SUMMARY
Self-fertile hermaphrodites have evolved independently several times in the genus Caenorhabditis [1, 2]. These XX hermaphrodites make smaller sperm than males [3, 4], which they use to fertilize their own oocytes. Since larger sperm outcompete smaller sperm in nematodes [3–5], it had been assumed that this dimorphism evolved in response to sperm competition. However, we show that it was instead caused by a developmental bias. When we transformed females of the species C. remanei into hermaphrodites [6], their sperm were significantly smaller than those of males. Because this species never makes hermaphrodites in the wild, this dimorphism cannot be due to selection. Instead, analyses of the related nematode C. elegans suggest that this dimorphism might reflect the development of sperm within the distinct physiological environment of the hermaphrodite gonad. These results reveal a new mechanism for some types of developmental bias — the effects of a novel physical location alter the development of ectopic cells in predictable ways.
RESULTS AND DISCUSSION
Most nematode species are gonochoristic — they produce XO males and XX females [7]. The males make small, round spermatids that activate following mating, extend pseudopods, and crawl towards the spermathecae of the female, where they compete to fertilize oocytes. However, some species have evolved an androdioecious mating system, with XO males and self-fertile XX hermaphrodites. In these species, the hermaphrodites make spermatids late in larval development, and then permanently switch to the production of oocytes. In almost all respects, the male and hermaphrodite sperm from these species appear identical [8]. However, one trait is sexually dimorphic — the male sperm are much larger than those of hermaphrodites [4].
Artificially produced Caenorhabditis hermaphrodites make smaller sperm than males
To study the characteristics of sperm in species that do not make hermaphrodites in the wild, we used RNA interference to manipulate the sex-determination pathway, to induce spermatogenesis in XX individuals. In C. elegans, tra-2 activity blocks both male development and spermatogenesis [9], so it must be repressed in the germlines of XX hermaphrodites to allow spermatogenesis to occur [10–12]. Although C. remanei uses a male/female mating system in the wild, we found that knocking down tra-2 activity with a dilute dose of dsRNA causes some XX animals to develop as pseudo-hermaphrodites [6]. These animals have female bodies, but produce sperm when they are young, and then switch to oogenesis during adulthood. Their sperm do not self-activate unless a second gene, swm-1, is also targeted.
The androdioecious nematodes that had been studied previously all have sexually dimorphic sperm — those from males are much larger than those from hermaphrodites [4]. To characterize the sperm produced by C. remanei pseudo-hermaphrodites, we isolated them by dissection (Fig. 1A). The spermatids appeared normal and could be activated by pronase [6]. However, they were smaller than those from C. remanei males (Fig. 1). Sperm from pseudo-hermaphrodites of the SB146 strain had an average cross-sectional area of 22.5 μm2, which was significantly smaller than that of male sperm (average 41.6 μm2, P<10−14, Mann-Whitney U test). We found a similar dimorphism for strain PB4641 (average 35.2 μm2 versus 49.2 μm2, P<10−8). Furthermore, when we constructed pseudo-hermaphrodites from another Caenorhabditis species, C. sp. 9, they also produced smaller sperm than related males (average 15.7 versus 38.6 μm2, P<10−11). Thus, male/female species of Caenorhabditis are predisposed to make sexually dimorphic sperm. Because the XX animals do not make sperm in the wild, this trait cannot have been produced by selection.
Figure 1. C. remanei is predisposed to make sexually dimorphic sperm.
A. Photomicrographs of sperm that had been isolated from dissected animals and activated with pronase. In these Differential Interference Contrast images, the cross-sectional areas of the sperm were calculated using Zeiss Axiovision software. B. Histograms showing the size of sperm isolated from individuals of the indicated species and sex. In both panels, the symbol Ψ stands for “pseudo”. For each genotype, “n” indicates the number of sperm that were measured, and these sperm were isolated from at least three different individuals. The sizes of sperm produced by males and hermaphrodites were compared using the Mann-Whitney U test [42].
The hermaphrodite soma and germ line co-operate to nurture small sperm
The regulatory genes that control sexual development are best known in the related nematode C. elegans [13]. Thus, to learn why nematodes are predisposed to make sexually dimorphic sperm, we studied C. elegans sex-determination mutants (Fig. 2).
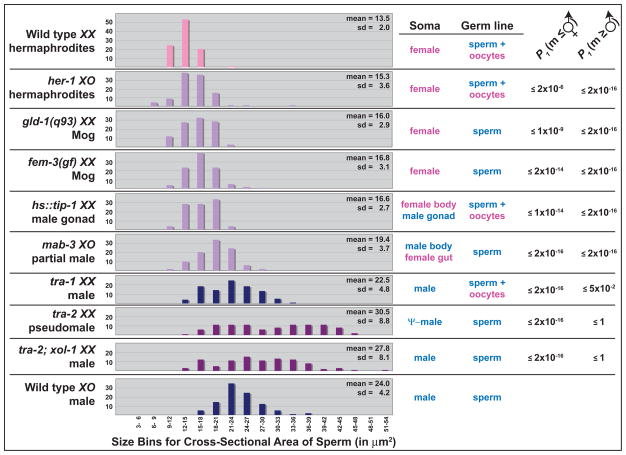
Figure 2. In C. elegans, somatic tissues and the germ line co-operate to specify sperm size.
Histograms showing the size of sperm isolated from individuals of the indicated genotype and sex. Each strain is described in Table S2. Data for wild-type hermaphrodites are shaded pink, and those for males blue. Genotypes that make larger sperm than hermaphrodites but smaller than males are lavender, and genotypes that make larger sperm than normal males are shaded maroon. For each genotype, 97 sperm were measured, and these sperm were isolated from at least three different individuals. The size distributions were compared to those of wild-type hermaphrodites in one set of assays, and to wild-type males in another, using the Mann-Whitney U test [42], with a correction for the False Discovery Rate due to multiple comparisons [43].
First, we examined the relationship between X chromosome dose and sperm size. In the wild type, XX animals are hermaphrodites and XO animals are males, but this relationship can be altered by mutations that perturb the sex-determination pathway [14]. For example, tra-2; xol-1 XX animals develop as perfect males [15]. These tra-2; xol-1 XX males made sperm that were about the same size as those from wild-type XO males (x̄ = 27.8 versus 24.0 μm2), but were much larger than sperm from XX hermaphrodites (x̄ = 13.5 μm2). Moreover, tra-2 XX pseudo-males [9] gave similar results. By contrast, her-1 XO hermaphrodites [16] produced sperm that were almost as small as those of wild-type XX hermaphrodites (x̄ = 15.3 versus 13.5 μm2). Thus, the ratio of X chromosomes to autosomes plays a minimal role in specifying sperm size. Instead, sperm size is highly correlated with the somatic sex of the animal.
Next, we examined XX animals that had a female body but a male somatic gonad (Fig. 2). These worms were produced using a hs::tip-1 construct (D. Zarkower, personal communication). Their sperm were larger than those of wild-type hermaphrodites (x̄ = 16.7 versus 13.5 μm2), but also smaller than sperm from wild-type males. Thus, the somatic gonad can influence sperm size, but does not act alone to determine it.
What other tissues might be important? The intestine is a major site for protein synthesis in worms, and can affect the germ line [17]. For example, the hermaphrodite intestine secretes yolk protein, which is taken up by developing oocytes [18]. Thus, we examined sperm from XO mab-3 mutants (Fig. 2), which develop a hermaphrodite intestine but retain a male somatic gonad [19]. Their sperm had an average size of 19.4 μm2, which was significantly smaller than wild-type male sperm (average 24.0 μm2). Taken together, these results imply that the somatic goand and intestine cooperate to nurture larger sperm in males.
Since these somatic effects were not large enough to explain the magnitude of the sexual dimorphism on their own, we examined the influence of oogenesis on sperm size, since hermaphrodite nematodes begin producing oocytes before some germ cells have completed spermatogenesis. First, we studied fem-3(q96ts), a gain-of-function mutation that causes XX animals to make sperm throughout their lives [20]. These XX mutants made sperm with an average size of 16.8 μm2, which was about 25% larger than normal hermaphrodite sperm (Fig. 2). We observed similar results with another mutation that prevents hermaphrodite oogenesis, gld-1(q93Mog) (Fig. 2). Finally, we studied animals with male bodies, by comparing sperm from tra-2; xol-1 XX males, which make only sperm, with those of tra-1 XX males, which also produce oocytes [21, 22]. As we had predicted, the tra-1 XX males produced smaller sperm (22.5 μm2 versus 27.8 μm2). Thus, sperm that develop in a germline that has initiated oogenesis are smaller, regardless of the somatic sex of the animal. Perhaps developing oocytes compete with spermatocytes for resources.
Both male and hermaphrodite sperm have decreased in size in androdioecious species
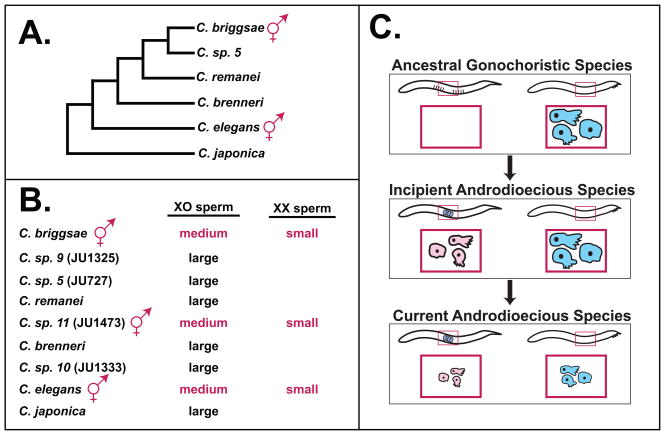
Since the pioneering studies of LaMunyon and Ward [4], the phylogeny of Caenorhabditis has been refined (Fig. 3A), and new species have been identified. Moreover, recent studies have shown that hermaphroditism evolved independently in C. sp. 11, as well as in C. elegans and C. briggsae (K. Kiontke, M.-A. Félix, M. Ailion, and D. H. A. Fitch, pers. comm.). We found that sperm in C. sp. 11 hermaphrodites were much smaller than those of related males (Fig. 3B), confirming that all androdioecious species in this group make sexually dimorphic sperm. Moreover, the sperm produced by males in each androdioecious species were much smaller than those produced by males from any of the gonochoristic species (Fig. 3B). Thus, not only was the ancestral species of the elegans group gonochoristic, its males must have produced large sperm.
Figure 3. In Caenorhabditis, sperm size has decreased in both androdioecious sexes.
A. Most recent phylogeny of Caenorahbditis [44]. The new species 9, 10 and 11 have not yet been described, but species 11 evolved self-fertile hermaphrodites independently from C. elegans and C. briggsae (K. Kiontke, M.-A. Félix, M. Ailion, and D. H. A. Fitch, pers. comm.). B. Summary of sperm size in members of the elegans group. Large > 30 μm2, medium >20 μm2 and small < 20 μm2 cross-sectional area. Data are presented in Table S3. C. Model for the evolution of sperm size in androdioecious species. The rectangular insets show sperm, colored pink for hermaphrodites or blue for males.
Selection and developmental biases cooperate to specify sperm size in nematodes
Based on these results, we propose the following scenario for the evolution of sperm size in androdioecious nematodes (Fig. 3C). First, newly evolved hermaphrodites produce smaller sperm than related males because of a developmental bias. Genetic tests imply that the cause of this bias is the inability of the hermaphrodite body and germ line to nurture the robust growth of sperm seen in males (Fig. 2). Next, selection acts to decrease sperm size in both sexes, since sperm from androdioecious males are smaller than those from males in other species (Fig. 3B), and sperm from established lines of androdioecious hermaphrodites are much smaller than those made by C. remanei XX pseudo-hermaphrodites (Fig. 1). If males were undergoing sperm competition with each other, selective pressure should not act directly to decrease male sperm size, since larger sperm are more competitive [3–5]. However, hermaphrodites appear to be under selective pressure to cease spermatogenesis early, so that they can begin oogenesis and reproduction as soon as possible [23]. Thus, selection might cause a further decrease in hermaphrodite sperm size, so that they can produce the maximum number of sperm in the brief time available. If so, the size of male sperm might decrease as well, either because a common genetic regulatory pathway controls spermatogenesis in both sexes [8], or because males become so rare that they are no longer in competition with each other, which might favor the production of smaller sperm that are just large enough to outclass those of hermaphrodites.
Ectopic expression of regulatory programs explains some developmental biases in evolution
Most analyses of developmental bias have focused on a negative trait — the ability of developmental processes to limit the types of variation that are present in a population and available to be sifted by natural selection [24–26]. In these analyses, mutations occur randomly, but they map onto a distorted range of variation around the population norm, with some phenotypes rare or absent. Thus, the developmental laws that result in the absence of these phenotypes constrain the course of future change. Data from wild populations of sticklebacks suggest that the phenotypic variation in a population is indeed related to long-term patterns of evolutionary change [27], and similar phenomena have been measured in populations of nematodes in laboratory studies [28]. As shown by our work, development also influences evolution in additional ways.
In total, three developmental mechanisms might affect the evolution of sperm size in nematodes. The first is so common that it is often ignored when discussing developmental biases — hermaphrodite nematodes have appropriated the use of genes that regulate spermatogenesis in males [8]. This pattern is the mirror image of the developmental constraints discussed above, because the ability to reuse existing regulatory programs in new contexts can generate very specific types of variation. It could involve the duplication and divergence of regulatory genes, as occurred with F-box proteins in nematode sex determination [29], or the alteration of cis-regulatory sequences in target genes, such as those that control sexually dimorphic color patterns in Drosophila [30]. The fact that specific programs are available in some phylogenetic lineages but not others should bias the course of evolution, and could explain why some groups evolve traits like self-fertility on repeated occasions, while other groups do not.
Second, the size of sperm in androdioecious males has decreased over time, even though data suggest that selection should favor males with larger sperm. This result implies that selection for smaller sperm in hermaphrodites might indirectly result in smaller male sperm. Such a correlation in growth was discussed by Darwin, and was described in one of the most famous examples of developmental bias — the decrease in the number of digits following selection for smaller size in amphibians [31].
Third, the ectopic expression of developmental programs occurs, by necessity, in novel settings. Thus, the new cellular or chemical environment will alter the trait in specific and predictable ways. For example, our data suggest that sperm made in hermaphroditic nematodes are predisposed to smaller size, because hermaphrodite tissues are not adapted to nurture the growth of larger sperm. Because the ectopic expression of developmental programs is common during evolution, we anticipate that this type of bias will prove to be a frequent factor in evolutionary change.
Alberch and Gale showed that experimental studies in comparative systems were the best way to identify developmental biases [31]. Given the rich array of resources now available for studying Caenorhabditis, Drosophila and other groups of species, we have entered a golden age for deciphering the rules by which development controls phenotypic variation and adaptation.
EXPERIMENTAL PROCEDURES
Nomenclature
We use the standard genetic nomenclature for C. elegans described by Horvitz, et al. [32]. However, C. remanei XX animals are females, since they produce oocytes but do not make sperm. We call C. remanei XX animals that have a female body but make both sperm and oocytes “pseudo-hermaphrodites.”
Strains
The wild-type androdioecious strains were C. elegans N2 [33], C. briggsae AF16 [34], and C. sp. 11 JU1473 (M. Felix, personal communication). The gonochoristic strains were C. remanei SB146 [35], C. remanei PB4641 (M. Felix, personal communication), C. brenneri CB5161 [36], C. sp. 5 JU800, C. sp. 9 JU1420, C sp. 10 JU1333 (M. Felix, personal communication) and C. japonica DF5098 [37].
The C. elegans mutations were him-1(e879) [38], dpy-10(e128) [33], mab-3(e1240) [19], tra-2(e1095) [9], tra-2(ar221) (J. Hubbard, personal communication), unc-4(e120) [33], tra-1(e1099) [22], fem-3(q93gf,ts) [20], him-5(e1490) [38], her-1(e1518) [16], xol-1(y9) [15] and ezEx18[Hs::tip-1(+); rol-6(dom)] (D. Zarkower, personal communication). Complete genotypes for each experiment are listed in Table S2. The C. briggsae mutation tra-2(nm1) [39] gave results that were similar to those for C. elegans (Table S3).
Animals were raised on agar plates on E. coli AMA00004 at 20º C, unless noted otherwise.
RNA interference
The primers listed in Table 1 were used to amplify templates by PCR. Each template was flanked by T7 promoters. We used the Mega-Script kit (Ambion) to transcribe each template, and allowed the two RNA strands to hybridize at 37º C. The RNA was then purified using a Mega-Clear kit (Ambion). Purified products were confirmed by gel-electrophoresis and the RNA concentration measured by UV spectroscopy. Each double-stranded RNA was injected into the gonads of young adult females [40]. These animals were then allowed to recover, mated with wild-type males, and their progeny examined for mutant phenotypes.
Heat shock
L4 larvae were picked to a plate and allowed to lay eggs at 20°C for 48 hours. The larvae were then shifted to 35° for 15 minutes, and returned to 20°C. Two to three additional heat shocks were applied at 12-hour intervals. Control hermaphrodites and males were also subject to heat shocks, after which the average size of hermaphrodite sperm was x̄ = 13.3 μm2 (sd = 2.0, n = 36) and of male sperm was x̄ = 23.7 μm2 (sd = 3.7, n = 46). Neither value differed from non-heat-shocked controls, and both differed significantly from hs-tip-1 sperm.
Sperm Size
Animals were placed in a 10 μl drop of sperm media that had been prepared for sperm activation [41] and dissected using sharp 18g needles. Young males were sliced across the vas deferens to release their spermatids into the media, whereas hermaphrodites were cut open through the spermatheca. After 5 minutes, slides were examined by DIC optics and pictures of fields of sperm were taken. The cross-sectional area of the sperm was measured using Zeiss AxioVision software.
Supplementary Material
HIGHLIGHTS.
The sexual dimorphism in the size of Caenorhabditis sperm is caused by developmental bias.
This bias is a result of the effects of female physiology on ectopic spermatogenesis.
Other types of developmental bias also influence the evolution of sperm size.
All sperm in androdioecious species undergo a further decrease in size during evolution.
Acknowledgments
This work was supported by National Science Foundation grant 0543828 and National Institutes of Health Grant GM085282. We thank Eric Moss for comments on this manuscript, D. Zarkower for the tip-1 constructs, and the Caenorhabditis Genetics Center, M.-A. Felix, D. Fitch and K. Kiontke for strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cho S, Jin SW, Cohen A, Ellis RE. A phylogeny of Caenorhabditis reveals frequent loss of introns during nematode evolution. Genome Res. 2004;14:1207–1220. doi: 10.1101/gr.2639304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci U S A. 2004;101:9003–9008. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaMunyon CW, Ward S. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc Biol Sci. 1998;265:1997–2002. doi: 10.1098/rspb.1998.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaMunyon CW, Ward S. Evolution of sperm size in nematodes: sperm competition favours larger sperm. Proc Biol Sci. 1999;266:263–267. doi: 10.1098/rspb.1999.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMunyon CW, Ward S. Evolution of larger sperm in response to experimentally increased sperm competition in Caenorhabditis elegans. Proc Biol Sci. 2002;269:1125–1128. doi: 10.1098/rspb.2002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldi C, Cho S, Ellis RE. Mutations in two independent pathways are sufficient to create hermaphroditic nematodes. Science. 2009;326:1002–1005. doi: 10.1126/science.1176013. [DOI] [PubMed] [Google Scholar]
- 7.Kiontke K, Fitch DH. Wormbook, T.C.e.R. Community. The Phylogenetic Relationships of Caenorhabditis and Other Rhabditids. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.L’Hernault SW. Spermatogenesis. WormBook; 2006. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgkin JA, Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977;86:275–287. [PMC free article] [PubMed] [Google Scholar]
- 10.Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jan E, Motzny CK, Graves LE, Goodwin EB. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 1999;18:258–269. doi: 10.1093/emboj/18.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford R, Lee MH, Nayak S, Ohmachi M, Giorgini F, Schedl T. FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development. 2000;127:5265–5276. doi: 10.1242/dev.127.24.5265. [DOI] [PubMed] [Google Scholar]
- 13.Ellis RE. Chapter 2 Sex Determination in the Caenorhabditis elegans Germ Line. Curr Top Dev Biol. 2008;83:41–64. doi: 10.1016/S0070-2153(08)00402-X. [DOI] [PubMed] [Google Scholar]
- 14.Zarkower D. Wormbook, T.C.e.R. Community. Somatic Sex Determination. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller LM, Plenefisch JD, Casson LP, Meyer BJ. xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell. 1988;55:167–183. doi: 10.1016/0092-8674(88)90019-0. [DOI] [PubMed] [Google Scholar]
- 16.Hodgkin J. More sex-determination mutants of Caenorhabditis elegans. Genetics. 1980;96:649–664. doi: 10.1093/genetics/96.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGhee JD. The C. elegans intestine. WormBook; 2007. pp. 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimble J, Sharrock WJ. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983;96:189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- 19.Shen MM, Hodgkin J. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell. 1988;54:1019–1031. doi: 10.1016/0092-8674(88)90117-1. [DOI] [PubMed] [Google Scholar]
- 20.Barton MK, Schedl TB, Kimble J. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics. 1987;115:107–119. doi: 10.1093/genetics/115.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schedl T, Graham PL, Barton MK, Kimble J. Analysis of the role of tra-1 in germline sex determination in the nematode Caenorhabditis elegans. Genetics. 1989;123:755–769. doi: 10.1093/genetics/123.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgkin J. A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev. 1987;1:731–745. doi: 10.1101/gad.1.7.731. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkin J, Barnes TM. More is not better: brood size and population growth in a self-fertilizing nematode. Proc Biol Sci. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- 24.Maynard Smith J, Burian R, Kauffman S, Alberch P, Campbell J, Goodwin B, Lande R, Raup D, Wolpert L. Developmental Constraints and Evolution. Q Rev Biol. 1985;60:265–287. [Google Scholar]
- 25.Yampolsky LY, Stoltzfus A. Bias in the introduction of variation as an orienting factor in evolution. Evol Dev. 2001;3:73–83. doi: 10.1046/j.1525-142x.2001.003002073.x. [DOI] [PubMed] [Google Scholar]
- 26.Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323:746–751. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schluter D. Adaptive Radiation Along Genetics Lines of Least Resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 28.Braendle C, Baer CF, Felix MA. Bias and evolution of the mutationally accessible phenotypic space in a developmental system. PLoS Genet. 2010;6:e1000877. doi: 10.1371/journal.pgen.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Lang S, Ellis RE. Independent recruitment of F box genes to regulate hermaphrodite development during nematode evolution. Curr Biol. 2009;19:1853–1860. doi: 10.1016/j.cub.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell. 2008;134:610–623. doi: 10.1016/j.cell.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberch P, Gale EA. A developmental analysis of an evolutionary trend: digital reduction in amphibians. Evolution. 1985;39:8–23. doi: 10.1111/j.1558-5646.1985.tb04076.x. [DOI] [PubMed] [Google Scholar]
- 32.Horvitz HR, Brenner S, Hodgkin J, Herman RK. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979;175:129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- 33.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fodor A, Riddle DL, Nelson FK, Golden JW. Comparison of a new wild-type Caenorhabditis briggsae with laboratory strains of C. briggsae and C. elegans. Nematologica. 1983;29:203–217. [Google Scholar]
- 35.Sudhaus W, Kiontke K. Phylogeny of Rhabditis subgenus Caenorhabditis (Rhabditidae, Nematoda) Journal of Zoological Systematics & Evolutionary Research. 1996;34:217–233. [Google Scholar]
- 36.Baird SE, Sutherlin ME, Emmons SW. Reproductive isolation in Rhabditidae (Nematoda: Secernentea); mechanisms that isolate six species of three genera. Evolution. 1992;46:585–594. doi: 10.1111/j.1558-5646.1992.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 37.Kionkte K, Hironaka M, Sudhaus W. Description of Caenorhabditis japonica n. sp (Nematoda: Rhabditida) associated with the burrower bug Parastrachia japonensis (Heteroptera:Cydnidae) in Japan. Nematology. 2002;4:933–941. [Google Scholar]
- 38.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction Mutants of the Nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill RC, de Carvalho CE, Salogiannis J, Schlager B, Pilgrim D, Haag ES. Genetic flexibility in the convergent evolution of hermaphroditism in Caenorhabditis nematodes. Dev Cell. 2006;10:531–538. doi: 10.1016/j.devcel.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 41.Ward S, Hogan E, Nelson GA. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev Biol. 1983;98:70–79. doi: 10.1016/0012-1606(83)90336-6. [DOI] [PubMed] [Google Scholar]
- 42.Mann HB, Whitney DR. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 44.Kiontke K, Barriere A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DH, Felix MA. Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol. 2007;17:1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.