was purchased from Enzo Life Sciences International, Inc. Lipids were dissolved at 0.4mg/ml in 0.5% Tween 20 and 0.9% NaCl and stored at 4°C.
Cell lines and culture conditions
iNKT hybridoma cell lines 1.2, 1.4 and 2C12 (12, 13) were cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 mg/ml each of penicillin and streptomycin, 50 mM 2-mercaptoethanol, and 10% FBS. The cell lines were maintained in an incubator with a humidified atmosphere containing 5% CO2 at 37 °C.
Cell-free Ag presentation assay
The cell-free Ag-presentation assay for stimulation of mouse iNKT cell hybridomas by soluble mCD1d was carried out following published protocols (12, 13) with the modifications described below. Briefly, 1 μg of soluble mCD1d, G155W and hCD1d protein was coated in 96-well flat bottom plate at 4°C overnight. The plates were blocked by PBS and 10% FBS for 1 h after washing and then the glycolipid of interest at various concentrations (shown in Figure 1B and S1) was added to each well and incubated for 24 h at 37°C. After washing, 5×104 hybridoma cells in 200 μl complete media were added to each well and incubated at 37°C for 16 hours in a CO2 incubator. IL-2 release in the supernatant was measured after 16 h of culture in a sandwich ELISA as previously described (12, 13).
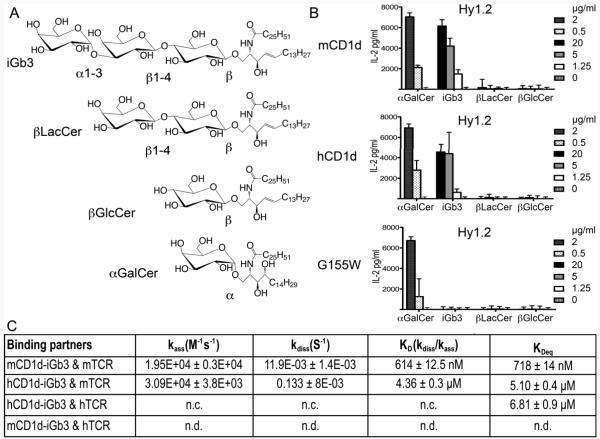
Figure 1. Recognition of β-linked glycolipids by iNKT cells.
(A) Lipid structures. Removal of the terminal α1-3 galactose of iGb3 leads to βLacCer. Further removal of the β1-4 glucose leads to βGlcCer. (B) iNKT cell hybridoma activation using CD1d coated plates and the lipids from (A). Insets show lipid concentrations. (C) SPR binding kinetics. Equilibrium dissociation constants (KDeq) were derived from steady state analysis. Abbreviations: n.c, not calculated; n.d, not detected.
Mouse CD1d/β2m expression and purification and Vα14-Vβ8.2 TCR Refolding
The expression and purification methods of fully glycosylated mouse and human CD1d/β2m heterodimer proteins were reported previously (11). Mouse TCR refolding was performed according to previously reported protocols, using the identical construct (11, 14). A representative human Vα24Vβ11 iNKT TCR (CDR3β: CASSDPNEQFF) was cloned in pET22b (Vα24 chain) and pET30a (Vβ11 chain) E. coli expression vectors. An additional interchain disulphide bond was engineered by mutating to cysteine Thr48 (TRBC 48) on the Cα domain and Ser57 on the Cβ domain. Moreover, a cysteine residue (TRBC 75) on the Cβ domain was mutated to serine to avoid incorrect oligomerization. The two chains were expressed, refolded and purified as described for to the mouse Vα14Vβ8.2 iNKT TCR.
Glycolipid loading and ternary complex formation
Mouse CD1d was loaded overnight with 4-6 molar excess of iGb3 (0.4mg/ml in 0.5% tween 20 and 0.9% NaCl). iGb3 loaded CD1d was purified by size exclusion chromatography and incubated with an equimolar amount of TCR for 1 hour at RT. The mixture was concentrated to 4.8 mg/ml in 10mM Hepes pH 7.5, 30mM NaCl before being used for crystallization.
Surface plasmon resonance studies
SPR studies were performed using a Biacore 3000 (GE Healthcare) according to the methods described previously (14). Human or mouse CD1d were expressed, purified and biotinylated as previously reported and loaded with iGb3 (8). CD1d -iGb3 complexes and empty CD1d as a control were immobilized on a streptavidin sensor chip (CAPture chip, GE Healthcare), while increasing concentrations (0.02–20 μM) of the mouse or human TCR were passed over the chip at 25 °C with a flow rate of 30 μL/min. Experiments were repeated twice. Kinetic parameters were calculated in the BIA evaluation software (version 4.1) after background substraction.
Crystallization and structure determination
Crystals of mCD1d-iGb3-TCR complexes were grown at 27.6°C by sitting drop vapor diffusion, mixing 0.5μl protein with 0.5μl precipitant (16% polyethylene glycol 3350, 8% v/v Tacsimate pH 5.0). Crystals were flash-cooled at 100 K in mother liquor containing 20% glycerol. Diffraction data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 7-1. Data processing and structure determination by molecular replacement using the CD1d and TCR coordinates from PDB ID 3QUY was carried out as reported for other CD1d-glycolipid-complexes (11). Model building and refinement was performed analogous to other CD1d-glycolipid-TCR structures (11). Data collection and refinement statistics are presented in Table SI.
Results and discussion
Mouse NKT cells recognize iGb3 presented by mouse or human CD1d
We tested the ability of iGb3 and its biosynthetic precursors β-LacCer and β-GlcCer, which lack one or two distal sugar rings compared to iGb3 (Figure 1A), to activate three Vα14 NKT hydridomas in a cell-free Ag presentation assay (Figure 1B and S1). Consistent with other studies (7, 15) the truncated ligands β-LacCer and β-GlcCer did not activate either hybridoma. However, iGb3 was able to induce significant IL-2 release from all three hybridomas. These results confirm the importance of the distal α1-3 linked sugar of iGb3 for its antigenicity. Interestingly, iGb3 was able to stimulate mouse iNKT cell hydridomas when presented by human CD1d, while presentation of iGb3 by the “humanized” mCD1d mutant G155W (in hCD1d, Trp153 is at the equivalent position compared to Gly155 in mCD1d) did not lead to mouse iNKT cell activation. Recognition of αGalCer was unimpaired when presented by the G155W mutant, indicating that this mutant was still able to bind and properly present glycolipids. Most residues lining the entrance to the mCD1d binding groove are conserved in hCD1d (except for the G155W exchange) but differences are observed in the area above the A’ pocket (i.e. Met69 in mCD1d to Ile, Gly155 to Trp, Met162 to Trp). The results obtained with the G155W mutant therefore suggest that the observed cross-reactivity of mouse iNKT cells toward human CD1d presented iGb3 is not dependent on a single residue, it is in fact abrogated by the single amino acid substitution, but it is rather the result of several contacts unique to the human CD1d protein surface that compensate for the G155W substitution, suggesting an alternative mode of iGb3 presentation by mouse and human CD1d.
iGb3 has a sub-micromolar affinity for the mouse iNKT TCR
Previous Surface Plasmon Resonance (SPR) experiments carried out using a short-chain version of iGb3 and an iNKT TCR with a truncated CDR3α loop reported a low affinity (KD~50μM) interaction (16). Here, we measured the affinity of both the mouse (Vα14Vβ8.2 derived from the 2C12 hybridoma (14) and human (Vα24Vβ11) iNKT cell TCR toward the full length iGb3 presented by either mouse or human CD1d (Figure 1 and S1B). The mouse TCR binds to mCD1d-iGb3 in with submicromolar affinity (KD of 718 nM). Binding was characterized by a relatively fast on-rate (ka 1.95×104 M−1s−1) and a slow off rate (kd 1.19×10−2 s−1), not dissimilarly from other previously characterized αGalCer analogues, but in contrast to microbial Ags that are characterized by much faster TCR dissociation rates (GalA-GSL from Sphingomonas spp., ka 1.43×105 M−1s−1, kd 9.4×10−2 s−1; BbGl2c from Borrelia burgdorferi, ka 2.16×104 M−1s−1, kd 0.165 s−1) (14). SPR measurements for β-LacCer and β-GlcCer did not result in measureable affinities, consistent with their failure to activate iNKT cells (data not shown). Human CD1d-iGb3 is bound with weaker affinities by both mouse and human iNKT TCRs (KD of 5.1 and 6.8 μM respectively), compared to mCD1d-iGb3, while no measurable binding was observed for the interaction of mCD1d-iGb3 with the human iNKT TCR. Taken together, this data confirm the pattern observed in the Ag presentation assay (Figure 1B and S1A) and further suggests a limited cross-reactivity between human and mouse iNKT cells. Finally, our data suggest that in vitro iGb3 is a surprisingly more potent iNKT Ag than previously appreciated.
Crystal structure of the mCD1d-iGb3-iNKT TCR complex
We determined the crystal structure of the mouse CD1d-iGb3-iNKT TCR complex at 2.8Å resolution (Figure 2, Table SI). The overall docking orientation of the iNKT TCR on mCD1d is highly similar to other previously determined ternary complexes with bound αGalCer (5), its various analogs (9, 11), as well as microbial Ags (8). In the mCD1d-iGb3-TCR complex the TCR adopts a parallel orientation on mCD1d characterized by a footprint centered above the F’ pocket. Key residues in the CDR3α, CDR2β and CDR3β loops (Figure S1C) mediate polar contacts with the α1 and α2 helices of mCD1d, while the TCR α–chain exclusively mediates contacts with the Ag.
Figure 2. Structure of the mouse CD1d-iGb3-TCR complex.

(A) Crystal structures of the mTCR-iGb3-mCD1d complex. iGb3 in yellow; mCD1d heavy chain and β2m in gray; TCR α-chain in cyan; TCR β-chain in orange. (B) Final 2Fo-Fc electron density map for iGb3. The 2FoFc map at 1 σ is shown as a blue mesh in side view (top panel) and top view (bottom panel). C) Superposition of αGalCer (purple) and iGb3 (yellow) in the mouse ternary complex illustrates the similar binding orientation of the β-linked glucose and the α-linked galactose sugars after TCR binding.
Consistent with all the sphingolipid Ags analyzed so far (3), the iGb3 molecule binds to mCD1d with its sphingosine chain in the F’ pocket and the C26 acyl chain in the A’ pocket. Surprisingly, upon TCR binding, the trisaccharide headgroup of iGb3 gets squashed over the α2 helix of CD1d, with each sugar making polar and VdW contacts with CD1d residues (Figure 3A). In particular, the proximal β-Glc residue contacts Asp153 through its 2′-OH, while the second β-Gal sugar contacts Thr 159 through its 2″-OH. On the other hand, the terminal α-Gal residue nestles in a relatively hydrophobic pocket formed between Ala158, Thr159 and Met162, while packing against the side chain of Met162 (Figure 3A). The flat binding orientation of the trisaccharide molds the proximal β-linked glucose of iGb3 to bind into a highly similar position compared to α-linked glycolipids such as αGalCer, with the 2′-OH forming a H-bond with Asp153 of CD1d (Figure 2C). The same residues on mCD1d (Asp153) and the TCR (Asn30α, Gly96α) are involved in recognizing the iGb3 β-linked proximal sugar and the sugar of other α-linked Ags, therefore suggesting remarkably conserved recognition logic for both α- and β-anomeric glycosphingolipids. Surprisingly, considering the essential role of the terminal sugar of iGb3 in determining its antigenicity, contacts between the Ag and the TCR α–chain involve only the β-Glc and β-Gal sugars but not the terminal α-Gal (Figure 3B). In addition, unlike any previously characterized exogenous iNKT cell Ag, all three CDR loops from the Vα14 chain are involved in contacting the iGb3 molecule. In particular, Gly96 and Asn30 on the CDR3α and CDR1α loops, respectively, form H-bonds with the 2′ and 3′ hydroxyl groups of β-Glc. At the same time, the carbonyl group of both Asn30 on CDR1α and Val50 on CDR2α, as well as the framework residue Lys68 are interacting with the 6″-OH of the β-Gal residue (Figure 3B). Interestingly, a similar interaction pattern with the iNKT TCR was recently reported for the self-Ag phosphatidylinositol (PI) (10), which is not a β-linked glycosphingolipid self-antigen but instead a phosphoglycerolipid and, as such presented differently by CD1d. As is the case for iGb3, PI also contacts all three CDR loops of the invariant alpha chain either directly or through water-mediated contacts. In particular, the novel interactions involving the residues near the CDR2α loop (Val50 and Lys68) are remarkably conserved, suggesting that those novel contacts, which are absent in all other studies iNKT antigens so far are used to discriminate self antigens, such as iGb3 and PI, from foreign antigens, notwithstanding that most of those interactions involve backbone atoms of the TCR and are therefore largely independent of the TCR sequence. Therefore, previous mutational studies of the TCR did not characterize CDR2α residues as important for antigen recognition (17). In summary, we speculate that the iNKT cell recognition logic toward self-antigens is expanded compared to the recognition of αGalCer or microbial antigens through those additional contacts involving CDR2α residues, while all other contacts involving CDR1α and CDR3α are conserved between self and foreign antigens.
Figure 3. Binding mode of iGb3 to mCD1d and TCR.

(A iGb3 (in yellow) stick forms extensive polar contacts with CD1d residues D80, D153, T156, T159 and M162, mostly from the α2-helix. (B) The TCR (in cyan) forms polar interactions with iGb3 through residues N30 (CDR1α), V50, K68 (CDR2α), as well as G96 (CDR3α). (C) Superposition of the mCD1d-iGb3 and mCD1d-iGb3-TCR complexes. iGb3 is shown as green sticks before TCR binding (from PDB ID 2Q7Y) and in yellow after TCR binding. TCR is shown as a slightly transparent molecular surface with electrostatics surface potentials (from −30kT/e to +30kT/e) with the underlying CDR loops in cyan. (D) A proposed “stairway” conformation involving the α2-helix residues of CD1d (D153, T156, T159 and M162), support and bind the trisaccharide headgroup of iGb3.
Conformational changes on mCD1d and iGb3 upon TCR binding and implications for recognition of β-linked Ags
The availability of the crystal structure of the mCD1d-iGb3 complex (16) allowed us to compare the conformation of the ligand and mCD1d before and after TCR ligation (Figure 3C). Superposition of the two structures on mCD1d shows how the TCR is able to squash the trisaccharide headgroup of iGb3 over the α2 helix of CD1d while maintaining the position of the ceramide backbone in a relatively conserved orientation. Consistent with the requirement of an additional energetic contribution for iGb3 upon complex formation, SPR measurements of the kinetics of binding in the murine complex show a considerably slower association rate for iGb3 compared to αGalCer (Figure 1). Interestingly, the well-defined density observed for iGb3 in the mCD1d-TCR complex suggests that in the presence of the TCR the Ag adopts an ordered conformation involving in particular several contacts with the mCD1d α2 helix, as well as the TCR. In presence of the TCR the trisaccharide moiety nicely packs against the surface of the helix, which forms a “stairway” generated by the sidechains of Asp153, Thr156, Thr159 and Met162 to support and bind the three sugars of iGb3 (Figure 3D). It therefore appears that when the TCR squashes the iGb3 headgroup onto the CD1d surface, the third sugar anchors the trisaccharide moiety of iGb3 to the CD1d surface, resulting in a stable conformation of the ligand, as implied by the relatively slow TCR dissociation rate and the well defined electron density observed. Consistent with this binding mode, the shorter β-GlcCer and β-LacCer ligands that lack the terminal sugar are not antigenic. Moreover, the closely related compounds iGb4 (GalNAcβ1-3Galα1-3LacCer) and Gb3 (Galα1-4LacCer) are not antigenic because of the additional sugar moiety or the suboptimal orientation of their distal sugar that cannot fit or reach the anchoring pocket on the mCD1d surface. In conclusion, we hypothesize that complex β-linked glycolipids that cannot anchor the sugar moiety to the CD1d surface after TCR flattening require more binding energy to stabilize the CD1d-TCR complex and as such have significantly weaker potency.
Finally, as described for other iNKT Ags, this TCR is also able to force mCD1d in a conserved binding mode by inducing the formation of a hydrophobic roof above the F’ pocket (Figure S1D). The F’ roof formation involves the repositioning of the side chains of residues Leu84 and Val149 and Leu150 of mCD1d and was previously shown to affect the stability of the mCD1d-iNKT TCR complex (8). Although TCR-induced conformational changes for MHC-peptide and CD1d-glycolipid have been reported (8, 18), the extent of reorientation observed for iGb3 is unprecedented among TCR Ags, highlighting once again the unique nature of the iNKT TCR.
Supplementary Material
Acknowledgements
We would like to thank SSRL BL 7-1 for remote data collection and Dr. Kronenberg (LIAI) for providing the Vα24Vβ11 TCR cDNA.
Footnotes
D.M.Z is supported by a CRI Investigator award and by NIH grant RO1 AI074952.
Structure factors and coordinates have been deposited in the Protein Data Bank under accession code 3RZC.
References
- 1.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Zajonc DM, Wilson IA. Architecture of CD1 proteins. Curr Top Microbiol Immunol. 2007;314:27–50. doi: 10.1007/978-3-540-69511-0_2. [DOI] [PubMed] [Google Scholar]
- 4.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 5.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gapin L. iNKT cell autoreactivity: what is ‘self’ and how is it recognized? Nat Rev Immunol. 2010;10:272–277. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM. The V alpha 14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–2389. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, Richardson SK, Howell AR, Illarionov PA, Brooks AG, Besra GS, McCluskey J, Gapin L, Porcelli SA, Godfrey DI, Rossjohn J. A Molecular Basis for the Exquisite CD1d-Restricted Antigen Specificity and Functional Responses of Natural Killer T Cells. Immunity. 2011;34:327–339. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aspeslagh S, Li Y, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Van Beneden K, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr., Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 16.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate V alpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 18.Tynan FE, Reid HH, Kjer-Nielsen L, Miles JJ, Wilce MC, Kostenko L, Borg NA, Williamson NA, Beddoe T, Purcell AW, Burrows SR, McCluskey J, Rossjohn J. A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat Immunol. 2007;8:268–276. doi: 10.1038/ni1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.