Abstract
Receptors encoded within the Trem locus have been shown to play an important role in modulating the cellular response to PRR signaling. TLT2 is a member of the Trem locus that is conserved in mouse and human. TLT2 exhibits a unique expression pattern in that it is expressed on cells of the myeloid and lymphoid lineage, suggesting that it plays a role in both innate and adaptive immunity. Here, studies reveal that TLT2 plays an important role in potentiating neutrophil antibacterial activity and chemotaxis. TLT2 ligation enhances the neutrophil response to the formylated peptide FMLP leading to increased ROS production, degranulation and chemotaxis. Moreover, TLT2 has the ability to specifically potentiate neutrophil activation and chemotaxis in response to a range of agonists that bind to G protein-coupled receptors, as it does not potentiate the response of cells to growth factor receptor-, Fc receptor- or TLR-mediated signaling. Finally, TLT2 ligation potentiates the recruitment of neutrophils to sites of inflammation in vivo. These findings reveal a novel functional role for TLT2 that involves potentiation of neutrophil responses to G protein-coupled receptor signaling. Thus TLT2 appears to play an important role in enhancing the innate immune response via a novel molecular mechanism.
Introduction
Cells of the innate immune system rely on a range of receptors to detect and respond to pathogens. The direct recognition of pathogens or products of cellular stress by pattern recognition receptors (PRRs) such as the toll-like receptors and the nod-like receptors are well characterized (1, 2). Additionally, other families of receptors serve to modulate the response of immune cells to signals derived from PRRs including those encoded within the triggering receptor expressed on myeloid cells (Trem) locus (3). Receptors encoded within the Trem locus are expressed by a wide range of innate immune cells including neutrophils, monocytes, macrophages, microglia, osteoclasts and dendritic cells, as well as megakaryocytes and platelets (4). TREM-like Transcript 2 (TLT2) is a type 1 transmembrane receptor, with a single extracellular immunoglobulin domain followed by a serine/threonine-rich membrane-proximal segment, and a short cytoplasmic tail following a single transmembrane domain. Unlike other TREM family members, murine TLT2 does not possess an obvious signaling motif within its 39 amino acid cytoplasmic tail, nor does it possess charged amino acids in the transmembrane domain, suggesting that it does not interact with prominent adaptor proteins such as DAP12, FcRγ, or TIRAP. In mice, TLT2 has been shown to be expressed on B cells, macrophages, and neutrophils and thus is the only member of the TREM locus to be expressed by cells of both the innate and adaptive immune systems (5).
TREM-1, the most well characterized TREM protein, is the only TREM family member other than TLT2 that is expressed on neutrophils. TREM-1 ligation mediates increased production of inflammatory cytokines, chemokines, lactoferrin, reactive oxygen species (ROS) and myeloperoxidase (MPO) (6). Functional studies have found that TREM-1 cross-linking induces only a modest increase in specific antibacterial activities, whereas it predominately acts in a synergistic fashion to amplify the inflammatory response in conjunction with toll-like receptor and nod-like receptor signaling (7, 8). Ligation of TREM-1 in concert with LPS stimulation potentiates cytokine secretion more than either stimulus alone. Thus, TREM-1 modulates immune cell function by effectively amplifying the magnitude of the response to signals delivered via PRRs. TREM-2 is expressed on a variety of cell types, including monocytes, macrophages, osteoclasts, bone marrow-derived dendritic cells, immature dendritic cells, and microglia (9, 10). TREM-2 has recently emerged as an important negative regulator of autoimmunity and appears to exert an anti-inflammatory activity on microglia and macrophages. Thus, TREM family members appear to predominantly function as modulatory proteins that control innate immune cell function in response to other stimuli.
TLT2, like TREM-1, is highly expressed on neutrophils and its expression is upregulated in response to inflammatory conditions in vivo (5). However, the functional role of this receptor on cells of the innate immune system has not been described. Because the TREM-1 and TREM-2 receptors have been shown to modulate the functional response of cells to signals derived from pattern recognition receptors, it was of interest to determine if engagement of the TLT2 receptor would also modulate neutrophil function in response to signals derived from receptors involved in the induction of, or response to, inflammatory conditions. In this study, agonistic mAbs against TLT2 were used to demonstrate that ligation of TLT2 on neutrophils potentiates the respiratory burst and degranulation in response to FMLP and C5a, as well as the chemotactic response to several chemokines. The chemokines MIP-2 and KC, the complement component C5a, and the bacterial product FMLP all signal via G protein-coupled receptors (GPCR). These receptors contain seven membrane-spanning helices that undergo conformational changes upon ligand binding, which in turn transduces a signal via activation of heterotrimeric G-proteins. GPCRs make up a large family of proteins with broad functions in biological systems. In the context of innate immunity, signals delivered via GPCRs influence many aspects of neutrophil function including promoting the production of reactive oxygen species and the release of antimicrobial mediators, as well as chemotaxis (11). The finding that TLT2 regulates neutrophil function by potentiating the response to agonists that signal via GPCR-dependent pathways represents a novel mechanism whereby antibacterial responses and recruitment of phagocytic cells to sites of infection and inflammation are enhanced.
Materials and Methods
Isolation of mouse neutrophils
C57BL/6 mice 8–10 wk of age were used for isolation of bone marrow cells from the tibias and femurs. All mice were housed in specific pathogen-free conditions in University of Alabama at Birmingham animal facilities, and all procedures were approved by an institutional review committee. The bone marrow was passed through 25 and 20 gauge needles (Becton Dickinson, Franklin Lakes, NJ) to generate a single cell suspension in 1X HBSS (136 mM NaCl, 5.55 mM Glucose, 5.36 mM KCl, 4.16 mM NaHCO3, 1.66 mM KH2PO4, 0.338 mM Na2HPO4, pH 7.2). Red blood cells were lysed by incubation in AKC (0.15 M NH4Cl, 12 mM NaHCO3, .1 mM EDTA, pH 7.2) for 5 min on ice, leukocytes were then separated by density sedimentation using a Percoll (Amersham, Piscataway, NJ) gradient (60%/80%) in 1X HBSS centrifuged at room temperature at 1500 RPM for 25 min. The cells at the 60/80 interface, which was comprised of >95% neutrophils as assayed by flow cytometry were collected, counted with a hemocytometer, and resuspended in media at appropriate concentrations. The media used in all experiments was RPMI-1640, supplemented with pen/strep, sodium pyruvate, mercaptoethanol, L-glutamine, and 5% FBS. Anti-CD11b-FITC (BD Pharmingen, San Diego, CA), anti-Gr-1-PE and anti-Gr-1-APC mAbs (Southern Biotech, Birmingham, AL) were used to determine the purity of isolated neutrophils.
Respiratory burst assay
The αTLT2 mAbs 1H4 and 1C5 were generated as previously described (5) and were prepared under LPS-free conditions. The antibody preparations used in these studies were subjected to the limulus amebocyte lysate test, and were demonstrated to contain no detectable endotoxin (limit of detection is 0.03 EU/mL). Where indicated, antibodies were biotinylated using EZ-Link NHS-LC-biotin (Pierce, Rockford, IL). Following isolation, 1×106 purified neutrophils in 100 μl of RPMI-1640 were incubated with αTLT2 mAb, isotype control mAb, LPS, or were left untreated for the specified times. Extracellular ROS scavengers (2000 units/ml catalase, Worthington, Lakewood, NJ) and 50 units/ml superoxide dismutase (Sigma; St. Louis, MO) and 10% luminol (final concentration 5 μM) were added to the samples. After a 5 min equilibration period at 37°C, FMLP or GM-SCF (Calbiochem, Darmstadt, Germany) was added and the respiratory burst response was monitored using an Envision Multi-label Plate reader (Perkin Elmer, Waltham, MA) in the ultrasensitive luminescence mode.
Neutrophil degranulation assay
A total of 1×106 neutrophils per sample in 1 ml of RPMI-1640 were incubated with the appropriate αTLT2 mAb, isotype control mAb or medium alone as indicated for 10 min at 37°C. Following this incubation the FMLP agonist, WKYMVm (Calbiochem) C5a, GM-CSF or the TLR agonists LPS (Sigma), monophosphoryl lipid A (MPLA) (Dr. John Kearney, Microbiology, UAB), Poly(I:C) (Invitrogen), flagellin (Dr. Charles Elson, Medicine, UAB), imiquimod (Invitrogen) and CpG-ODN (Invitrogen) were added at the indicated concentrations for the indicated times. After stimulation, all reactions were terminated by addition of ice cold 1X PBS. To examine the effect of secondary cross-linking of TLT2, 1 μg of biotinylated αTLT2 mAb was preincubated with varying concentrations (2–40 μg) of streptavidin (Pierce), which was then added to the neutrophil preparations for 10 min prior to the addition of triggering stimuli. After the indicated period of time, the neutrophils were then stained for the surface markers CD11b and Gr-1. Degranulation was measured by a specific increase in the cell surface expression of CD11b.
Phagocytosis assay
A total of 5×106 2.0 micron biotinylated polystyrene beads (Polysciences, Inc, Warrington, PA) were incubated with a 1:100 dilution of streptavidin-FITC (Biosource, Carlsbad, CA) in 100 μl PBS for 15 min at room temperature in the dark. The beads were then washed 3 times with 1X PBS. Subsequently, 50% of the FITC-labeled beads were incubated at RT with mouse serum containing anti-FITC antibodies for 30 min in the dark. The FITC-specific antiserum was generated by immunizing C57BL/6 mice with FITC conjugated protein antigen, after which serum was harvested on day 10. After opsonization with the antiserum, the beads were washed 3 times with 1X PBS and warmed to 37°C prior to addition to neutrophils. Neutrophils (1×105 cells/sample) in 500 μl RPMI-1640 were preincubated with the indicated stimuli for 10 min. 1×106 fluorescent beads were then introduced to the cell suspensions followed by a 15 min incubation. Trypan blue was added to quench extracellular FITC fluorescence and phagocytosis of the beads was assayed by flow cytometry.
Chemotaxis assay
Neutrophil chemotaxis was assayed using 3.0 micron, 6.5 mm transwell inserts (Costar, Corning, NY) placed in 24-well cell culture plates. 1×106 neutrophils were placed in 100 μl of RPMI-1640 in the upper chamber and the chemotactic factors, FMLP, MIP-2, KC, IL-8, C5a, or GM-CSF were added at the indicated concentrations to 600 μl in the bottom chamber in 37°C RPMI-1640. The murine recombinant proteins KC, MIP-2, GM-CSF and human recombinant IL-8 were obtained from PeproTech (Rocky Hill, NJ), and murine C5a was obtained from eBioscience (San Diego, CA). αTLT2 or isotype control mAb was added to the upper chamber with the neutrophils at specified concentrations, and the samples were incubated for 60 min at 37°C in a humidity controlled incubator under 5% CO2. Migrating neutrophils were removed from the bottom chamber and counted. The normalized chemotactic index was calculated by dividing the number of cells migrating in samples containing αTLT2 or isotype control mAbs by the number migrating in response to the chemotactic agent alone.
Ear model of inflammation
To induce inflammation in the ear, 20 μl of 2% croton oil (Sigma, St. Louis, MO) in acetone was applied to both sides of the pinna of one ear, whereas acetone alone was applied to the control ear. Either 100 μg of αTLT2 mAb or 1X PBS alone was injected intravenously (IV) into mice 30 min prior to the application of croton oil. Four hours following the application of croton oil, 4 mm biopsies were obtained from the proximal portion of the ear, mechanically homogenized with a Tissue Tearor homogenizer (Biospec Products, Bartlesville, OK), and assayed for MPO activity. MPO levels were determined by the addition of the chromogenic substrate 3,3′, 5,5′-tetramethylbenzidine (TMB) (Sigma) to 100 μl of the ear lysate. The absorbance at 450 nm was then measured using a Vmax plate reader (Molecular Devices, Sunnyvale, CA). These samples were assayed with a standard curve of known concentrations of the enzyme HRP. Comparing the MPO activity present in specific numbers of purified neutrophils to this standard curve allowed for the estimation of the number of neutrophils present in the biopsy samples. To examine the effect of administration of aTLT2 mAb on the recruitment of cells and architecture of the ear, mice were sacrificed and the ears were removed, fixed overnight in 10% NBF, paraffin embedded, and 8 micron thick sections prepared. The sections were treated to remove the paraffin and rehydrated, after which they were stained with H&E (Harris Hematoxylin and Eosin, Sigma). The stained sections were subsequently dehydrated, cleared, and mounted with Permount (Fisher #SP15). The slides were examined with a Zeiss AX10 microscope using a 20x objective and pictures were taken using a Zeiss AxioCam MRC.
Lung model of inflammation and adoptive transfer of neutrophils
To induce inflammation in the lung 5 μg of LPS was introduced intratracheally. Bone marrow neutrophils were isolated from CD45.1 donor mice as previously described. Once isolated, the neutrophils were divided equally into 3 groups of 3×107 neutrophils and subsequently incubated for 10 min in 500 μl of 1X PBS containing either 5 μM CSFE, 5 μM Cell Tracker CMPTX Red or without fluorescent additives. CSFE and CMTPX cell permeable tracking dyes were obtained from Invitrogen (Carlsbad, CA). Each group of neutrophils was then subjected to the indicated treatment for 15 min at 37°C, followed by 3 washes in prewarmed 1X PBS and resuspended in 100 μl. After loading, the cells were mixed in a 1:1:1 ratio and approximately 3×107 total neutrophils in 100 μl were injected IV into CD45.2 mice 2 h after the initiation of lung inflammation. After an additional 4 h, mice were sacrificed and cells were isolated from lungs by bronchoalveolar lavage. The isolated cell suspensions were stained with anti-CD45.1-APC (eBioscience). Flow cytometry was preformed to indentify CD45.1 expressing cells and neutrophils subjected to the indicated treatment conditions were then resolved based on of CSFE or CMTPX fluorescence, or the absence of fluorescent labeling.
Flow cytometry
Cells were washed 3 times with FACS buffer (1X PBS, 0.01% NaN3+, 0.5% FBS) and then incubated with the appropriate fluorescent Ab mixture in 96-well microtiter plates for 15 min on ice. After this incubation, the cells were again washed 3 times with FACS buffer. When necessary, samples were incubated with secondary antibodies for an additional 15 min on ice. Samples were analyzed immediately following labeling on either a FACScan, FACSCalibur or LSR II flow cytometer (BD Biosciences), and the resultant data were analyzed using FlowJo (Tree Star, Ashland OR.
Statistical analysis
Statistical analyses were computed via the student two-tailed t-test. The CD45.1 adoptive transfer experiment was analyzed with the one-way anova. The p-value of significant differences is reported, with a p-value < 0.05 considered statistically significant.
Results
TLT2 ligation potentiates the functional response of neutrophils to FMLP
Previous studies have demonstrated that TLT2 is constitutively expressed by both macrophages and neutrophils (5). Under experimental inflammatory conditions, including administration of thioglycolate, LPS, or staphylococcal enterotoxin B in vivo, responding macrophages and neutrophils exhibit significant upregulation of TLT2 on their surface (5). This rapid upregulation of TLT2 by neutrophils suggests that TLT2 may play a role in regulation of neutrophil function at sites of inflammation. A principle function of neutrophils is to respond to bacterial pathogens; therefore experiments were performed to determine if ligation of TLT2 with mAb enhances the antimicrobial activity of neutrophils by inducing ROS generation. To determine the effect that engagement of TLT2 has on ROS production, neutrophils were isolated from murine bone marrow and were analyzed using a chemiluminescence assay to measure the generation of ROS. As seen in Figure 1A, and Supplemental Figure 1, ligation of TLT2 with either of the mAbs 1H4 or 1C5 alone does not induce a respiratory burst response.
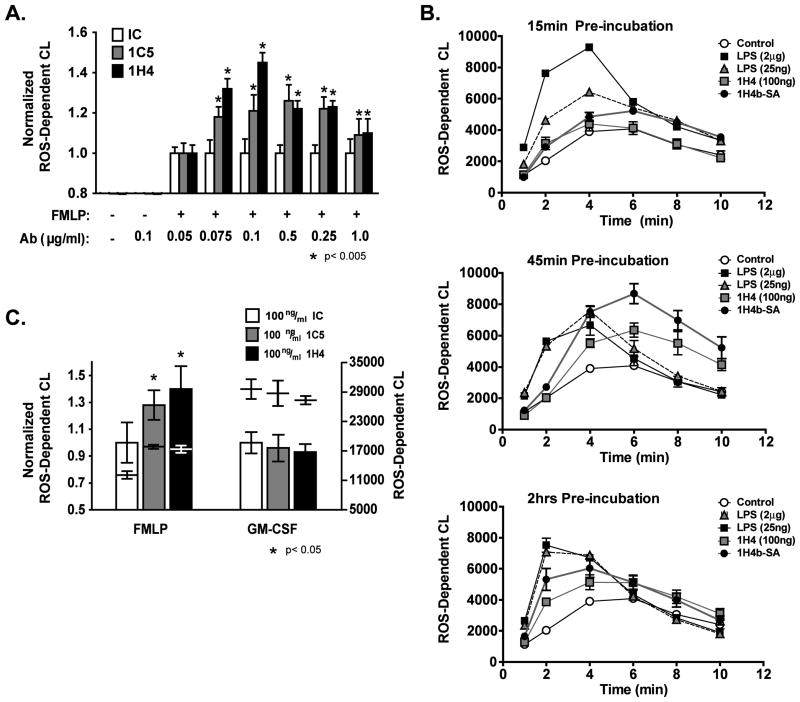
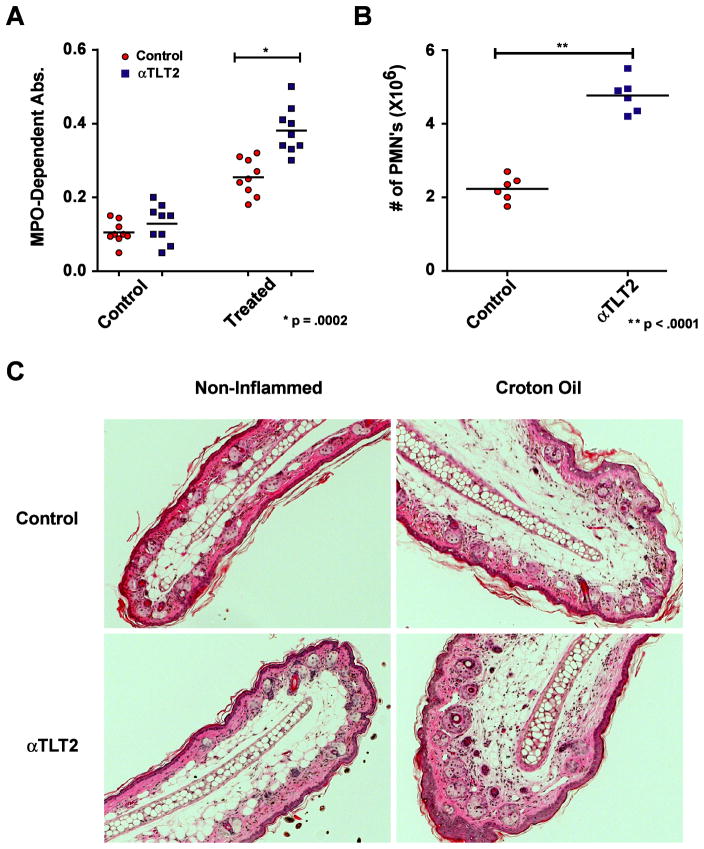
Figure 1. TLT2 ligation potentiates ROS production by murine neutrophils in response to FMLP.
(A) Treatment with distinct αTLT2 mAbs (1H4 or 1C5) enhances the production of ROS in a dose dependent manner following the addition of FMLP. Neutrophils (1×106) were preincubated for 45 min with αTLT2 or isotype control (IC) mAb at the indicated concentrations, and then stimulated with FMLP (1 μM). (B) Purified neutrophils were incubated in the presence of αTLT2 mAb or LPS for either 15 min (upper panel) 45 min (middle panel), or 2 h (lower panel) then stimulated with 1 μM FMLP. Alternatively, neutrophils were preincubated for 15, 45 or 120 min with biotinylated 1H4 mAb (1 μg) that had been premixed with SA (20 μg) followed by addition of FMLP. ROS production was monitored at the indicated times. (C) TLT2 ligation does not alter ROS generation in response to GM-CSF. Purified neutrophils were incubated in the presence of αTLT2 or isotype control mAb for 45 min followed by stimulation with either FMLP (1 μM) or GM-CSF (1 ng/ml). For (A) the maximum ROS production for pretreated samples was normalized to controls that received FMLP alone. For (C) the data were normalized to samples that received either FMLP or GM-CSF alone. The absolute values for ROS-dependent chemiluminescence are shown as well. All data represent the average of triplicate samples with mean ± standard deviation shown and are representative of at least three independent experiments. Asterisks denote significance of values for αTLT2 mAb-treated samples compared to the respective control sample.
Neutrophils generate ROS in response to a variety of extracellular stimuli including inflammatory cytokines and growth factors, as well as bacterial products such as LPS and formylated peptides derived from prokaryotic pathogens. FMLP is a short, formylated bacterial peptide that is released during degradation of the bacterial membrane and is a strong chemoattractant for phagocytic cells such as neutrophils (11). The response of innate immune cells to formylated peptides is mediated by binding to the FMLP receptor, a GPCR, resulting in activation of phospholipase C, protein kinase C, and calcium mobilization (12). Experiments were performed to determine if preincubation of neutrophils with αTLT2 mAb exerts a priming effect resulting in a change in the kinetics or magnitude of the respiratory burst elicited in response to FMLP. Preincubation with αTLT2 mAb prior to stimulation with FMLP resulted in a substantial increase in ROS production compared to neutrophils incubated with FMLP alone (Fig 1, S1). In contrast, preincubation of neutrophils with isotype control mAb had no effect on ROS production (Fig. S1) or on the subsequent response following addition of FMLP, regardless of the concentration of isotype control antibody used (Fig. 1). The potentiation of ROS production occurs over a broad range of αTLT2 mAb concentrations (Fig. 1A). When the potentiation of FMLP induced ROS generation induced by αTLT2 mAb pretreatment is compared to that induced by pretreatment with LPS, a prototypic priming agent, the effects, although similar in magnitude, differ in their kinetics (i.e. initiation and duration). The priming effect of LPS is very rapid, as seen in Figure 1B (upper panel). After a 15 min preincubation with LPS, the kinetics of the response to FMLP are shifted, and the overall magnitude of the response is substantially increased, compared to treatment of cells with FMLP alone. In contrast, preincubation with αTLT2 mAb for 15 min fails to significantly alter ROS production induced by FMLP. However, after a 45 min preincubation with αTLT2 mAb, ROS production in response to FMLP is similar to that observed with LPS pretreatment in terms of magnitude. Whereas cells pretreated with LPS exhibited an accelerated, transient potentiation of ROS production, TLT2 ligation did not accelerate the kinetics of the response, but was observed to potentiate ROS production for a prolonged period of time. Finally, pretreatment of neutrophils for 2 h with αTLT2 mAb resulted in a slight shift in the kinetics of the response similar to that observed with LPS treatment, as well as a prolongation of the potentiated response (Fig. 1B). Potentiation of ROS production can be further enhanced by secondary cross-linking as demonstrated by treatment of neutrophils with biotinylated 1C5 in the presence of streptavidin, which results in a further enhancement of ROS production in response to FMLP at all time points assayed (Fig. 1B). This observation suggests that cross-linking of TLT2 may be important for eliciting an enhanced response to FMLP. In summary, whereas ligation of TLT2 alone does not induce the generation of ROS by neutrophils, cross-linking of this receptor serves to potentiate ROS production in response to FMLP. Although ROS generation was similar for neutrophils primed either with αTLT2 mAb or LPS in terms of magnitude, significant differences were observed in the kinetics of ROS production. TLT2 ligation primarily acts to enhance the magnitude of ROS production over an extended period of time, but does not alter the kinetics of the response. In contrast, LPS accelerates the kinetics and increases the magnitude of the response in a transient manner. These data suggest that the mechanism by which TLT2 ligation potentiates ROS production is different from that associated with the priming effect of LPS.
In addition to FMLP, other stimuli, including growth factors induce a respiratory burst response in neutrophils. Therefore, experiments were performed to determine if the significant enhancement in ROS generation induced by TLT2 ligation in response to FMLP would be observed in response to other agonists. The GM-CSF receptor (also known as CD116) is expressed on several cell types, including mature neutrophils. GM-CSF, like FMLP, has been shown to elicit ROS production by neutrophils; however the GM-CSF receptor mediates a phosphotyrosine-based signal leading to ROS production (13). As seen in Figure 1C, unlike the response to FMLP, ROS generation by neutrophils in response to GM-CSF was not potentiated by ligation of TLT2.
In addition to promoting the generation of ROS, FMLP is a potent chemotactic agent for neutrophils (11). Because ligation of TLT2 was observed to potentiate ROS production in response to FMLP, experiments were performed to determine if the chemotactic response of neutrophils to FMLP is also potentiated. Medium containing varied concentrations of FMLP receptor agonist were placed in the bottom chamber of a 3 μm transwell device and neutrophils were placed in the upper chamber. Anti-TLT2 or isotype control mAb, or medium alone was added to the neutrophils just prior their addition to the upper chamber and after a 1 h incubation at 37° C, cells that migrated to the lower chamber were collected and counted. As shown in Figure 2A, inclusion of αTLT2 mAb in these assays resulted in a substantial increase in the number of cells migrating through the transwell in response to FMLP. Of note, the concentration of αTLT2 mAb that maximally potentiates the chemotactic response of neutrophils is the same as that which provides maximal potentiation of ROS production. To rule out the possibility that Fc receptor-mediated signaling generated by the binding of intact antibodies to neutrophils was responsible for these observed effects, F(ab′)2 fragments of the 1H4 mAb were generated and used in these assays (Fig. 2B). The observed potentiation of cell migration in response to FMLP was identical whether cells were treated with F(ab′)2 fragments or intact 1H4, suggesting that signals delivered via TLT2 were responsible for the observed effects on ROS production and cell migration.
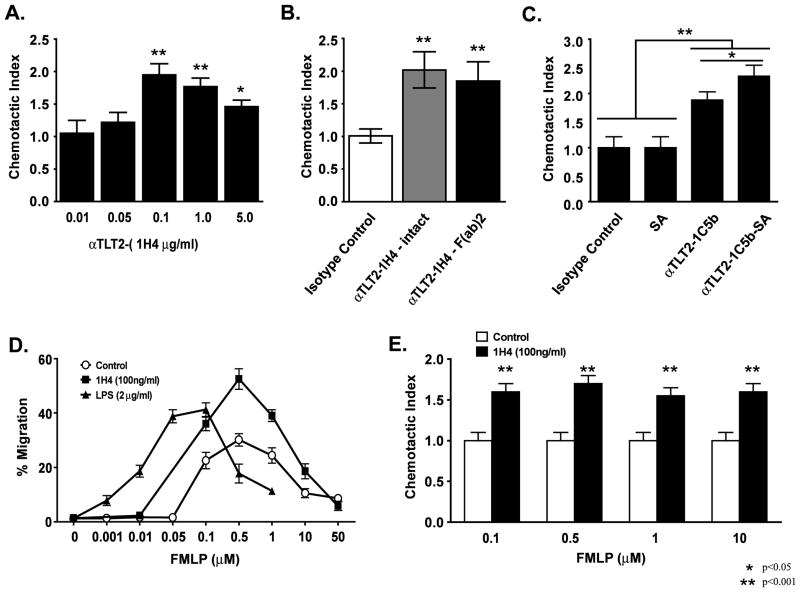
Figure 2. TLT2 ligation enhances the chemotactic response of neutrophils to FMLP.
Purified neutrophils (1×106) were placed in the upper chamber of a 3 μm transwell device in the presence or absence of the indicated concentration of the αTLT2 mAb 1H4 (A) or 100 μg of either intact or F(ab′)2 fragments of 1H4 (B) or 1 μg of the αTLT2 mAb 1C5 coupled to biotin with or without streptavidin (20 μg) (C). For panels B and C, isotype control indicates samples in which 0.1 or 1 μg/ml of rat mAb was added to the upper chamber, respectively. For panels (A–C) 1 μM FMLP was added to the lower chamber for all samples. After 1 h, the cells were harvested from the lower chamber and counted. The number of cells present in the lower chamber for each experimental condition was divided by the number of migrating cells in control samples (FMLP alone) to determine the chemotactic index. The mean ± SD is shown for triplicate samples and the experiments depicted are representative of at least 3 independent experiments. (D) TLT2 ligation enhances neutrophil migration in response to a wide range of FMLP concentrations. Neutrophils (1×106) in medium alone, or containing 100 ng/ml of 1H4 mAb or 2 μg/ml LPS were placed in the upper chambers of transwell devices. The indicated concentrations of FMLP or medium alone were placed in the lower chambers. The percentage of cells migrating into the lower chamber are the average of triplicate samples with the mean ± SD shown, and are representative of at least three independent experiments. (E) TLT2 potentiation of FMLP-mediated chemotaxis is proportional across a wide dose response. The chemotactic indices for αTLT2 mAb pretreated versus control neutrophils in response to FMLP are depicted. The values are derived by dividing the % migration data for αTLT2 pretreated samples by the % migration for neutrophils incubated in medium alone as shown in (D). Asterisks denote significance of values for αTLT2 mAb-treated samples compared to the respective control sample.
Additional experiments were performed to determine if secondary cross-linking would further enhance this effect using biotinylated 1C5, either alone or in the presence of streptavidin. As seen in Figure 2C, the inclusion of streptavidin alone has no effect on cell migration in response to FMLP, whereas the inclusion of biotinylated 1C5 mAb alone results in a nearly two-fold increase in the number of migrating cells. As seen in ROS assays, the inclusion of streptavidin and biotinylated 1C5 mAb results in a further enhancement, suggesting again that secondary cross-linking of TLT2 is responsible for this effect.
A possible mechanism by which TLT2 ligation potentiates the response to FMLP is by decreasing the neutrophil threshold of sensitivity for agonist binding to the FMLP receptor. If this were true, then one would expect to see a shift in the dose response to FMLP in neutrophils on which TLT2 has been ligated. To determine if this is the case, neutrophil migration was assayed over a wide range of FMLP concentrations in the presence or absence of αTLT2 mAb. As seen in Figure 2D, the inclusion of an optimal concentration of αTLT2 mAb potentiates neutrophil migration over a wide range of FMLP concentrations. However, TLT2 ligation does not result in a shift in the dose response to FMLP. Indeed, the observed potentiation in the number of migrating neutrophils treated with αTLT2 mAb is proportional regardless of the FMLP concentration as the ligation of TLT2 resulted in a chemotactic index (~1.7) that is nearly identical for all concentrations of FMLP tested (Fig. 2E). In contrast, inclusion of LPS shifts the chemotactic response curve to FMLP, suggesting that it lowers the threshold of sensitivity to this chemoattractant (Fig. 2D). Collectively, these results suggest a potential role for TLT2 in amplifying the cellular response to FMLP, as opposed to altering the threshold of sensitivity of the FMLP receptor. This suggests that TLT2 ligation could amplify the magnitude of the signal delivered via the FMLP receptor and/or prolong the duration of that signal thereby enhancing the cellular functional response. Alternatively, TLT2 ligation could in theory activate a parallel signaling pathway that amplifies the functional response of the neutrophil. Although, if this were true, one would expect that ligation of TLT2 alone would drive ROS production and chemotaxis in the absence of FMLP triggering, which was not observed. These data further support the conclusion that the mechanism by which TLT2 modifies the response to GPCR-mediated signaling is distinct from that of LPS.
Ligation of TLT2 specifically potentiates the neutrophil response to GPCR-mediated signaling
Besides triggering both ROS production and neutrophil migration, FMLP and GM-CSF induce degranulation of murine neutrophils. The release of specific granules in response to these stimuli has been shown to mediate the rapid expression of the integrin CD11b resulting in an increase in cell adhesion and is thought to be a mechanism to promote transmigration of neutrophils into inflamed tissues (14). Because the rapid upregulation of CD11b is associated with cellular activation, experiments were performed to determine if TLT2 ligation alone is sufficient to induce the upregulation of CD11b, or if ligation of the TLT2 receptor potentiates upregulation of CD11b induced by FMLP or the complement component C5a, which bind to GPCRs, versus GM-CSF or TLR agonists, which activate distinct signaling pathways that do not utilize heterotrimeric G-proteins.
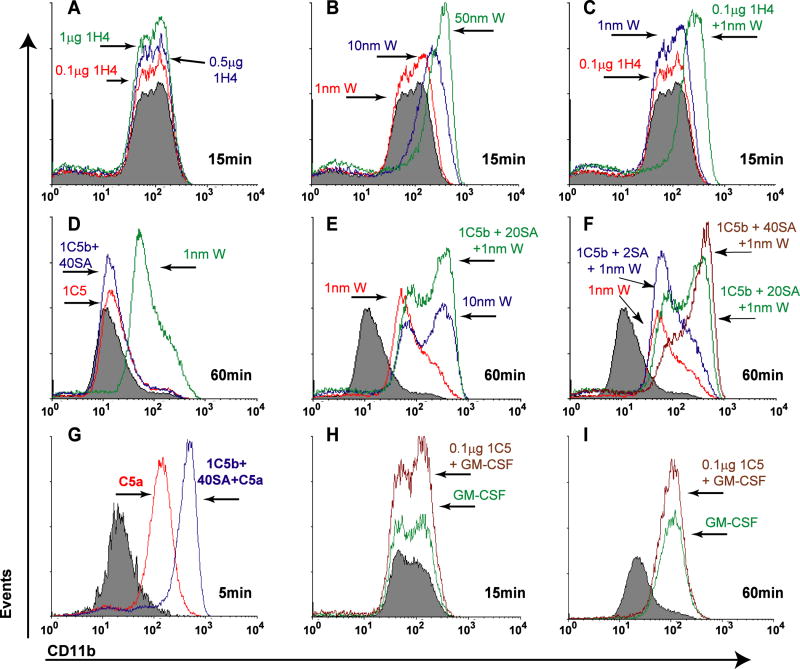
As seen in Figure 3A, ligation of TLT2 alone has no effect on CD11b expression on purified neutrophils, regardless of the mAb concentration used, compared to the FMLP receptor agonist WKYMVm, which induced CD11b expression in a dose dependent manner (Fig. 3B). However, when neutrophils were preincubated with 100ng/mL of 1H4 mAb and were subsequently stimulated with 1 nM WKYMVm, a concentration that does not significantly alter CD11b expression, a synergistic upregulation of CD11b was observed (Fig. 3C). As was seen in the ROS assay, the potentiation of CD11b expression in response to FMLP following TLT2 ligation was further enhanced when neutrophils were preincubated in the presence of biotinylated 1C5 mAb plus streptavidin as a secondary cross-linking agent (Fig. 3D–F). Exposure to C5a also results in neutrophil degranulation, and like FMLP, activation of cells by C5a is the result of signal transduction mediated by the C5a receptor, a GPCR. Therefore experiments were performed to determine if TLT2 ligation would potentiate the cellular response to C5a. As seen in Figure 3G, preincubation of neutrophils with anti-TLT2 mAb potentiates degranulation in response to C5a resulting in the upregulation of CD11b. In contrast, ligation of TLT2 does not affect CD11b upregulation when used in conjunction with GM-CSF (Fig. 3H, I). Pretreatment of neutrophils with isotype control mAb alone or in conjunction with any of the agonists tested did not alter CD11b expression (data not shown).
Figure 3. TLT2 ligation potentiates neutrophil degranulation and upregulation of the activation marker CD11b in response to GPCR-mediated signaling.
Purified neutrophils (1×106) were incubated either with the αTLT2 mAb 1H4 (at the concentrations indicated) or biotinylated 1C5 (1μg/ml). Where indicated, streptavidin (2–40 μg) was added to mediate secondary cross-linking of TLT2. Neutrophils were stimulated with the FMLP agonist WKYMVm (W) at the indicated concentrations (A–F), the activated complement component C5a (2 ng/ml) (G), or GM-CSF (1 ng/ml) (H–I). At the indicated time points the cells were harvested and stained to detect surface expression of CD11b and analyzed by flow cytometry. Filled histograms represent CD11b expression on neutrophils incubated in medium alone. Representative histograms of CD11b expression are shown. All data are representative of at least three independent experiments.
To determine if ligation of TLT2 potentiates the ability of TLR agonists to induce degranulation of neutrophils, experiments were performed in which neutrophils were preincubated in the presence of αTLT2 or isotype control mAb followed by addition of TLR agonists, including LPS, poly(I:C), MPLA, flagellin, imiquinod and CpG-ODN, which bind to TLR2, 3, 4, 5, 7/8 and 9, respectively. Neutrophil degranulation in response to these TLR agonists was not affected by preincubation of neutrophils with αTLT2 or isotype control mAb (data not shown). A second assay was performed to monitor the effect that TLT2 ligation has on the ability of TLR agonists to prime neutrophil degranulation in response to low concentrations of GM-CSF. TLT2 ligation had no effect on the priming activity of any of the TLR agonists examined (data not shown).
The fact that TLT2 ligation specifically potentiates the response of neutrophils to FMLP, a bacterial component, and is upregulated on neutrophils at sites of inflammation suggests that it may play an important role in the innate immune response to bacterial infection. Phagocytosis of bacteria by neutrophils is mediated by multiple receptors including integrins, lectins, and scavenger receptors (15). However, the phagocytosis of bacteria by neutrophils is greatly enhanced in the presence of opsonizing antibodies, which promote engulfment of particles via Fc receptors (FcR). Thus experiments were performed to determine if the FcR-dependent phagocytic activity of neutrophils is enhanced by ligation of TLT2. To experimentally determine the effect of TLT2 ligation on the engulfment of opsonized particles, two-micron polystyrene beads were conjugated to FITC to facilitate visualization, and then incubated with purified neutrophils either alone or following opsonization with polyclonal αFITC antibody. After 15 min, trypan blue was added to quench extracellular FITC fluorescence and phagocytosis was assayed by flow cytometry. As seen in Supplemental Figure 2, ligation of TLT2 had no measurable effect on the percentage of neutrophils that phagocytosed beads via a FcR-dependent mechanism, as αTLT2 mAb pretreated samples were identical to non-pretreated controls, regardless of whether intact mAb, F(ab′)2 fragments, or secondary cross-linking was used.
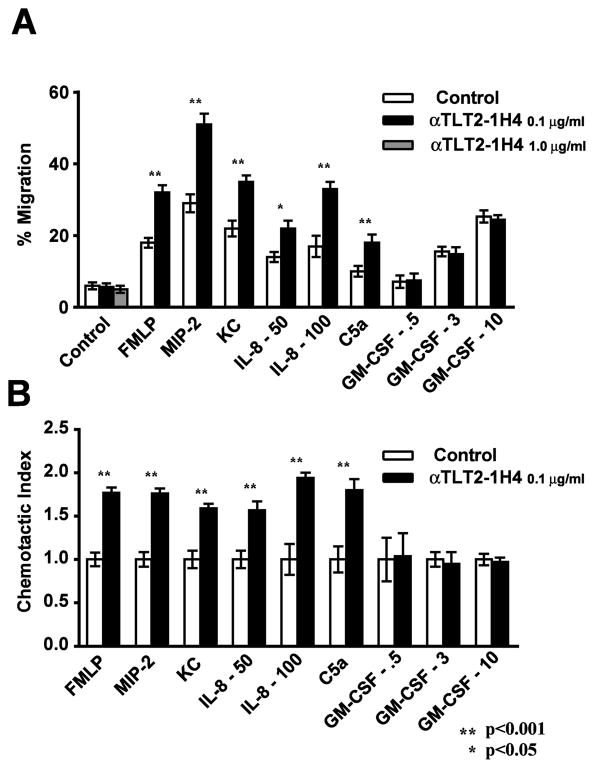
TLT2 ligation potentiates the functional response of neutrophils to FMLP and C5a, including cell migration, ROS production, and upregulation of CD11b, but exhibits no effect on neutrophil functional responses to TLR agonists, GM-CSF or FcR-mediated activation. Given the important role that GPCRs play in regulating neutrophil migration in vivo, it was of interest to determine if TLT2 ligation potentiates the migration of these cells in response to other chemokines. Chemotaxis of purified neutrophils was assayed in response to a variety of chemoattractants including the murine chemokines, KC and MIP-2, their human homologue IL-8, as well as the activated complement component C5a. In this assay, TLT2 ligation in the absence of chemoattractant has no measurable effect. However, in the presence of chemokine, αTLT2 mAb resulted in a 1.7- to 2.0-fold increase in the number of cells that migrate in response to these chemoattractants (Fig. 4A, B). Importantly this effect is not observed in response to GM-CSF, which is the only member of this panel of chemoattractants that does not signal via a GPCR. Treatment with isotype control mAb had no effect on neutrophil chemotaxis nor did it potentiate migration in response to any of the chemoattractants tested (Fig. 2 and data not shown). Interestingly, although the percentages of cells migrating in these assays varied depending on the chemoattractant utilized, the magnitude of the effect associated with TLT2 ligation was constant. This demonstrates that TLT2 ligation potentiates the response of neutrophils to multiple GPCRs, as the effect of TLT2 ligation is similar whether neutrophils are responding to signals delivered via the FMLP receptor, CXCR2, or the C5a receptor.
Figure 4. TLT2 ligation results in enhanced migration of neutrophils in response to a variety of chemoattractants in vitro.
Purified neutrophils were subjected to migration assays using 3 μm transwell filters. Neutrophils (1×106) were placed in the upper chamber in medium alone (control) or in the presence of the indicated concentration of αTLT2 mAb. Medium alone (control) or containing chemoattractants (FMLP, 1 μM; Mip-2, 5 ng/ml; KC, 5 ng/ml; IL-8, 50–100 ng/ml or GM-CSF, 0.5–10 ng/ml) was placed in the lower chamber, as indicated. After a 1 h incubation at 37°C, the neutrophils present in the lower chamber were harvested and counted. The percentage of cells present in the lower chamber (A) and the chemotactic index (B) are shown. The chemotactic index was calculated by dividing the % migration for neutrophils incubated with each chemoattractant in the presence or absence of αTLT2 mAb by the % migration for neutrophils incubated with medium alone. Data represent the average of triplicate samples with the mean ± SD shown.
TLT2 ligation promotes neutrophil recruitment to sites of inflammation in vivo
Because ligation of TLT2 potentiates the chemotactic response of neutrophils to agonists that bind GPCRs, and given the importance of these receptors in controlling the migration and recruitment of neutrophils, it was of interest to determine if TLT2 ligation potentiates neutrophil recruitment to sites of experimentally induced inflammation in vivo. Croton oil (2% in acetone) was used to induce a nonspecific inflammatory response in the ear. Thirty minutes following i.v. administration of either 1H4 mAb in the experimental group or saline in control mice, croton oil was applied to the pinna of one ear while acetone alone was applied to the other. After 4 h the mice were sacrificed and a 4 mm biopsy was removed from both ears. These biopsies were mechanically dissociated and the resulting lysate was analyzed for the presence of MPO (Fig. 5A) using the colorimetric substrate tetramethylbenzidine (TMB) (16). Assaying the MPO activity present in these samples against standard curves of horse radish peroxidase (HRP) facilitates the estimation of the number of neutrophils present in these samples. As seen in Figure 5B, administration of 1H4 mAb resulted in a two-fold increase in the number of neutrophils present in the inflamed ears, whereas there was no significant difference in the number of neutrophils present in samples derived from non-inflamed ears. To further examine the effect that administration of αTLT2 mAb in vivo has on neutrophil recruitment and the inflammatory process in control and croton oil-treated ears, H&E staining of sections taken from the ear was performed to assess the cellular infiltrate as well as to examine the architecture of the ear. As seen in Figure 5C, administration of αTLT2 mAb was observed to increase the recruitment of neutrophils to croton oil-treated ears in comparison to mice that received a sham injection of saline. The cellular influx consisted of primarily neutrophils in both treated and control animals based on examination of the sections at high magnification (data not shown). Administration of αTLT2 mAb had no effect on the recruitment of neutrophils to the control ear (Fig. 5C) and as demonstrated by an analysis of MPO activity (Fig. 5A). Finally, examination of the architecture of the ear did not reveal any significant differences at 3 h between the control and αTLT2-treated animals for either the control or croton oil-treated ear. These data support the conclusion that TLT2 engagement in vivo results in an enhanced chemotactic response to signals generated during inflammation thereby increasing the number of neutrophils accumulating in the inflamed tissue.
Figure 5. Administration ofαTLT2 mAb results in enhanced accumulation of neutrophils at sites of inflammationin vivo .
Either 100 μg of the αTLT2 mAb 1H4 or an equivalent volume of PBS was administered to groups of mice via i.v. injection. After 30 min, a solution of 2% Croton Oil in acetone was applied to the pinna of one ear, whereas only acetone was applied to the other (control ear). After 4 h, the mice were sacrificed and a 4 mm biopsy was removed from the center of the ears using a biopsy punch. These samples were homogenized and assayed for MPO activity using the colorimetric substrate TMB. The measured absorbance values are depicted for αTLT2 mAb treated and control mice for both ears (A). To determine the number of neutrophils recruited, lysates from known numbers of purified neutrophils were compared to serial dilutions of HRP to develop a standard curve. Using this standard curve for the MPO dependent conversion of TMB, the numbers of neutrophils present in the Croton Oil treated ears for αTLT2 mAb treated and control mice were calculated (B). The data presented represent a minimum of 5 mice and the mean ± SD, and significant p values are shown for both (A) and (B). (C) H & E staining of ear sections taken at 3 h from mice injected with saline (control) or αTLT2 mAb. Representative sections are shown for both control ears that were treated with acetone alone as well as inflamed ears treated with croton oil in acetone. The sections are representative of at least three independent experiments.
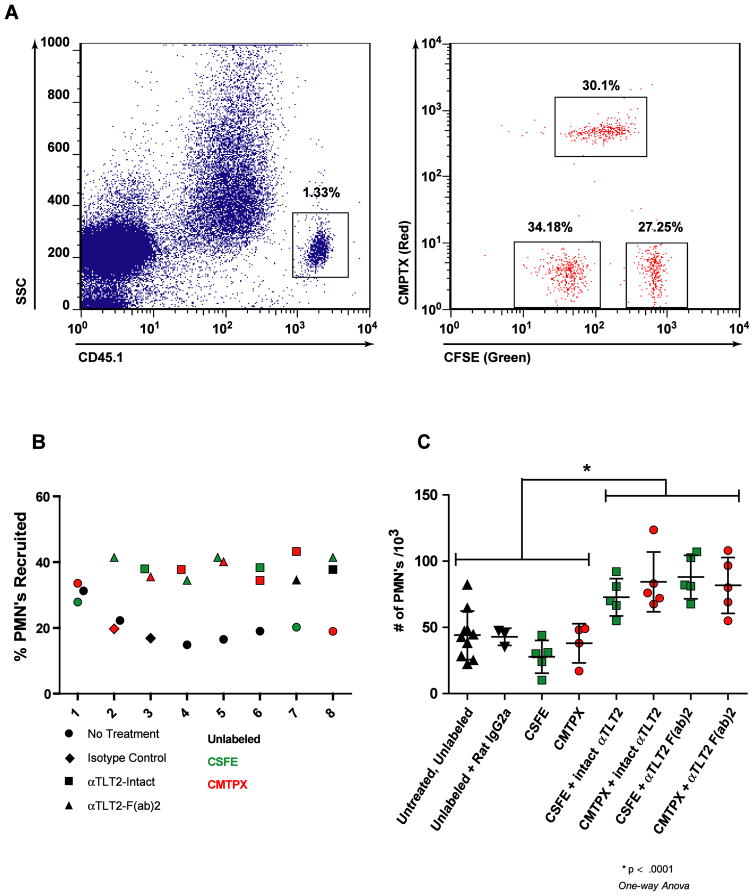
Because TLT2 is broadly expressed by immune cells, including macrophages, neutrophils, and B lymphocytes, it is difficult to determine if enhanced recruitment of neutrophils following administration of αTLT2 mAb is the result of a direct effect of TLT2 ligation on neutrophils or due to an indirect effect resulting from TLT2 ligation on other cell types. In an effort to determine if the observed enhancement of neutrophil accumulation at sites of inflammation is a direct effect of TLT2 ligation on responding neutrophils in vivo, a series of adoptive transfer experiments were performed in which neutrophils were isolated from the bone marrow of CD45.1 C57Bl/6 mice and labeled with either CFSE or CMPTX, or left unlabeled. These cells were then treated either with isotype control antibodies, F(ab′)2 fragments of 1H4 αTLT2 mAb or intact 1H4. An equal mixture of treated cells was then adoptively transferred into CD45.2 C57Bl/6 recipient mice, which had been challenged intratracheally with 5 μg of LPS to induce inflammation in the lung 2 h prior to adoptive transfer. Four hours after adoptive transfer of treated neutrophils, the recipient mice were sacrificed, and cells present in the lung were isolated by lavage, stained with the appropriate antibodies, and analyzed by flow cytometry. Adoptively transferred neutrophils were discriminated by the expression of CD45.1, and these cells were then analyzed based on the dye they were loaded with; green, red, or unlabeled, allowing for the identification of these populations present in the lungs of recipient mice (Fig. 6A). As seen in Figure 8B, neither pretreatment with isotype control antibody nor dye loading affected the recruitment of adoptively transferred neutrophils into the inflamed lung. Pretreatment with αTLT2 mAb, however, increased the frequency of treated neutrophils recovered in comparison to untreated or isotype control cells, regardless of the labeling conditions (Fig. 6B). The difference in frequencies between controls and αTLT2 mAb treated neutrophils results in approximately a two-fold increase in the absolute number of αTLT2 treated cells isolated from the lungs of the recipient mice (Fig. 6C). The observed increase in the number of neutrophils that migrated occurs whether neutrophils were treated with intact 1H4 mAb or F(ab′)2 fragments of this mAb. As these experiments utilized pooled neutrophils isolated from the same group of donor animals and represent a specific enhancement in the recruitment of αTLT2 mAb treated neutrophils compared to control cells within the same recipient animal, these data strongly support the conclusion that ligation of TLT2 potentiates the chemotactic response of neutrophils to chemokines in vivo via a direct effect on the neutrophil itself.
Figure 6. TLT2 ligation enhances neutrophil recruitment into sites of inflammation in vivo.
(A) Purified neutrophils from CD45.1 mice were labeled with either CFSE, CMPTX, or left unlabeled. These cells were then incubated in the presence of the αTLT2 mAb as indicated, or an isotype control antibody, or medium alone. After treatment, the purified, labeled neutrophils were mixed at equivalent ratios and 3×107 total cells were adoptively transferred into CD45.2 recipient mice, which had received an intratracheal challenge with 5 μg of LPS to induce inflammation in the lung 30 min prior to adoptive transfer. After 4 h, the recipient mice were sacrificed and the neutrophils present in the lung were isolated by lavage. Donor derived neutrophils were indentified based on the expression of CD45.1, and these cells were analyzed for the presence of the fluorescent dyes, which discriminated the pre-transfer treatment conditions. (B) Ligation of TLT2 increases the relative frequency of neutrophils recruited into the lungs of recipient mice. A total of 8 mice are depicted and for each mouse the % PMNs recruited to the lung for each of 3 pre-treatment conditions is shown. The pretreatment condition is coded by the symbol used and the dye used is reflected by the color of the symbol. (C) TLT2 ligation causes an absolute increase in the number of neutrophils that migrate into inflamed lungs. The absolute number of neutrophils for each treatment condition was calculated based on flow cytometric analysis. The mean ± SD for a minimum of 5 mice is shown for each treatment condition.
Discussion
The Trem locus encodes several receptors expressed on myeloid lineage cells that function to modulate the response of cells to ligands for PRRs. In the case of TREM-1, ligation potentiates the systemic inflammatory response following stimulation of cells via TLRs and in some instances can exert a direct effect on macrophages leading to the production of inflammatory cytokines (7, 8, 10, 17). In contrast, TREM-2, which is expressed on a range of cell types, including macrophages has been shown to exert an inhibitory effect on TLR-mediated signaling resulting in attenuation of activation and cytokine production (9, 18). TLT2 is expressed on cells of the myeloid lineage, including neutrophils and macrophages, and is upregulated in response to inflammatory conditions in vivo (5). Based on these observations, it was of interest to determine if TLT2, like other members of the TREM family, modulates the functional response of myeloid lineage cells to signals derived from PRRs. This study demonstrates that TLT2 ligation potentiates ROS production, degranulation and chemotaxis in response to numerous GPCR agonists, including FMLP, C5a, MIP-2, KC and IL-8. Importantly, TLT2 ligation was not observed to potentiate the cellular response to GM-CSF receptor or FcR-mediated signaling, which involve reversible protein tyrosine phosphorylation. Moreover, TLT2 ligation did not potentiate neutrophil degranulation in response to TLR. Thus, TLT2 appears to mediate signaling processes that selectively potentiate the response to GPCRs. Importantly, ligation of TLT2 was not observed to decrease the threshold of sensitivity to GPCR agonists, supporting the conclusion that may act by amplifying or prolonging the GPCR signal, which in turn potentiates the functional response of the cell. Alternatively, because studies have yet to be performed demonstrating that TLT2 ligation affects the qualitative or quantitative nature of the biochemical signals associated with GPCRs, it is formally possible that TLT2 initiates a parallel signaling pathway that amplifies the functional response to GPCRs without directly intersecting those pathways. Regardless, the role of TLT2 as a receptor that potentiates the response of neutrophils to GPCR-mediated signals is comparable to the functions of TREM-1 and TREM-2 which modulate TLR-mediated signaling.
As mentioned above, the mechanism by which TLT2 potentiates the response to GPCR signaling is currently not known. TLT2 does not possess charged transmembrane residues like TREM-1 and TREM-2 that would promote the interaction with transmembrane signaling effectors such as DAP12. Unlike TLT1, TLT2 does not contain an ITAM or ITIM in its cytoplasmic domain, which have the ability to recruit SH2 domain-containing effector proteins to mediate signal transduction (4, 19). Indeed, the cytoplasmic tail of human and mouse TLT2 are not highly conserved and there are no apparent signaling motifs that would, a priori, be predicted to be important for potentiating GPCR-mediated signaling. The TLT2 cytoplasmic domains from human and mouse are proline-rich and it is possible that this could confer the ability to interact with WW or SH3 domain-containing proteins. Alternatively, the proline residues may play a role in providing tertiary structure to the cytoplasmic domain that is important for mediating interactions with key effector proteins. Regardless, it is clear that ligation of TLT2 does not potentiate the cellular response to signals delivered via the GM-CSF receptor, which is a receptor protein tyrosine kinase, or Fc receptors, which also engage protein tyrosine kinases. Moreover, studies do not support the conclusion that TLT2 ligation modulates the response to TLR signaling, as is the case for TREM-1 and TREM-2. Therefore, it appears that TLT2 selectively modulates the cellular response to GPCR-mediated signaling. However, studies have yet to determine if this is true for receptors coupled to a wide range of heterotrimeric G proteins as the receptors examined to date engage G proteins of the Gαi subclass. Whether TLT2 can modulate the response to signaling via receptors coupled to other subclasses of heterotrimeric G proteins, such as Gαs or Gαq, remains to be determined.
An equally important question that remains to be determined concerns the identity of the ligand for TLT2. Previous studies have suggested that TLT2 binds to B7-H3 and that TLT2 plays a role in modulating the function of CD8+ T cells in response to engaging its ligand (20). However, it is unlikely that this is the case. First, studies by our group have consistently failed to detect expression of TLT2 on cells of the T lineage (5). Regardless of whether CD4+ or CD8+ T cells are examined in their resting or activated state, TLT2 expression is not observed using two anti-TLT2 mAbs that bind to distinct epitopes. Secondly, it has been reported by an independent group that TLT2 does not bind B7-H3 (21). In conclusion, the identity of the physiological ligand for TLT2 remains to be determined. Currently, little is known regarding the ligands for other TREM family receptors. Studies using recombinant TREM-1 fusion proteins have detected putative ligands on the surface of neutrophils and platelets as well as on necrotic cells and in the serum of patients with sepsis, but their identity has not been determined (22–24). Similarly, TREM-2 fusion proteins have detected putative ligands on the surface of peritoneal and bone marrow-derived macrophages (18). More recently, TREM-2 has been shown to exist in a complex with Plexin-A1, which binds to Sema6D (25). Thus DAP12-mediated signaling can be initiated by virtue of the TREM-2:Plexin-A1 interaction via binding of Plexin-A1 to Sema6D. This observation explains the phenotypic consequences of a genetic loss of the TREM-2 receptor and DAP12 and demonstrates that at least one member of the Trem locus is responsible for mediating signals in response to a ligand that binds to a distinct, associated receptor (25). Because TLT2 is expressed on both neutrophils and macrophages, which are critical effectors of the innate immune response against bacteria, and its expression is increased on these cells in response to inflammation, one might predict that the ligand for TLT2 could be derived from infectious organisms, or be generated as a byproduct of the innate immune response against pathogens (e.g. an acute phase protein). This concept is also supported by the fact that TLT2 is most highly expressed on marginal zone and B1 B cell subpopulations, which are involved in mounting the natural/innate humoral immune response against bacterial pathogens (5).
In summary, this study demonstrates that TLT2 plays an important role in potentiating the neutrophil response to GPCR agonists leading to enhanced ROS production, degranulation and chemotaxis. Importantly, TLT2 does not appear to potentiate the cellular response to other types of receptors. Because TLT2 is upregulated on myeloid lineage cells in response to inflammatory conditions in vivo, and plays a role in potentiating the cellular response against agonists derived from pathogens or generated during the innate immune response, it is clear that this member of the TREM locus is important in potentiating the anti-bacterial innate immune response via a novel mechanism that involves the potentiation of the response to GPCR signaling.
Supplementary Material
Acknowledgments
We thank Dr. Lynn Rasmussen at Southern Research Institute for generously allowing the use of their Envision Multi-label Plate reader for the Respiratory Burst assay.
M. M. Halpert was supported by training grants from the HHMI Med into Grad Initiative (Grant 56005705) and a NIH Institutional Training Grant in Immunology (T32 AI 007051-33).
Abbreviations used in this paper
- GPCR
G protein-coupled receptor
- MPO
myeloperoxidase
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- SA
streptavidin
- TLT2
TREM-like transcript 2
- TMB
tetramethylbenzidine
- TREM
triggering receptor expressed on myeloid cells
References
- 1.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 3.Klesney-Tait J, I, Turnbull R, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 4.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J Immunol. 2006;176:6012. doi: 10.4049/jimmunol.176.10.6012. [DOI] [PubMed] [Google Scholar]
- 6.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 7.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- 8.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 10.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyduk SJ, Chan JR, Duffy ST, Chen M, Peterson MD, Waddell TK, Digby GC, Szaszi K, Kapus A, Cybulsky MI. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109:176. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shami A, Mahanna W, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Selective activation of Jak2, Stat3, and Stat5b. J Biol Chem. 1998;273:1058. doi: 10.1074/jbc.273.2.1058. [DOI] [PubMed] [Google Scholar]
- 14.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 33:657. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 16.Menegazzi R, Zabucchi G, Knowles A, Cramer R, Patriarca P. A new, one-step assay on whole cell suspensions for peroxidase secretion by human neutrophils and eosinophils. J Leukoc Biol. 1992;52:619. doi: 10.1002/jlb.52.6.619. [DOI] [PubMed] [Google Scholar]
- 17.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187:S397. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 18.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 19.Barrow AD, Astoul E, Floto A, Brooke G, Relou IA, Jennings NS, Smith KG, Ouwehand W, Farndale RW, Alexander DR, Trowsdale J. Cutting edge: TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J Immunol. 2004;172:5838. doi: 10.4049/jimmunol.172.10.5838. [DOI] [PubMed] [Google Scholar]
- 20.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105:10495. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong-Baeza I, Gonzalez-Roldan N, Ferat-Osorio E, Esquivel-Callejas N, Aduna-Vicente R, Arriaga-Pizano L, Astudillo-de la Vega H, Villasis-Keever MA, Torres-Gonzalez R, Estrada-Garcia I, Lopez-Macias C, Isibasi A. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients. Clin Exp Immunol. 2006;145:448. doi: 10.1111/j.1365-2249.2006.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 24.Gibot S, Massin F, Marcou M, Taylor V, Stidwill R, Wilson P, Singer M, Bellingan G. TREM-1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37:456. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- 25.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP 12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.