Abstract
Developmental alcohol exposure can permanently alter brain structures and produce functional impairments in many aspects of behavior, including learning and memory. This study evaluates the effect of neonatal alcohol exposure on adult neurogenesis in the dentate gyrus of the hippocampus and the implications of such exposure for hippocampus-dependent contextual fear conditioning. Alcohol-exposed rats (AE) received 5.25 g/kg/day of alcohol on postnatal days (PD) 4-9 (third trimester in humans), in a binge-like manner. Two control groups were included: sham-intubated (SI) and suckle-control (SC). Animals were housed in social cages (3/cage) after weaning. On PD80, animals were injected with 200 mg/kg BrdU. Half of the animals were sacrificed two hours later. The remainder were sacrificed on PD114 to evaluate cell survival; separate AE, SI, and SC rats not injected with BrdU were tested for the context preexposure facilitation effect (CPFE; ∼PD117). There was no difference in the number of BrdU+ cells in AE, SI and SC groups on PD80. On PD114, cell survival was significantly decreased in AE rats, demonstrating that developmental alcohol exposure damages new cells' ability to incorporate into the network and survive. Behaviorally tested SC and SI groups preexposed to the training context 24h prior to receiving a 1.5mA 2s footshock froze significantly more during the context test than their counterparts preexposed to an alternate context. AE rats failed to show the CPFE. The current study shows the detrimental, long-lasting effects of developmental alcohol exposure on hippocampal adult neurogenesis and contextual fear conditioning.
Keywords: Fetal alcohol spectrum disorders, hippocampus, development, neurogenesis, contextual fear, animal model
1. Introduction
Alcohol use during pregnancy can lead to numerous birth defects including adverse development of the central nervous system (CNS). Offspring of women who abused alcohol during their pregnancies have a broad constellation of symptoms known as fetal alcohol spectrum disorders (FASD). The most severe of these disorders, Fetal Alcohol Syndrome, affects 1 out of 1000 live births each year internationally (Sampson et al., 1997; Abel, 1998; Morleo et al., 2011). A salient feature of FASD is brain dysfunction that results in learning and memory deficits, hyperactivity and motor deficits (Miller and Spear, 2006). In particular, alcohol exposure in utero has been shown to target select regions of the CNS, including the cerebellum, corpus callosum and the hippocampus (Riley et al., 1995; Mattson et al., 1996; Archibald et al., 2001; Auti-Ramo et al., 2002). Although the classic facial abnormalities of fetal alcohol syndrome result from alcohol exposure during the first trimester or equivalent of development (Sulik et al., 1981; Sulik, 2005), the brain remains vulnerable to the teratogenic effects of alcohol throughout gestation. For example, alcohol exposure during the brain growth spurt, which occurs during the third trimester in humans, damages the brain and impairs behavior (Chen et al., 2003). Alcohol exposure during the third trimester-equivalent in the rat (postnatal days [PD] 4-9) results in widespread apoptotic neurodegeneration in the developing rat forebrain (Ikonomidou et al., 2000), which could explain the reduced brain mass and neurobehavioral disturbances associated with FASD. Third trimester alcohol exposure also leads to loss of hippocampal CA1 pyramidal cells (Tran and Kelly, 2003; Livy et al., 2003) and to several pathological changes in the dendritic arborization of these neurons (Gonzalez-Burgos et al., 2006). Cell number and density reductions in CA3 and dentate gyrus regions have been reported in PD10 pups (Livy et al., 2003) while differences have not been found in adult rats (Tran and Kelly, 2003). Collectively, these findings highlight the vulnerability of the developing hippocampus to the neurotoxic effects of alcohol exposure, especially during the brain growth spurt in both human and rodent models.
In addition to hippocampal cell loss, recent studies have reported reductions in adult hippocampal neurogenesis resulting from developmental alcohol exposure (Klintsova et al., 2007; Ieraci and Herrera, 2007; for a recent review see Gil-Mohapel et al., 2010). Adult neurogenesis begins with cell proliferation and ends with cell migration and integration of a functional neuron into a preexisting circuit. There are two brain regions in which this occurs: the subgranular zone of the hippocampal dentate gyrus (DG) and the subventricular zone producing precursors for olfactory bulb neurons (Altman and Das, 1965; Lois and Alvarez-Buylla, 1993; Palmer et al., 2000). In particular, research shows that DG adult neurogenesis is regulated by numerous intrinsic and extrinsic factors including genetic background, age, sex, neurotransmitters, behavior, physical exercise, stress, hormones and drugs (Gould et al., 1997; Kempermann, Kuhn and Gage, 1997; Kuhn, Dickinson-Anson and Gage, 1996; Malberg and Duman, 2003; Nacher et al., 2001; Nixon and Crews, 2002; Tanapat et al., 1999; van Praag et al., 1999). Alcohol exposure during the neonatal period appears to have long-term effects on neurogenesis in rats. Our lab has previously demonstrated that binge-like alcohol exposure during the neonatal period (PD4-9) decreases adult neurogenesis in adult (PD50 and PD80) rats (Klintsova et al., 2007).
Alterations in cellular structure and function are likely to contribute to the behavioral deficits often reported in alcohol-exposed rats. For example, both juvenile and adult rats exposed to alcohol during gestational or neonatal development show impairments on traditional “hippocampus-dependent” behavioral tasks, such as spatial water maze and contextual fear conditioning (Goodlett and Peterson, 1995; Goodlett and Johnson, 1997; Allan et al., 2003; Murawski and Stanton, 2010). In light of normal performance of alcohol-exposed rats on control tasks (e.g., visible platform trials and cued fear conditioning), such behavioral deficits are attributed to alcohol-induced disruptions of hippocampal function. An attempt to link these behavioral deficits to alterations in adult neurogenesis has been made in rodent studies of prenatal alcohol exposure (reviewed in Gil-Mohapel et al., 2010). However, this study is the first to address both adult cell- and neurogenesis (Experiment 1) and learning (Experiment 2) in adult animals exposed to alcohol on PD4-9. We employ a variant of contextual fear conditioning, the context preexposure facilitation effect (CPFE), in which conditioning to an immediate shock requires preexposure to the training context (Rudy & O'Reilly, 2001). We have previously demonstrated that performance of this task is disrupted in juvenile rats exposed to alcohol over PD4-9 (Murawski & Stanton, 2010, 2011). Here we further determine whether this disruption persists into adulthood.
The purpose of this study was to determine whether postnatal alcohol exposure (PD4-9) affects hippocampal adult neurogenesis and behavioral performance in hippocampal-dependent CPFE in adult rats. An overview of the study design appears in Figure 1. Experiment 1A examined the effect of PD4-9 alcohol exposure on cell proliferation in adult male rats (∼PD80). We predicted a decrease in cell proliferation as a result of alcohol exposure. Experiment 1B examined the survival of newly generated neurons 35 days after a single injection (200mg/kg) of bromodeoxyuridine (BrdU) in adult male rats (PD115). We predicted alcohol-exposed animals would have decreased levels of surviving cells. Finally, Experiment 2 examined the effect of postnatal alcohol exposure on behavioral performance in hippocampal-dependent CPFE in adult male and female rats (∼PD112). All female rats were in proestrus on the first (preexposure) day of the CPFE protocol. We predicted contextual fear deficits in alcohol-exposed rats of both sexes.
Figure 1.
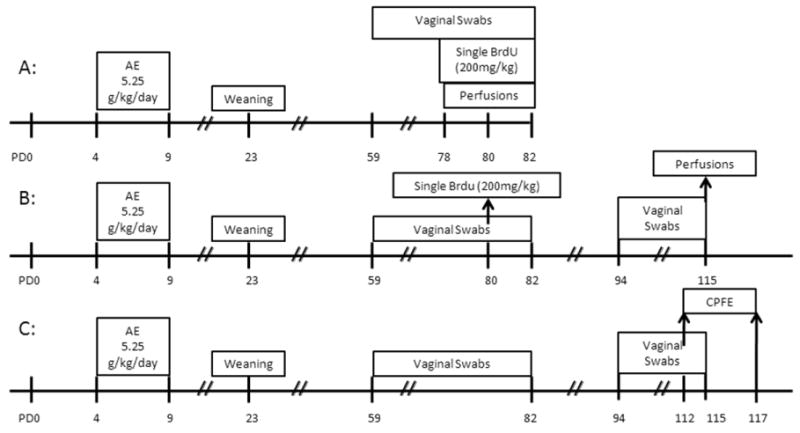
Schematic timeline of experimental procedure. Alcohol exposure was consistent across all Experiments, wherein AE animals received 5.25 g/kg/day on PD4-9. All animals were weaned on PD 23 (2-3 animals per cage). In Experiment 1A, swabs occurred on PD59-PD82. Beginning on PD 78 (through PD82), animals were given a single injection of bromodeoxyuridine (BrdU) and perfused 2-3hrs later. In Experiment 1B, swabbing occurred on PD59-PD80 and PD94-PD115. On PD80, animals received a single BrdU injection and were perfused 35 days later (PD115). In Experiment 2, swabbing occurred on PD59-PD80 and PD94-PD117. Males and proestrus females were tested on the CPFE beginning on PD112.
2. Results
2.1. Experiment 1: Adult Neurogenesis
Experiment 1 asked whether PD4-9 alcohol treatment would impair the proliferation and the survival of newly generated DG neurons in adult male rats, as assessed both two hours (∼PD80) and 35 (PD115) days after a single injection (200mg/kg) of bromodeoxyuridine (BrdU). This study extends previous work (Klintsova et al., 2007) by using a single BrdU injection (rather than multiple injections) to examine the effects of neonatal alcohol exposure on neurogenesis in adult rats.
2.1.1. Body Weights and BACs
Body weights for animals in all experiments and the BAC averages from all alcohol-exposed (AE) animals are summarized in Table 1. All animals continued to gain weight throughout treatments.
Table 1. Body Weights and BACs.
| Body Weights | BACs | ||||
|---|---|---|---|---|---|
| Experiment 1A | |||||
| PD4 (g) | PD9 (g) | PD78-PD82 (g) | |||
| Group | Male (g) | Male (g) | Male (g) | (mg/dl) | |
| SC | 11.5 ± 0.2 | 21.0 ± 0.4 | 427.4 ± 7.7 | N/A | |
| SI | 11.4 ± 0.3 | 20.6 ± 0.7 | 417.0 ± 11.3 | N/A | |
| AE | 11.9 ± 0.3 | 18.3 ± 0.8bc | 431.2 ± 13 | 373.73 ± 12.05 | |
| Experiment 1B | |||||
| PD4 (g) | PD9 (g) | PD 80 (g) | PD 115 (g) | ||
| Group | Male (g) | Male (g) | Male (g) | Male (g) | (mg/dl) |
| SC | 9.9 ± 0.3 | 17.7 ± 0.4 | 391.9 ± 7.5 | 482.7 ± 9.8 | N/A |
| SI | 10.0 ± 0.3 | 17.6 ± 0.6 | 412.5 ± 8.8 | 498.0 ± 10.3 | N/A |
| AE | 10.2 ± 0.5 | 14.8 ± 1.1bc | 398.6 ± 21.1 | 513.7 ± 18 | 360.1 ± 22.2 |
| Experiment 2 | |||||
| PD4 (g) | PD9 (g) | ∼PD112(g) | |||
| Group | Male & Female(g) | Male & Female(g) | Female (g) | Male (g) | (mg/dl) |
| SC | 10.9 ± 0.4 | 19.3 ± 0.9 | 272.1 ± 4.3 | 475.0 ± 13.4 | N/A |
| SI | 10.2 ± 0.3 | 18.4 ± 0.5 | 275.3 ± 7.1 | 490.8 ± 11.5 | N/A |
| AE | 10.5 ± 0.4 | 16.1 ± 0.7bc | 271.5 ± 5.0 | 489.7 ± 21.7 | 396.9 ± 11.5 |
PD: postnatal day, AE: alcohol exposed, SI: sham intubated, SC: suckle control
p<0.05 compared to that of SI group
p<0.05 compared to that of SC group
Body weight and BAC data from Experiment 1A appear in the Top section of Table 1. The data from the neonatal period (PD4 and 9) versus adulthood (PD78-82) were analyzed separately so as not to violate the assumption of ANOVA concerning homogeneity of variance. A 3 (dosing condition) × 2 (day) repeated measures ANOVA on neonatal body weights revealed a main effect of day [F(1, 31)=1202.16, p<.001] and a dosing condition × day interaction [F(2, 31)=16.17, p<.001]. On PD9, AE animals' weights were significantly lower than those of both sham-intubated (SI) (p<.05) and suckle control (SC) (p<.05) animals. A univariate ANOVA on adult weights revealed no difference in weight across postnatal condition (F<1), indicating that the initial reduction of body weight exhibited by AE animals at the end of the dosing period (PD9) was no longer present in adulthood at the time of perfusion. Blood samples collected from 10 alcohol-exposed pups on PD4 (10 males) show an average BAC of 373.73 ± 12.05mg/dl.
Body weight and BAC data from Experiment 1B appear in the middle section of Table 1. A repeated measures 3 (dosing condition) × 2 (age) ANOVA on neonatal body weights, revealed a main effect of days [F(1,34)=603.40, p<.001] and a dosing condition × days interaction [F(2, 34)=12.43, p<.001]. On PD9, AE animals' weights were significantly lower than those of SI (p<.05) and SC (p<.05) animals. Additionally, a repeated measures 3 × 2 ANOVA on adult body weights (PD80 and PD115) revealed a main effect of age [F(1, 26) = 516.54, p<.001], with body weight being higher on PD115 than PD80. No effect of dosing condition was evident in adults (F<1), again suggesting that the initial weight reduction seen in alcohol-exposed pups at the end of dosing (PD9) is no longer evident later in life (PD115). Blood samples collected from 9 alcohol-exposed pups show an average BAC of 360.1 ± 22.2 mg/dl.
2.1.2. BrdU Cell Counts
In order to assess the influence of neonatal alcohol exposure, all rats were given a one-time intraperitoneal injection of BrdU (5-bromo-2-deoxyuridine, 200mg/kg in 0.9% sterile saline solution [20mg/ml]), a synthetic analog of thymidine that can be incorporated into the newly synthesized DNA during the S phase in the cell cycle. In Experiment 1A, injections occurred on PD78-82 and rats were perfused two hours after injection. A univariate ANOVA was performed to examine the role of dosing condition on BrdU+ cell counts in male rats (Figure 2A,B). Postnatal alcohol exposure did not affect BrdU+ cell counts in adult rats (Fs<1). Thus, neonatal alcohol exposure does not influence adult hippocampal cell proliferation in male rats at PD78-82.
Figure 2.
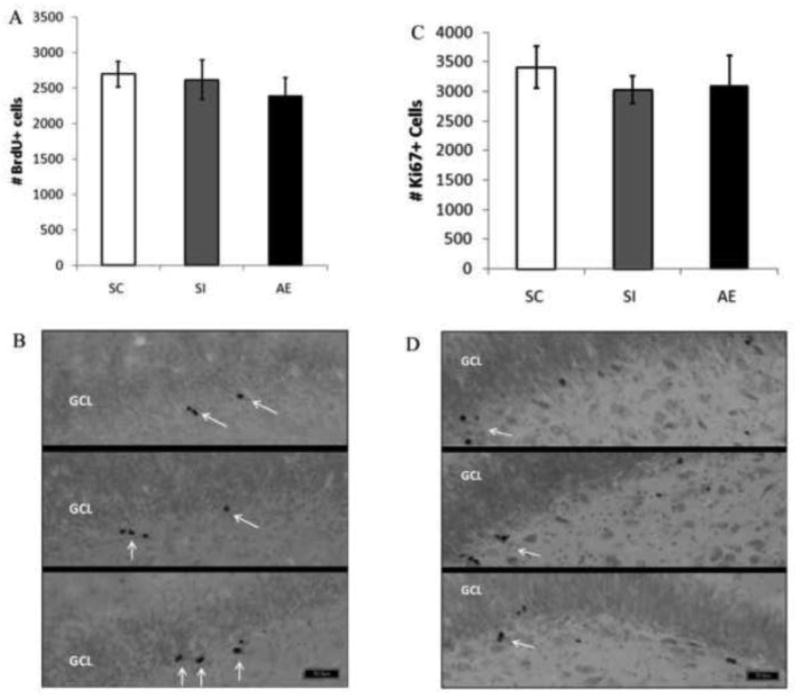
Influence of postnatal alcohol exposure on PD78-82 proliferation. A: Total number of BrdU+ cells in males 2 hours post BrdU injection. No significant difference in number of BrdU+ cells was found across dosing condition in males. (SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM. B: Images of BrdU+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. Top is taken from a SC, middle from a SI, and bottom from an AE animal. Arrows point to BrdU+ cells. C: Total number of Ki67+ cells in PD78-82 males. No significant difference in the number of Ki67+ cells was found across dosing condition in males. (SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM. D: Images of Ki67+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. Top is taken from a SC, middle from a SI, and bottom from an AE animal. Arrows point to BrdU+ cells. (SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM.
Cell survival of newly generate neurons was assessed on PD115, thirty five days after BrdU injections. Cells that successfully complete the S phase are the most likely to complete the cell cycle and go on to differentiate and incorporate into the preexisting network. In Experiment 1B, all animals received BrdU injections on PD80 and were perfused on PD115. A univariate ANOVA was performed to examine the role of dosing condition on BrdU+ cell counts, which is reported as a percentage of control (Figure 3). Results indicate a main effect of dosing condition [F (2, 24) = 3.477, p=.05]. Post-hoc tests (Tukey HSD) revealed that AE animals had significantly fewer surviving cells than SI animals (p < .05). These results illustrate the long lasting impact neonatal alcohol exposure has on cell survival in adult male rats.
Figure 3.
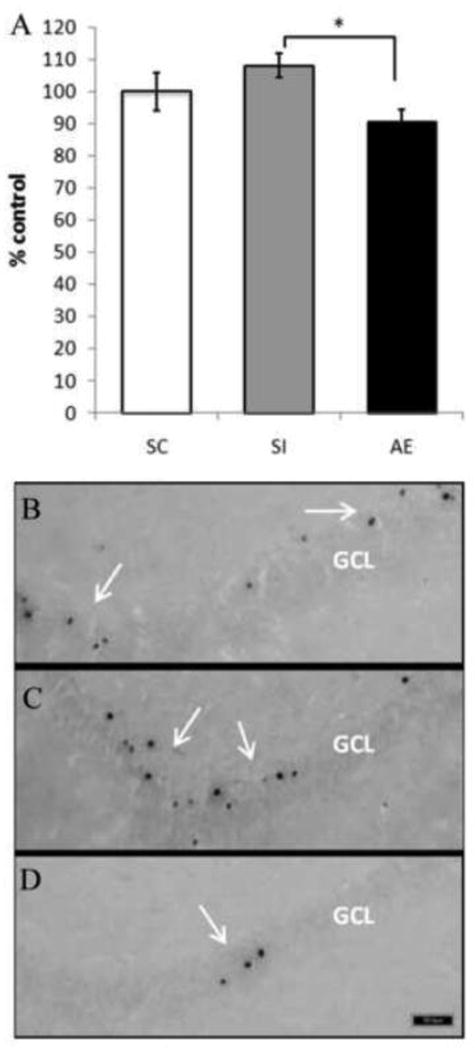
Total number of BrdU+ cells as a percent of control in males 30 days post mitosis. A significant main effect of dosing condition was evident, in which alcohol exposed animals had significantly lower numbers of BrdU+ cells than sham intubated controls thirty days after injection with BrdU. B-D: Images of BrdU+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. B was taken from a SC, C was taken from a SI and D was taken from an AE animal. Arrows point to groups of BrdU+ cells. *indicates p<0.05. (SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM.
2.1.3. Ki67 Cell Counts
In addition to BrdU labeling, we examined the effects of postnatal alcohol exposure on the number of Ki67+ cells in PD78-82 males (Figure 2C,D). Ki67 is an endogenous marker expressed during all active stages of the cell cycle. In Experiment 1A, similar to our BrdU results, dosing condition had no effect on the number of Ki67+ cells in adult males (F<1), with mean (+/- SE) values of 3407.00 (+/-358.01) for the SC group, 3026.55 (+/- 229.47) for the SI group, and 3102.22 (+/- 501.32) for the AE group. Neonatal alcohol exposure did not influence Ki67+ cell proliferation in males on PD78-82.
Furthermore, the long-term effect of dosing condition on levels of cell proliferation in males was evaluated in Experiment 1B on PD115 through the use of the endogenous marker Ki67 (Figure 4). A univariate ANOVA was performed to determine the influence of dosing condition on levels of cell proliferation at the time of perfusion (PD115). Results show that there was no difference among conditions in the number of cells in the active stages of the cell cycle (Ki67+ cells), [F(2, 25) = 0.283, p= .76], with mean (+/- SE) values of 3180.25 (+/- 664.34) for the SC group, 2853.75 (+/- 636.01) for the SI group, and 3028.89 (+/- 492.55) for the AE group. These results indicate that PD4-9 alcohol exposure does not have a long lasting effect on the proliferation of newly generated neurons in male rats.
Figure 4.
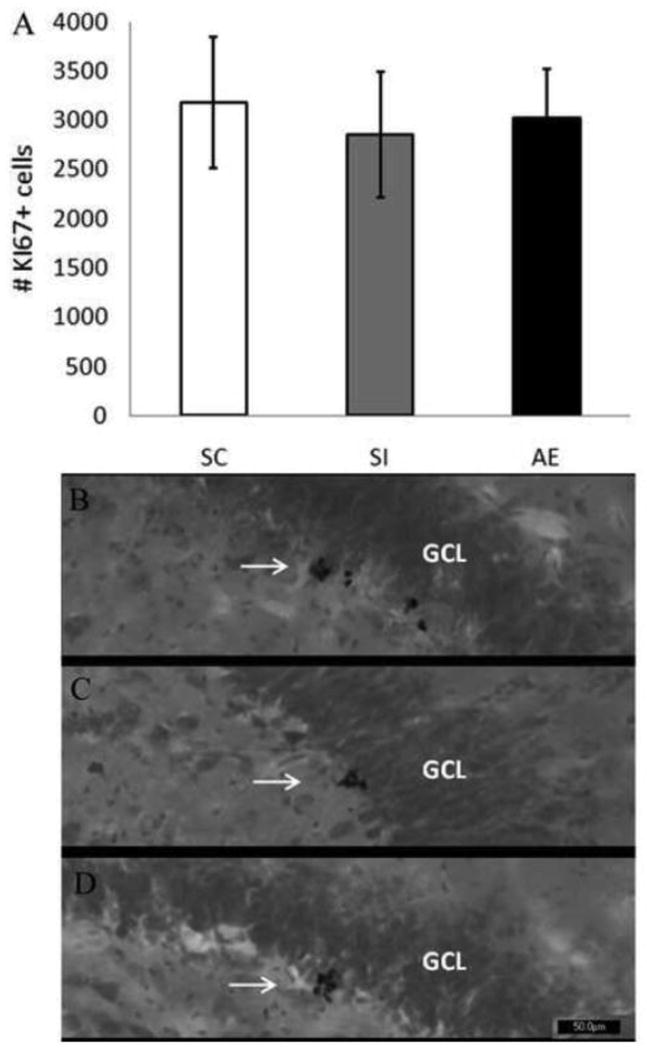
Total number of Ki67+ cells in PD115 males. No significant difference in the number of Ki67+ cells was found across dosing condition in males. B-D: Images of Ki67+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. B was taken from a SC, C was taken from a SI and D was taken from an AE animal. Arrows point to groups of BrdU+ cells. *indicates p<0.05. (SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM.
2.1.4. Doublecortin (DCX) Cell Counts
Finally, sections were stained with the immature neuronal marker DCX in order to determine whether the number of immature neurons was affected by postnatal alcohol exposure in PD78-82 males (Experiment 1A) and PD115 males (Experiment 1B). A univariate ANOVA indicated no significant difference across dosing conditions on the number of DCX+ cells in PD78-82 males [F(2,11) = 1.03, p= .40] with mean (+/- SE) values of 12745 (+/- 1043) for the SC group, 16303 (+/- 1333) for the SI group, and 14632.17 (+/- 2542) for the AE group (Figure 5A,B). Similarly, no significant difference was found across postnatal conditions on the number of DCX+ cells in PD115 males [F(2,14) = .107, p= .90] with mean (+/- SE) values of 18094 (+/- 3511) for the SC group, 19489 (+/- 2560) for the SI group, and 18001 (+/- 786) for the AE group (Figure 5C,D). Together, these data suggest that postnatal alcohol exposure does not affect the generation of immature neurons.
Figure 5.
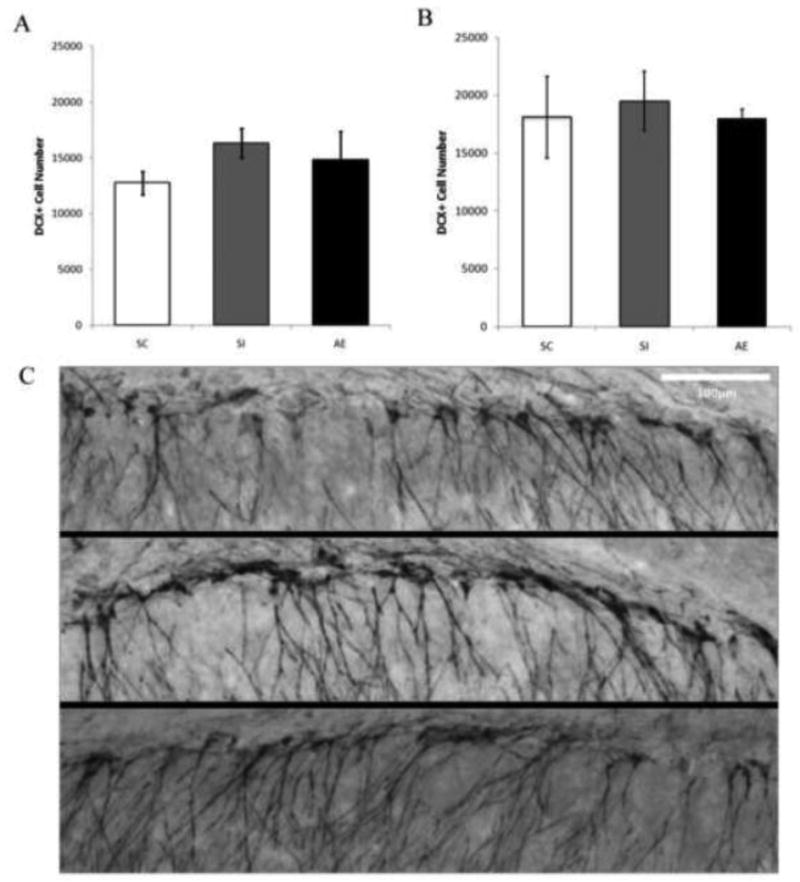
Total number of DCX+ cells in both PD78-82 males and PD115 males. A: No significant difference in the number of DCX+ cells was found across dosing condition on PD78-82. B: No significant difference in the number of DCX+ cells was found across dosing condition on PD115. C: Image of DCX+ cells recognized with diaminobenzidine and counterstained with Pyronin Y. Images taken at 10×, scalebar 100μm. Top image is from a SC, middle from a SI animal and bottom image from an AE animal.
(SC, suckle control; SI, sham intubated; AE, alcohol exposed). Values indicate means ± SEM.
2.2. Experiment 2: Contextual Fear Conditioning
Experiment 2 examined the effect of postnatal alcohol exposure on performance of the CPFE, a hippocampal-dependent contextual fear conditionings task in adult male and female rats (∼PD112). The CPFE is much more sensitive to hippocampal injury (Rudy, 2009) and to neonatal alcohol (Murawski & Stanton, 2010) than conventional context conditioning.
2.2.1 Body weights and BACs
Body weight and BAC data for Experiment 2 appear in the bottom section of Table 1. A repeated measures 3 × 2 × 2 ANOVA on neonatal body weight showed a main effect of dosing condition [F(2, 66)=3.57, p<0.03], a main effect of days [F(1, 66)=1783.26, p<0.01] and a dosing condition × days interaction [F(2, 66)=23.16, p<0.01]. As in Experiment 1a and 1b, no weight differences between dosing conditions were evident at PD4 (ps>0.4); however, AE animals had significantly lower weights than both SC and SI rats on PD 9 (ps< 0.01). On PD112, a main effect of sex [F(1, 66)=484.21, p<0.01] was shown, with female body weights significantly lower than males (272.6 ± 3.3 vs. 488.3 ± 8.7). No weight differences were observed between dosing conditions (F<0.3) and there was no interaction between sex and dosing condition (F<0.2), again demonstrating that the initial weight reduction seen in AE pups on PD9 did not persist into adulthood. Blood samples collected from 23 alcohol-exposed pups on PD4 show an average BAC of 396.9 ± 11.5 mg/dl.
2.2.2. Behavioral measures
In the CPFE, separate groups of rats are preexposed to the test context (Pre) or an alternate context (No Pre) on Day 1, given immediate shock in the test context on Day 2, and then tested for freezing in the test context on Day 3 (see Methods for full details). The CPFE is defined by an increase in freezing time in Group Pre relative to No Pre. The percentage of time spent freezing during the 5 minute context testing phase of the CPFE is shown as a function of dosing condition (SC, SI, and AE) and preexposure group (Pre and No Pre) in Figure 6. The CPFE was present in the SC and SI groups but not the AE group. A 2 (sex) × 3 (dosing condition) × 2 (preexposure) factorial ANOVA revealed a significant main effect of dosing condition [F(2, 60)=6.50, p<0.01], a main effect of preexposure [F(1, 60)=24.6, p<0.01], and a significant dosing condition × preexposure interaction [F(2, 60)=3.23, p<0.05). There was a non-significant trend for lower contextual freezing in females compared to males (p>0.07, data not shown) but sex did not interact with the effects of treatment or preexposure. Post-hoc (Newman-Keuls) analysis of the dosing condition × preexposure interaction indicated that SC and SI rats preexposed to the training context (Group Pre) differed significantly from all rats preexposed to an alternate context (ps<0.01), indicating a clear CPFE in these control groups. AE rats preexposed to the training context showed significantly less freezing (8.71% ± 2.58) than control rats preexposed to the training context (SC=27.11% ± 3.91; SI= 29.53% ± 6.20; ps<0.01) and AE-Pre rats failed to differ from AE rats preexposed to an alternate context (No Pre; 8.71% ± 2.58 vs. 4.35% ± 1.20, respectively). No Pre rats preexposed to the alternate context showed low levels of freezing (<10.2%) across all dosing conditions, which did not differ significantly from one another (ps>0.68). Thus, neonatal alcohol exposure abolished this variant of contextual fear conditioning.
Figure 6.
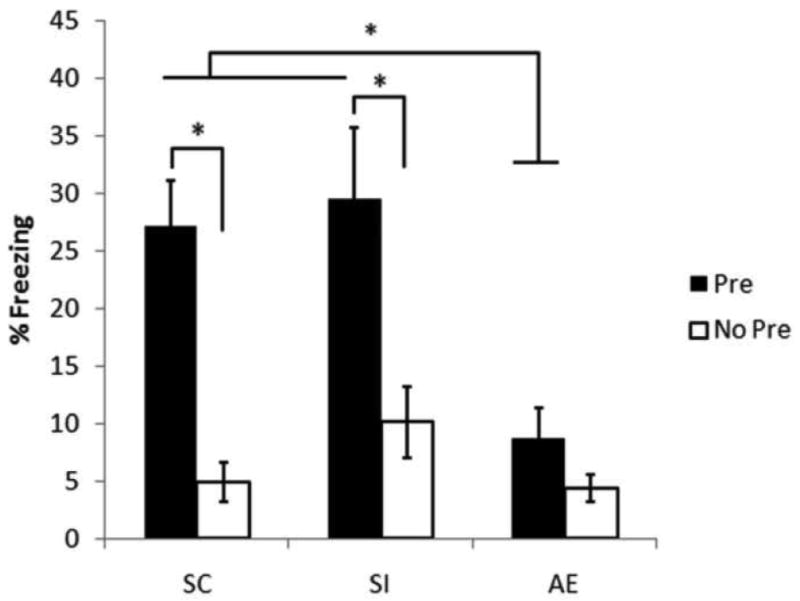
Mean (±SE) percent freezing during a 5 minute testing of contextual fear conditioning in adult male (and female) rats. During the postnatal days (PD)4-9, rat pups were given a 5.25g/kg/day binge dose of alcohol (AE) or sham intubated (SI). Separate litters provided pups social controls (SC). Starting around PD112, pups were either preexposed to the training context (Pre) or to an alternate context (No Pre). 24h following preexposure rats were returned to the training context where they received an immediate footshock. They were tested for contextual conditioning the following day. Both SC and SI rats preexposed to the training context showed contextual conditioning relative to their No Pre counterparts. AE rats failed to condition regardless of preexposure experience.
3. Discussion
The results of Experiment 1 demonstrate that binge-like alcohol exposure during the third trimester equivalent significantly decreases the number of surviving BrdU+ cells (35 days post mitosis) in the dentate gyrus of adult male rats without affecting cell proliferation or generation of immature neurons. Previous work has shown that in the hippocampal dentate gyrus the vast majority of surviving cells are neurons (van Praag et al., 1999a, 1999b; Holmes et al., 2004; Helfer et al., 2009; Clark et al., 2009) and that in the dentate gyrus the number of glial cells comprises a single digit percentage of new cells (van Praag et al., 1999a, 2005; Crews et al., 2006). Additionally, exposure to alcohol during development does not appear to influence the percentage of neurons in a new cell population (Helfer et al., 2009; Choi et al, 2005). Furthermore, we demonstrate that both adult male and female rats exposed to alcohol over the early postnatal period show contextual fear conditioning deficits. During the CPFE, preexposure to the training context facilitates conditioning to an immediate shock relative to being preexposed in an alternate context. Both SI and SC rats demonstrated the CPFE. However, the CPFE was absent in AE rats. These behavioral results did not differ between males and females that were in proestrus when the training protocol began.
3.1. Neonatal alcohol exposure does not affect cell proliferation in the adult DG
Binge-like alcohol exposure on PD4-9 does not affect cell proliferation later in adulthood (PD80, PD115). These results mirror those found previously by our lab, where the same alcohol exposure (PD4-9, 5.25g/kg/day) produced no effect on cell proliferation in adolescent rats (PD50- Klintsova et al., 2007; PD42- Helfer et al., 2009). This finding has also been reported in three month old mice exposed to alcohol throughout gestation (Choi, Allan & Cunningham, 2005). Still, there are a few studies investigating the effects of neonatal alcohol exposure on cell proliferation that offer conflicting results. Redila and colleagues (2006) administered a liquid diet containing ethanol (36.5%) derived calories to dams. Offspring were injected with a single dose of BrdU (200mg/kg) on PD57 and 24 hours later there was evidence of decreased levels of DG cell proliferation in the AE pups compared to non-handled controls. Still, no significant difference between AE and pair-fed animals was evident; suggesting additional factors (i.e. procedural stress, caloric restriction) may affect cell proliferation. Additionally, a single dose of alcohol on PD7 (5g/kg) has been found to decrease dorsal but not ventral cell DG proliferation in 4 month old rats (Ieraci & Herrera, 2007). These contradictory results may reflect methodological differences such as timing of the alcohol administration (i.e. PD4-9 vs. PD7), the interval between injection and perfusion (24 hours in the study by Redila and colleagues (2006), while we used a two hour interval) and the number of BrdU administrations (single dose of 200mg/kg we used vs. the 7 consecutive days of 50mg/kg used by Ieraci and Herrera (2007). In addition, studies using mice have shown genetic influences over components of neurogenesis including cell proliferation, with each of the commonly used strains of mice exhibiting different rates of dentate gyrus cell proliferation (for review see Crews & Nixon, 2003). Differences in rodent strains may also be a reason neonatal alcohol exposure results in decreases in cell proliferation in some but not all studies. In any event, the present findings indicate that neonatal alcohol effects on survival of DG neurons (Experiment 1B) are not secondary to effects on proliferation in adult animals.
3.2. Neonatal alcohol exposure decreases cell survival in the adult DG
Binge-like alcohol exposure on PD4-9 significantly impairs the survival of hippocampal DG cells in adult rats without disrupting the total number of immature DCX+ neurons. Decreases in cell survival have also been found in PD85 rats exposed to alcohol throughout the entire gestational period (Redila et al., 2006) and in 4/5 month old mice after a single alcohol exposure (5g/kg) on PD7 (Ieraci and Herrera, 2007). Additionally, previous work in our lab demonstrated that PD4-9 alcohol exposure decreased cell survival in PD80 rats (Klintsova et al., 2007). The current data is the first to show this effect of neonatal alcohol exposure on adult rats extends to age PD115.
Still, previous work has found conflicting results of the effects of alcohol exposure on cell survival. One study examined survival on PD72 and found no effect of alcohol exposure (5.25 g/kg/day) on cell survival (Helfer et al., 2009). Additionally, a single dose of alcohol (5g/kg) on PD7 had no effect on PD54 cell survival (Wozniak et al., 2004). Further, a recent study by Gil-Mohapel and colleagues (2011) showed that alcohol administration throughout the gestational period (4.3g/kg) and during the first ten postnatal days (4g/kg) alters neither cell proliferation nor cell survival in PD60 and PD90 rats. The PD90 results of this study are in contrast to the aforementioned studies and may result from differences in methodology such as alcohol exposure and also the inclusion of female rats in the Gil-Mohapel study. Sex hormones influence cell proliferation. Normally cycling female rats in proestrus (high estrogen levels) show significantly higher numbers of proliferating cells than females in either estrus or diestrus or gonad-intact male rats (Tanapat et al., 1999). Collectively, these data suggest, as proposed by Ieraci and Herrera (2007) that alcohol exposure during the perinatal period is accelerating the process of aging by decreasing hippocampal neurogenesis and the effects of this accelerated aging are only observable in adult male rodents. This view is supported by our current data, which demonstrates a significant decrease in the number of survival cells in PD115 adult male rats.
Recent work has explored different mechanisms that may play a role in inducing cell survival in the dentate gyrus. Wnt family members, such as wnt3, are expressed in adult hippocampal astrocytes. The signaling of Wnt in the adult central nervous system appears to act as a regulatory pathway in adult neurogenesis that is involved in the control of neuronal fate commitment and the proliferation of neuronally committed precursor cells. Disruption of this signaling decreases adult neurogenesis (Lie et al., 2005; Gage, 2010). Singh and colleagues (2009) examined the role of Wnt signaling pathways in ethanol-induced abnormalities in proliferation and differentiation of adult-brain progenitor cells isolated from adult brains exposed to ethanol during early development. Ethanol exposure was shown to modulate Wnt signaling in progenitor cells and significantly delay neuronal maturation.
In addition, the lack of influence alcohol exposure has on cell proliferation (Experiment 1A) suggests that it may be interfering with the maturation of granule cells. Early in development, new granule cells are functionally ‘silent’ and are physiologically distinct from mature cells (Aimone, Deng & Gage, 2010). The new neurons receive depolarizing GABAergic inputs, which are ultimately critical for the survival of new neurons (Aimone, Deng & Gage, 2010). The action of alcohol as a GABA antagonist could be influencing the survival of these neurons in adult male rats.
3.3 Neonatal alcohol exposure disrupts contextual fear conditioning
Alcohol administered in a binge-like manner over the neonatal period (PD4-9) results in significant cell loss in the hippocampus (Bonthius et al., 2001; Tran and Kelly, 2003; Livy et al., 2003; Marino, Aksenov and Kelly, 2004) and disrupts behaviors negatively affected by hippocampal damage (Goodlett and Johnson, 1997; Hunt et al., 2009; Murawski and Stanton, 2010). In the current study we utilized a variant of contextual fear conditioning that is especially sensitive to anterograde hippocampal insult (Rudy and O'Reilly, 2001; Rudy, Huff and Matus-Amat, 2004) to examine behavioral effects of neonatal alcohol exposure on the CPFE in adult male and female rats. SC and SI control rats showed robust contextual fear conditioning to an immediate shock if they were preexposed to the training context. AE rats, however, failed to condition to an immediate shock regardless of preexposure experience. Rats from each dosing condition that were preexposed to an alternate context failed to condition to the immediate shock, a result known as the immediate shock deficit (Fanselow, 1990). The deficit in the CPFE in AE rats reflects a disruption of hippocampus-dependent context learning and not other processes that contribute to learned performance because alcohol-exposed rats do not differ from controls on discrete-cue, delay fear conditioning that is independent of hippocampal function (Weeber et al., 2001; Allan et al., 2003; Wagner and Hunt, 2006; Hunt, Jacobsen and Torok, 2009; Murawski and Stanton, 2010). Previous studies demonstrate CPFE disruptions in juvenile rats exposed to alcohol over the neonatal period (PD4-9 or PD7-9), deficits that correlate with PD4 BAC levels (Murawski and Stanton, 2010; 2011). The findings of the current study agree with these earlier findings and demonstrate that these deficits persist into adulthood.
Adult male rats develop stronger contextual fear conditioning than females (Maren, De Oca and Fanselow, 1994; Gupta et al., 2001; Kudo et al., 2004; Barker and Galea, 2010). Since estrogens can modulate contextual fear conditioning (Gupta et al., 2001) we sought to control for the stage of estrus and preexposed female rats during the proestrus phase of their cycle (see Methods). When controlling for stage of estrus we did not find any significant differences in contextual fear conditioning between males and females. The comparable levels of context learning exhibited by adult male and female rats may occur because female rats were preexposed during proestrus, a time when 17β-estradiol quickly elevates then decreases to baseline levels over a short time frame (Barha and Galea, 2010). 17β-estradiol influences CA1 pyramidal cells in ways that might influence context conditioning in female rats, such as increasing CA1 spine density and lowering CA1 pyramidal long term potentiation threshold (Rudick and Woolley, 2001; Smith, Vedder and McMahon, 2009). In a CPFE experiment, learning about the context occurs during the preexposure phase, while associating that learned context with the immediate shock occurs during training (Rudy and O'Reilly, 2001). Future work may examine how preexposure during different phases of the estrus cycle influence the CPFE in adult female rats and whether this pattern is disrupted by alcohol in a manner that further implicates neurogenesis in this effect.
The contextual fear conditionin impairments in alcohol-exposed rats shown in Experiment 2 may, in part, be related to the alterations in hippocampal neurogenesis seen in alcohol-exposed rats shown in Experiments 1A and 1B. A number of studies examining the functional significance of adult hippocampal neurogenesis on contextual fear conditioning have produced inconsistent results (Shors et al., 2002; Saxe et al., 2006; Winocur et al., 2006; Hernandez-Rabza et al., 2009). The discrepancies found in these studies may involve the method of arresting neurogenesis, the different tasks or experimental parameters used, or the timing between arresting neurogenesis and testing. Recently the importance of experimental parameters has been highlighted in studies of the functional significance of adult hippocampal neurogenesis. Drew and colleagues (2010) examined the effects of arresting adult neurogenesis using x-irradiation on contextual fear conditioning by manipulating a number of training parameters and found disruptions in context conditioning when a single, but not multiple, shock was used. Interestingly, preexposure to the training context eliminated this effect when a single shock was used (Drew et al., 2010). We have previously reported deficits in the contextual fear conditioning paradigm when 120s of context exposure terminates with a single shock in juvenile rats neonatally exposed to alcohol over PD4-9, an effect that is absent if preexposure to the training context is involved (Murawski and Stanton, 2010). Using the CPFE paradigm to map out the influence of the different stages of conditioning on DG cell proliferation, Pham et al. (2005) report a transient 33% reduction in BrdU+ cells following the training phase of the CPFE, where the previously learned context is associated with immediate shock. No differences in BrdU+ cells were reported for groups following preexposure, following immediate shock without preexposure, or during testing phases (Pham et al., 2005). Although it is beyond the scope of the present report, studies examining the effect of proliferation and survival of adult DG granule cells in alcohol-exposed rats following different stages of the CPFE are warranted. It should also be noted that newly generated granule cells make functional connections with CA3 pyramidal neurons within the first two weeks post mitosis (Hastings et al., 1999; Zhao et al., 2006) and showed enhanced plasticity within 1-1.5 months after birth (Schmidt-Hieber, Jonas and Bischofberger, 2004; see Deng, Aimone and Gage, 2010 for review), properties which would be likely to influence learning and memory of tasks that recruit the hippocampus. Additionally, tasks that recruit the hippocampus increase the survival of newly generated cells (Gould et al., 1999; Dupret et al., 2007). We show that male AE rats have normal proliferation but decreased survival of granule cells. It is not yet clear how the effects of developmental alcohol exposure on adult neurogenesis and contextual fear conditioning are related. It is, of course, possible that alcohol effects on some other aspect of morphology or function in the hippocampus (and/or other brain structures) causes the behavioral deficits reported here (Bellinger et al., 1999; Moore et al., 2004; Whitcher and Klintsova, 2008). Future studies which examine neurogenesis and CPFE performance in a larger number of rats exposed to a broader range of alcohol doses could reveal statistical correlations between disrupted neurogenesis and behavioral impairment. Such a correlation could mean that a decrease in cell survival negatively influences behavior or the lack of learning may prevent cell survival. Employing interventions to improve developmental alcohol-induced memory deficits (e.g., running wheel; enriched environments [Gil-Mohapel et al., 2010]) may provide a means to answer this question.
3.4. Conclusion
Data from the current study support and extend previous work using animal models that show the detrimental effects of neonatal alcohol exposure on the hippocampus (Redila et al., 2006; Klintsova et al., 2007; Ieraci & Herrera, 2007). In addition, our results provide evidence for structural changes that can underlie some of the behavioral deficits observed in individuals with FASD. In humans, FASD is associated with deficits in the formation of the central nervous system, including reduced hippocampal volume (Autti-Ramo, 2002). The current results illustrate both deficits in hippocampal adult neurogenesis and hippocampal dependent contextual fear conditioning as a result of neonatal alcohol exposure in the rat.
4. Experimental procedures
All procedures were done in accordance with the University of Delaware Institutional Animal Care and Use Committee.
4.1. Subjects
For experiment 1A, twelve Long Evans rat litters from timed pregnancies were obtained from Harlan on Gestational Day (GD5). After arrival at the animal facility these litters were housed and treated exactly as described below (Experiments 1B and 2).
For Experiment 1B, a total of 31 timed pregnancy litters from the animal housing colony at the University of Delaware's Office of Laboratory Animal Medicine facility were used. Females were housed overnight with breeder males and were checked for an ejaculatory plug the following morning. If a plug was found, that day was designated as gestational day (GD) 0. Pregnant females were housed in clear polypropylene cages (45×24×21 cm) with standard bedding and ad lib access to water and rat chow and maintained on a 12:12h light cycle. The date of birth was determined by checking for births during the light cycle. If births were detected, that day was designated postnatal day (PD) 0 (all births occurred on GD22). On PD3, litters were culled to eight pups (5 female, 3 males when possible) and pups received subcutaneous injections of a non-toxic black ink into one or more paws to aid in identification. A total of thirty-four male Long-Evan rat pups were used in this study. All pups were weaned on PD 23 and subsequently housed in social conditions of 2-3 rats per cage (45×24×17cm) with ad lib access to water and rat chow. On PD30, animals were housed in larger 45 × 24 × 21cm cages with ad lib access to food and water for the remainder of the study. All subjects were treated in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Delaware based on NIH standards. A total of thirty-seven male Long-Evans rat offspring were used in this study.
Experiment 2 followed the same breeding and housing procedures as Experiment 1B. A total of ninety-two Long Evans rats (46 females and 46 males) were derived from 15 time-bred litters in the UD animal colony.
4.2. Alcohol Exposure
Beginning on PD 4, pups were assigned to one of three conditions: suckle control (SC), sham intubated (SI) or alcohol-exposed (AE). SC rats were derived from separate litters than SI and AE rats, which were littermates randomly assigned (within each sex) to treatment group such that no more than one same-sex littermate was represented in a given postnatal sampling or behavioral condition. SC animals were weighed daily but otherwise left undisturbed. Intragastric intubations occurred from PD4-9 for both the SI and AE groups. SI animals were intubated, on the same schedule as the AE animals, and the tube was removed after approximately ten seconds without the infusion of any solution. AE animals were intubated and given a daily dose of 5.25g/kg of alcohol, (11.9% v/v in milk formula) which was divided into two feedings each day, two hours apart. Preparation of milk formula followed the protocol described previously (Kelly & Lawrence, 2008). A third intubation of milk (no ethanol) was administered two hours after the second alcohol dose. Solely on PD 4, a fourth intubation of milk (no ethanol) was given four hours after the second alcohol dose in order to compensate for reduced milk intake.
4.3. Blood Alcohol Concentrations
Blood samples were collected from a tail clip of each AE and SI pup 90 minutes after the second alcohol intubation on PD 4 in order to determine the blood alcohol concentration (BAC). BACs for the AE animals were assayed from the plasma of each blood sample using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA).
4.4. Swabs
For experiments 1A and 1B, beginning on PD 59 (until PD 83) and on PD 94 (until PD 115) males were removed from the cage and the genital area was swabbed with a saline-moistened Puritan cotton-tipped applicator for approximately 5 seconds. This was done as a control for procedures for smear collection of females in another study not reported here.
For experiment 2, Beginning on PD59 (until PD 80) and then again on PD94 (∼PD 117), vaginal swabs were collected from female rats daily at 0900AM. The tip of a Fisherbrand diagnostic swab (0.6mm) was moistened in 0.9% saline, gently inserted in the vagina and then rolled across a pre labeled microscopy slide. Slides were then immersed in 95% alcohol for 2 seconds and left overnight to dry before being stored. For the males, the animal was removed from the cage and the genital area was swabbed with a saline-moistened Puritan cotton-tipped applicator for approximately 5 seconds.
Vaginal smears were analyzed daily after collection in order to monitor the estrous cycle. Stages were determined based on the predominance of cell types found in the smear. A predominance of nucleated cells designated the rat was in proestrus. A predominance of cornified cells signified the stage of cycle to be estrus, while the presence of leukocytes signaled the rat was in diestrus. Swabs were collected to ensure that rats were cycling regularly and also to predict the stage of the estrous cycle for the next day in order to assure animals were preexposed during proestrus.
4.5. BrdU Injections
In Experiment 1A, rats were weighed and received a single injection of BrdU (200mg/kg in 0.9% sterile saline solution (20mg/ml), i.p.) at least two hours prior to perfusion. There were five days of perfusions (PD78-PD82). Injections typically occurred between 1100-1200 hrs on select days as indicated in Fig 1A.
In Experiment 1B, all animals received a single injection of BrdU (200mg/kg in 0.9% sterile saline solution (20mg/ml), i.p.) on PD80. Injections occurred between 0900-1000 hrs (Fig 1B). Perfusions occurred 35 days after injection (PD115; Figure 1B).
No BrdU injections occurred in Experiment 2.
4.6. Tissue Preparation
All animals assigned to neurogenesis experiments were anesthetized with a mixture of ketamine and xylazine. In Experiment 1A, this occurred two to three hours following injection with BrdU (see timeline in Fig 1A). In Experiment 1B, this occurred 35 days post injection (0900hrs-1200hrs; timeline in Fig 1B). All animals were transcardially perfused with heparinized 0.2M phosphate buffer followed by 4% paraformaldehyde in 0.2M phosphate buffer. Brains were stored in 30% sucrose in 4% paraformaldehyde solution. Serial horizontal sections (40μm) containing the dentate gyrus were obtained on a cryostat and collected in wells containing a cryoprotectant solution (glycerol and ethylene glycol in phosphate buffer solution). Brain tissue was stored at -20°C.
4.7. Immunhistochemistry
Every 16th section (640μm apart) of the entire dentate gyrus was chosen in a systematic random manner (first section chosen randomly from the first sixteenth consecutive sections containing the dentate gyrus) and processed for immunocytochemistry. Immunoreaction with Ki67, DCX and BrdU antibodies was visualized with diaminobenzidine.
For both Ki67 and BrdU visualization, sections were washed in Tris Buffer Solution (TBS) followed by incubation in 0.6% hydrogen peroxide and then washed in TBS. For the BrdU staining only, sections were then incubated in 50% Formamide in 2× concentrate of saline-sodium citrate buffer (SSC) at 65°C for 2hrs followed by a 2× SCC wash after which sections were quickly transferred into 2N hydrochloric acid for 30 mins at 37°C. This was followed by a 10 minute wash in 0.1M Boric acid in TBS wash and then a TBS wash. Next, for both stainings, sections were placed in blocking solution (3% normal goat serum-0.1% Triton X100 in 50mM TBS) for 1 hour. Sections were transferred into primary antibody (1:100 dilution, anti Ki67 (NCL-L-Ki67-MM1) Novacastra, Norwell, MA) (1:500 dilution, anti-BrdU made in rat (OBT0030) Accurate, Westbury, NY) in washing solution (3% normal goat serum in TBS) and left for 48hrs (Ki67) or blocking solution for 72hrs (BrdU) at 4°C. Next, sections were washed in TBS and then washing solution (Ki67 only), after which sections were incubated in secondary antibody (1:1000 dilution, goat anti-mouse biotinylated (BA-9200) Vector, Burlingame, CA) for 1hr or (1:250 dilution, goat anti-rat biotinylated (BA-9400) Vector, Burlingame, CA) for 2hrs at RT. This was followed by two TBS washes and one wash in washing solution (Ki67) or blocking solution (BrdU). Next, sections were incubated in ABC solution (Vector Laboratories, Burlingame, CA) for 1hr. Sections were then rinsed in TBS and then washed in DAB in TBS for at least five minutes (until reaction was visible under microscope). Sections were then rinsed quickly (1-2 sec) in TBS followed by two five minute TBS washes. Sections were then mounted onto slides and left to dry. The following day, slides were counterstained with 0.1% Pyronin Y and coverslipped using DPX mountant.
For DCX visualization, sections were washed with TBS followed by incubation in 0.3% hydrogen peroxide for 1r. Next, sections were washed with TBS and then transferred into primary antibody (1:500 dilution, goat anti-DCX(C-18) Santa Cruz Labs, CA). Twenty-four hours later, sections were rinsed in TBS and then incubated in secondary antibody (1:250 dilution, anti-goat biotinylated (Vector Labs, Burlingame, CA) for 1hr at RT. Next, sections were rinsed in washing solution, blocking solution and then incubated in ABC solution for 1hr. Sections were then rinsed in TBS and stained with DAB and counterstained according to methods described above.
Quantification of BrdU+, Ki67+ and DCX+ cells was performed in accordance with unbiased stereology approach. A series of sections (every 16th section) throughout the entire dentate gyrus was used for each staining batch. All cell counts were made on coded slides by an investigator blind to the treatment conditions. The whole granule cell layer including subgranular zone was outlined on the digitized image on each systematically random chosen section using StereoInvestigator software. Counts were made in an unbiased manner within a known volume of the dentate using the optical fractionator workflow (StereoInvestigator, MicroBrightField Inc., Williston, VT). The grid frame was set to 200 × 200 μm and the counting frame set to 200 × 200 μm. A guard zone of 2 μm and a dissector height of 16 μm were used. The frozen sections were originally cut at a nominal thickness of 40 μm. Immunostaining and mounting result in altered section thickness, which was measured at each counting site. An average section thickness was computed by the software and used to estimate the total volume of the DG sample region and total number of BrdU+ and Ki67+cells.
4.8. Contextual Fear Conditioning
4.8.1. Apparatus and stimuli
The apparatus and stimuli of the current study have been previously described (Burman, et al. 2009; Murawski & Stanton, 2010). Fear conditioning occurred over three consecutive phases spaced 24h apart: preexposure; training; and testing. During preexposure subjects were exposed to one of two contexts (Context A or Context B). Context A was one of four clear Plexiglas chambers (16.5×21.1×21.6 cm) as described by Murawski & Stanton (2010) All behavior in Context A was recorded by a video camera and fed into a Dell computer that ran FreezeFrame software (Actimetrics, Wilmette, IL) for analysis of freezing behavior (Murawski & Stanton, 2010). Context B consisted of separate chambers (22×22×26 cm) as described previously (Murawski & Stanton, 2010). All training and testing occurred in Context A.
4.8.2. Design and procedures
Subjects from each of the three dosing conditions were further assigned to one of two behavioral groups. Group Pre was preexposed to the training context (Context A). Group No Pre was preexposed to the alternate context (Context B). No more than one same-sex litter mate per dosing condition was assigned to any behavioral condition.
Starting on PD107, all subjects were handled for 3 minutes per day. For each subject, handling continued until preexposure (minimum of 5 days). Preexposure to Context A or B started on PD112. Female rats were only preexposed during the proestrus phase of their cycle (as close to PD122 as possible). Swabbing continued each day until a female rat was found to be in proestrus and then it was preexposed to either Context A or B. Male rats were yoked to females so that both sexes were preexposed on similar days. During preexposure, rats were transported two at a time in yellow ice buckets and placed into either Context A or B where they were allowed to freely explore the context for a 5 minute preexposure period, after which they were returned to their home cage. During training, all subjects were briefly placed into Context A where they received an immediate 2s 1.5mA footshock (<5s upon placement) and were then returned to their home cage. Finally, during testing, subjects were again placed into Context A and levels of contextual freezing were monitored over a 5 minute testing period. Context A was wiped down with a 5% ammonium hydroxide solution following removal of subjects during each phase of testing.
4.9. Data analysis
For Experiment 1, the data were imported into PASW Statistics 18 data analysis software. The level of significance was set at p < 0.05 for all tests. Body weight analysis utilized a repeated measure ANOVA with between-subjects factor of dosing condition and the within-subjects factor of age for PD4 and PD9 weights. BrdU analysis was performed through a Univariate ANOVA at both the proliferation and survival time points in order to examine the role of dosing condition on BrdU+ cell counts. Post-hoc tests (Tukey HSD) was used to characterize treatment effects at the survival timepoint. Ki67 analysis also required a Univariate ANOVA at both the proliferation and survival time points.
For Experiment 1A, a total of 2 pups were excluded from analysis as they died due to improper intubations. The total N's for the study were: SC: 12; SI: 12; AE: 10. For experiment 1B, a total of 4 pups were excluded from analysis as they died due to improper intubations. The total N's for the study were: SC: 8; SI: 9; AE: 9.
For Experiment 2, the data were analyzed using FreezeFrame software (Actimetrics, Wilmette IL). The bout length was set at 0.75 s and the freezing threshold (change in pixels/frame) was initially set as described in the instructions. A human observer blind to the animal groups verified the setting by watching the session and adjusting the threshold if necessary to ensure that small movements were not recorded as freezing. Freezing behavior was scored as a percentage of time spent freezing during the 5min testing session.
The data were imported into Statistica 8 data analysis software. Body weight analysis utilized a repeated measure ANOVA with between-subjects factors of sex and dosing condition and the within-subjects factor of age for PD4 and PD9 weights. Adult (preexposure) weights were analyzed with a factorial ANOVA with Sex and Dosing Condition as between subjects' factors. Freezing behavior was analyzed with a 2 (sex) ×3 (dosing condition) ×2 (preexposure group) factorial ANOVA. No significant main effects or interactions concerning the factor of sex appeared (p>0.07) and so the remaining statistics utilized a 3×2 (dosing condition×preexposure) factorial design. Post-hoc analyzes (Newman–Keuls) were used to characterize treatment and interaction effects, when statistically significant (alpha set at p≤0.05, two-tailed).
A total of 18 animals were excluded from analysis. Six rats died due to improper intubations. Four animals were excluded due to equipment failure/experimenter error. Outliers were defined a priori as individual freezing scores that differed by ± 2 standard deviations from the mean of the other rats in their respective group. Eight animals meet the criteria for outliers and were thus excluded from further analysis. Outliers included 2 animals each from Groups SC-Pre and SI-No-Pre, and one animal from each of the following Groups: SC-No-Pre, SI-Pre, AE-Pre, and AE-No-Pre. Statistics were run on the remaining 74 rats.
Highlights.
Neonatal alcohol exposure does not affect hippocampal adult cell proliferation
Neonatal alcohol exposure decreases hippocampal adult cell survival
Neonatal alcohol exposure alters hippocampus-dependent context fear conditioning
Acknowledgments
This work was supported by the University of Delaware Research Foundation (UDRF) award to AYK and MES and in part by NIH grant number AA09838. Authors are grateful to Jennifer Helfer and Jon Marc Finamore for their help with initial planning the study (JLH), animal generation (JLH, JMF) and immunocytochemistry (JMF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Fetal alcohol syndrome: The American paradox. Alcohol Alcohol. 1998;33:195–201. doi: 10.1093/oxfordjournals.alcalc.a008382. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;7:325–37. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcoholism-Clinical and Experimental Research. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54. [PubMed] [Google Scholar]
- Autti-Rämö I. Foetal alcohol syndrome--a multifaceted condition. Dev Med Child Neurol. 2002;44(2):141–4. doi: 10.1017/s0012162201001839. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800:1056–67. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Males show stronger contextual fear conditioning than females after context pre-exposure. Physiology & Behavior. 2010;99:82–90. doi: 10.1016/j.physbeh.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bedi KS, Wilson P, Wilce PA. Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse. 1999;31(1):51–58. doi: 10.1002/(SICI)1098-2396(199901)31:1<51::AID-SYN7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Woodhouse J, Bonthius NE, Taggard DA, Lothman EW. Reduced seizure threshold and hippocampal cell loss in rats exposed to alcohol during the brain growth spurt. Alcohol Clin Exp Res. 2001;25:70–82.1. [PubMed] [Google Scholar]
- Chen WJ, Maier SE, Parnell SE, West JR. Alcohol and the developing brain: neuroanatomical studies. Alcohol Res Health. 2003;27(2):174–80. [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29(11):2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health. 2003;27(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–54. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. Plos Biology. 2007;5:1683–1694. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Gage FH. Molecular and cellular mechanisms contributing to the regulation, proliferation and differentiation of neural stem cells in the adult dentate gyrus. Keio J Med. 2010;59(3):79–83. doi: 10.2302/kjm.59.79. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Patten A, Cox A, Kainer L, Giles E, Brocardo PS, Christie BR. Altered adult hippocampal neuronal maturation in a rat model of fetal alcohol syndrome. Brain Res. 2011;1384:29–41. doi: 10.1016/j.brainres.2011.01.116. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64(3):265–75. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- González-Burgos I, Alejandre-Gómez M, Olvera-Cortés ME, Pérez-Vega MI, Evans S, Feria-Velasco A. Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal CA1 field of juvenile rats: a stereology and Golgi study. Neurosci Res. 2006;56(4):400–8. doi: 10.1016/j.neures.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17(7):2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Seth MI, Tanapat P, Rydel TA, Gould E. Granule neurons generated during development extend divergent axon collaterals to hippocampal area CA3. Journal of Comparative Neurology. 2002;452:324–333. doi: 10.1002/cne.10386. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Research. 2009;1294:1–11. doi: 10.1016/j.brainres.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Rabaza V, Llorens-Martín M, Velázquez-Sánchez C, Ferragud A, Arcusa A, Gumus HG, Gómez-Pinedo U, Pérez-Villalba A, Roselló J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76(2):216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol. 2009;43:465–74. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26(3):597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods Mol Biol. 2008;447:101–10. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94(19):10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Kudo K, Qiao CX, Kanba S, Arita J. A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain Res. 2004;1024:233–43. doi: 10.1016/j.brainres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–7. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22:363–77. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(6):1088–93. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Spear LP. The alcoholism generator. Alcohol Clin Exp Res. 2006;30(9):1466–9. doi: 10.1111/j.1530-0277.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Moore DB, Madorsky I, Paiva M, Heaton MB. Ethanol exposure alters neurotrophin receptor expression in the rat central nervous system: Effects of neonatal exposure. J Neurobiol. 2004;60(1):114–26. doi: 10.1002/neu.20010. [DOI] [PubMed] [Google Scholar]
- Morleo M, Woolfall K, Dedman D, Mukherjee R, Bellis MA, Cook PA. Under-reporting of foetal alcohol spectrum disorders: an analysis of hospital episode statistics. BMC Pediatr. 2011:11–14. doi: 10.1186/1471-2431-11-14. 2011 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4-9. Behav Brain Res. 2010;212:133–42. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35(6):1–11. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13(3):512–20. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pham K, McEwen BS, LeDoux JE, Nader K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience. 2005;130:17–24. doi: 10.1016/j.neuroscience.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19(5):1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Gupta S, Jiang Y, Younus M, Ramzan M. In vitro neurogenesis from neural progenitor cells isolated from the hippocampus region of the brain of adult rats exposed to ethanol during early development through their alcohol-drinking mothers. Alcohol Alcohol. 2009;44(2):185–98. doi: 10.1093/alcalc/agn109. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34S:S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214(4523):936–8. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230(6):366–75. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–28. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running enhances cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature. 1999b;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behavioral Neuroscience. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Savage DD, Sutherland RJ, Caldwell KK. Fear conditioning-induced alterations of phospholipase C-beta1a protein level and enzyme activity in rat hippocampal formation and medial frontal cortex. Neurobiol Learn Mem. 2001;76:151–82. doi: 10.1006/nlme.2000.3994. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62(8):566–73. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17(3):403–14. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


