Abstract
CD8+ T cells become exhausted, inducing cell surface protein programmed cell death-1 (PD-1) as chronic virus diseases or tumors progress, but underlying mechanisms of this are unclear. We previously showed that M-CSF is important for developing tolerogenic dendritic cells (DCs) from human CD14+ monocytes. Here, we identify M-DC after stimulation with IL-10 as myeloid derived suppressor cells with additional tolerogenic activities to CD8+ T cells. IL-10 increased PD-L1 expression on M-DC, and M-DC/IL-10 cells induced expression of PD-1 on, and apoptosis of, CD8+ T cells, and phagocytosed CD8+ T cells. Enhanced phagocytic activity of M-DC/IL-10 required IFN-γ, which further increased PD-L1 and PD-L2 expression on M-DC/IL-10. M-DC/IL-10/IFN-γ cells were phenotypically macrophage-like cells with little or no expression of CD86, a costimulatory molecule, but with high expression levels of CD14, CD200R and CD80. No phagocytic activity was detected with GM-CSF-derived DC. We propose that phagocytosis by M-DC/IL-10/IFN-γ cells, which may be DCs or alternatively a unique subset of macrophages, may be a mechanism by which IFN-γ producing CD8+ T cells are tolerized following type 1 immune responses to chronic virus or tumor, and that IFN-γ links effector CD8+ T cells to their phagocytic clearance.
Introduction
Following some viral infections, virus specific CD8+ T cells often fail to differentiate into memory CD8+ T cells and rapidly lose their ability to lyse virally infected cells (1–2). Loss of T-cell responses to terminate infection is termed ‘CD8+ T cell exhaustion’(3). Programmed death-1 (PD-1) is implicated as a major cell-surface inhibitory receptor capable of regulating virus-specific CD8+ and CD4+ T cell exhaustion in mice, and in primates and humans during chronic virus infection (4–7). Blockade of the PD-1 signaling pathway in chronically infected mice rescues function of exhausted T cells (8–9). PD-1 is also induced on tumor-infiltrating T cells, and blockade of PD-1 increases tumor-specific T-cell proliferation and function, suggesting that PD-1 signaling may result in human tumor-specific T cell exhaustion (9–10).
PD-1 ligand (PD-L1, B7-H1, CD274), a cell surface glycoprotein, belongs to the B7 family of co-stimulatory molecules and is expressed on activated dendritic cells (DCs), macrophages, T cells, B cells, and monocytes (4, 10–12), as well as on human carcinomas of lung, ovary and colon, and in melanomas (13). PD-L1 is upregulated on myeloid DCs during virus infection (14), and contributes to poor control of chronic infections in mice (8) and humans, including HIV-1 (6, 15). PD-1/PD-L interactions regulate peripheral self-reactive CD8+ T cell tolerance upon encounter with DCs bearing self-antigen (16–17). PD-L1 promotes differentiation and maintains function of induced regulatory T cells (Tregs) by enhancing Foxp3 expression in Tregs (18). PD-L1 expression levels on myeloid DCs correlate with poorer cancer prognosis (19–20). Blockade of PD-1/PD-L1 interaction increases infiltration of CD8+ T cells to tumors (9), suggesting that PD-L1 induction is associated with tumor-specific T cell exhaustion (21).
Myeloid-derived suppressor cells (MDSC), described as CD11b+GR-1+ cells in mice suppress T cells in various cancer models (22–25). MDSCs recruited by tumors contribute to tolerance of anti-tumor CD8+ T cell responses to evade anti-tumor immunity (22–25). M-CSF is an important cytokine that promotes differentiation from DCs towards macrophages and contributes to differentiation of MDSCs (21, 26–27). MDSCs are abundant in local tumor environments, especially those enriched with M-CSF, which affect the suppressive capacity of MDSCs to tumor antigen-specific T cell immunity and possibly trigger PD-L1 expression (9, 21). However, whether or not PD-L1 plays a role in MDSC-mediated T cell suppression remains controversial (9, 28–29). PD-L1 is known to be expressed on Gr-1+CD11b+ MDSCs obtained from mice bearing tumor (9, 30), but in some reports, PD-L1 expression was not found on MDSC (31). This may be due to differences in tumor-derived factors, which may regulate expression of PD-L1 on MDSC. In fact, MDSCs are composed of a heterogeneous population of myeloid cells, including monocytes/macrophages, and DCs at different stages of differentiation (22).
IL-10 is a potent immunosuppressive cytokine that inhibits the ability of DCs to mature into functional APCs that have low level secretion of pro-inflammatory cytokines and expression of co-stimulatory receptors (25, 32). IL-10 is often increased in persistent infections in mice and humans (15, 33), and is implicated in impaired T cell response to chronic viral infections (32–34). Consistent with this, IL-10 receptor blockade increases proliferative capabilities of HIV- and HCV-specific T cells (35–36). IL-10 up-regulates PD-L1 expression on peripheral blood CD14+ monocytes (15, 37), and PD-L1 up-regulates IL-10 production (32, 38), as part of an immunosuppressive circuit. IL-10 and PD-L1 may cooperate to promote exhaustion of CD8+ T cells probably in synergy during persistent viral infections (15, 32, 39). Thus, IL-10 may be involved in switching functional properties of immunogenic DCs to tolerogenic DCs through mediation of PD-L1 signaling.
Although PD-1/PD-L1 signaling, IL-10 and MDSCs are important central immune suppressive mediators, the mechanisms by which the suppressive signaling pathways merge to execute exhaustion processes, particularly in CD8+ T cells, remain elusive. We previously demonstrated that M-CSF-derived DCs (M-DC) were tolerogenic to CD4+ T cells (40). Unlike GM-CSF derived DCs, M-DCs produce high levels of IL-10, but not IL-12. IL-10 producing macrophages preferentially take up apoptotic cells (41). This prompted us to postulate that M-CSF and IL-10 may instruct human CD14+ monocytes to an immune tolerance program by inducing PD-L1 for CD8+ T cell immune responses. We identify a novel subset of M-DCs with phagocytic activity, particularly in the presence of IFN-γ, an proinflammatory cytokine. Phagocytosis is dependent on PD-L1. Our results demonstrate that phagocytosis by tolerogenic DCs, mediated by PD-1/PD-L1 interaction, adds another dimension to CD8+ T cell tolerance.
Materials and Methods
Reagents
Recombinant human IL-15, IL-4 and M-CSF were purchased from PeproTech (Rocky Hill, NJ). Recombinant GM-CSF was obtained from BioVision (Exton, PA). LPS from Salmonella minnesota was purchased from Sigma-Aldrich. PD-1-PE (clones J105 and MIH4), anti-PD-L1-PE, anti-PD-L1-PE-Cy7 (clone MIH1), anti-PD-L2-PE (clone MIH18), anti-CD80 (clone 2D10.4), purified neutralizing anti-PD-L1 (clone MIH1) and corresponding isotype controls were purchased from eBioscience (San Diego, CA). Anti-PD-1-APC (clone MIH4), anti-CD14-FITC, anti-CD86- PE-Cy5 and anti-CD11c–APC were purchased from BD Pharmingen Flanklin Lakes, NJ). Anti-CD8-PE-Cy5.5 and ProLong Gold antifade reagent with DAPI was purchased from Invitrogen (Carlsbad, CA).
Purification of human CD14+ monocytes, CD8+ and CD4+ T cells
Human blood was obtained from the Indiana Blood Center (Indianapolis, IN). PBMC were isolated from the blood by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). CD14+ monocytes were isolated from PBMC by positive selection with magnetic beads (CD14 Mcrobeads, Miltenyi Biotec Inc. Auburn, CA) according to the manufacturer's manual. CD8+ T cells were purified from PBMC depleted of CD14+ cells by negative selection with magnetic beads (CD8+ T cell Isolation Kit II; Miltenyi Biotec) containing antibodies to CD4, CD14, CD16, CD19, CD36, CD56, CD123, TCRγδ, and Glycophorin A. CD4+ T cells were isolated from PBMC using MACS CD4 magnetic beads (Miltenyi Biotec) as described before (40). Isolated CD8+ T cells and CD4+ T cells were each >95% pure as analyzed by flow cytometry.
In vitro generation of DCs
CD14+ monocytes (5 × 105 cells/ml) were cultured in RPMI-1640 medium (BioWhittaker) supplemented with 10% heat-inactivated FBS (HyClone Laboratories), 2mM L-glutamine, 55 µM 2-ME, 100 U/ml penicillin, and 100 µg/ml streptomycin and 20 ng/ml IL-4 for 5 days in the presence of either recombinant human M-CSF (20 ng/ml) or 400 U/ml recombinant human GM-CSF to respectively generate M-DCs and GM-CSF-derived DCs (GM-DCs). 20 ng/ml IL-10 was included in M-DC and GM-DC generation cultures to produce M-DC/IL-10 and GM-DC/IL-10, respectively. In some cases, 50 ng/ml TNF-α (PeproTech, Rocky Hill, NJ) was added to the M-DC/IL-10 generation cultures. Half of culture medium was changed every 3 days, unless otherwise indicated. M-DC/IL-10 and GM-DC/IL-10 were further cultured for an additional 4 days with or without IFN-γ at 50 ng/ml to generate M-DC/IL-10/IFN-γ and GM-DC/IL-10/IFN-γ, respectively.
Coculture of DCs and CD8+ T cells
DCs harvested on day 5 were placed in 96-well flat bottom plates (Costar, Corning, NY) (1 × 104 cells in 100 ml medium). Prior to coculture with DCs, CD8+ or CD4+ T cells (1 × 106 cells/ml) were incubated for 5 days in complete RPMI-1640 medium supplemented with 10 ng/ml IL-15. IL-2 (100 units/ml) was added to CD4+ T cell cultures in addition to IL-15 in order to maintain cell viability. In some cases, CD8+ T cells were cultured in IL-15 (20ng/ml) for 5 days with or without dexamethasone (10−7 M) or anti-CD3 coated to culture plates at 0.1 µg/ml. DCs were mixed with allogeneic CD8+ T cells preincubated for 5 days in IL-15 and cultured for additional 4 days in 20 ng/ml IL-15, 20 ng/ml M-CSF and 20 ng/ml IL-10 in complete RPMI in the absence or presence of 50 ng/ml IFN-γ. CD8+ T cells were stained with CFSE (10µM) in PBS before being mixed with DCs. Cells in cocultures were examined under the microscope or harvested for flow cytometric analysis for expression of surface markers. In some cases, cell cultures were carried out on glass slides for observation under confocal microscopy.
Flow cytometric analysis
Harvested cells were washed with PBS supplemented with 1% BSA. Fc receptors on cells were preblocked with excess human IgG (Sigma-Aldrich) on ice for 15 minutes. Cells were stained for 30 minutes at 4°C with the following FITC-conjugated Abs: anti-CD14 (BD Pharmingen, CA), PE-conjugated Abs: PD-1 (J105 and MIH4, eBioscience), PD-L1 (MIH1 eBioscience), PE-Cy7-conjugated Ab: PD-L1 (MIH1, eBioscience), PE-Cy5-conjugated CD86 (BD Pharmingen, CA), PE-Cy5.5-conjugated Ab; CD8 (Invitrogen, CA), and APC-conjugated Abs: CD11c (BD Pharmingen, CA), PD-1(MIH4, BD Pharmingen, CA) or isotype controls (eBioscience, San Diego, CA). CD8+ T cells and DCs in coculture were analyzed using gating process for CD8+ and CD11c+ cells, respectively. CFSE stained CD8+ T cells and fluorescence in DCs were analyzed at channel 1 (FL1). Apoptosis of CD8+ T cells following culture with or without M-DC was measured by staining with Annexin V-APC. Cells were acquired with FACSCalibur (BD Sciences, CA), and data processed with FCS Version 3 software (De Novo Software, Los Angeles, CA).
Confocal microscopy
DCs cocultured with T cells for 2 to 4 days were allowed to adhere to glass slides (Lab-Tek Chamber Slide, Nunc, Rochester, NY), washed with PBS to remove loosely bound T cells and then fixed in 4% paraformaldehyde in PBS for 10 minutes at room temperature. Cells were then stained with DAPI and mounted with ProLong Gold antifade reagent (Invitrogen, Eugene, OR). Fluorescence analysis of cells was performed using the Zeiss LSM 510 confocal laser scanning system (Carl Zeiss, Heidelberg, Germany) at 100x magnification.
Quantification of CD8+ T cells phagocytosed by M-DC/IL-10/INF-γ cells
Numbers of CD8+ T cells phagocytosed by M-DC/IL-10 in the presence of INF-γ were obtained with an assumption that CD8+ T cell volume was about 1/27 of M-DC cell based on our microscopic observation of the diameter of CD8+ T cell being approximately 1/3 that of M-DC/IL-10. CFSE stained CD8+ T cells phagocytosed may contribute to an increase of green fluorescence in M-DC/IL-10 cells due to the CFSE incorporated and thus levels of green fluorescence increased by M-DC/IL-10 may be proportional to the numbers of CFSE+CD8+ T cells phagocytosed. Using this correlation, numbers of CD8+ T cells phagocytosed by M-DC/IL-10 were estimated by dividing the MFI gained by M-DC/IL-10 cells with 1/27 of the MFI detected in CD8+ T cells.
Statistical analysis
A 2-tailed paired Student t test (unless otherwise indicated) was used to determine statistical significance. Values of p less than 0.05 were considered significant.
Results
DCs derived by a combination of M-CSF and IL-10 increase PD-1 expression on CD8+ T cells upon coculture
We previously demonstrated that M-DCs showed tolerogenic potential to allogeneic CD4+ T cell proliferation. M-DCs were characterized by their ability to secrete IL-10 at high levels but not IL-12, upon LPS stimulation (40). However, whether M-DCs act as suppressor cells to CD8+ T cell functions has not been examined. PD-1 is involved in suppressing CD8+ T cell response to chronic infection and tumor (5, 42). We were interested in determining whether M-DCs affect PD-1 expression on CD8+ T cells, because no specific type of antigen presenting cells or DC subsets have been identified as being responsible for inducing PD-1 expression on CD8+ T cells. Although IL-10 is induced during chronic infection and has been reported to be a potential factor for PD-1-mediated viral persistence, there is no clear understanding of the specific cells responsible for inducing PD-1 on CD8+ T cells.
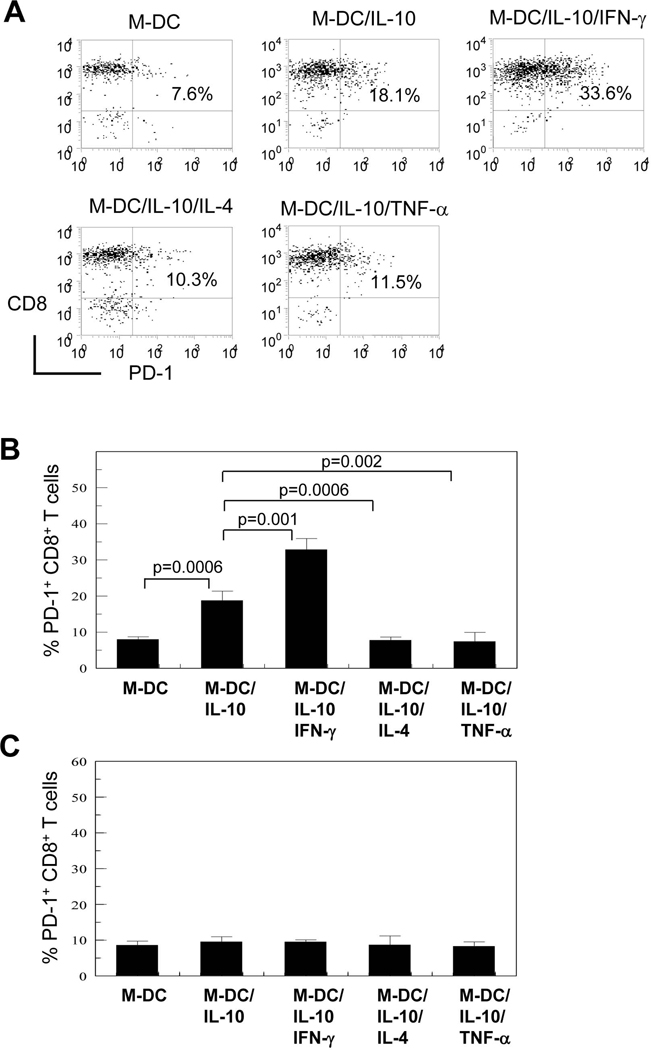
To determine potential effects of M-DCs and IL-10 treated M-DCs on PD-1 induction on CD8+ T cells, M-DCs were generated in vitro by culturing human peripheral blood CD14+ monocytes with M-CSF and IL-4 in the absence and presence of IL-10 for 5 days. These cells, respectively referred to as M-DC and M-DC/IL-10, were cocultured with allogeneic CD8+ T cells. Prior to coculture, allogeneic CD8+ T cells had been incubated for 5 days in IL-15 to simulate exhaustion processes of effector or memory CD8+ T cells. Following coculture for 4 days, PD-1 expression levels on CD8+ T cells were measured. Typically, less than 10% of CD8+ T cells expressed PD-1 after a 5-day culture in IL-15. PD-1was expressed on CD4+ T cells after a 5-day culture in IL-15 and IL-2 but their expression levels were lower compared to those on CD8+ T cells (< 5%). M-DC/IL-10 increased PD-1 levels on CD8+ T cells to greater than 20% as a result of coculture (Fig. 1A; 1B). This was in contrast to M-DC which had little effect on PD-1 expression on CD8+ T cells. GM-DC and GM-DC/IL-10 cells generated in the same way as M-DC or M-DC/IL-10, but with GM-CSF instead of M-CSF, did not enhance PD-1 expression on CD8+ T cells (data not shown). We then determined whether the presence of IFN-γ and IL-4, type 1 and type 2 cytokines respectively, and TNF-α, a proinflammatory cytokine, influence M-DC/IL-10 effects on PD-1 expression of cocultured CD8+ T cells. IFN-γ enhanced M-DC/IL-10 effects on PD-1 expressing CD8+ T cells (Fig. 1A; 1B). Of note, presence of IL-4 or TNF-α during M-DC/IL-10 generation abrogated the enhancing effect of M-DC/IL-10 on % PD-1 expression. Our results suggest that M-DC/IL-10 may be an important mediator cell population limiting IFN-γ producing CD8+ T cell responses. We next assessed if increased % PD-1 expression on CD8+ T cells by coculture with M-DC/IL-10 and M-DC/IL-10/IFN-γ was due to soluble factors secreted from these DCs. This was done by culturing CD8+ T cells with conditioned media collected from DC cultures at the 5th day of culture. Conditioned media from neither M-DC/IL-10 nor M/DC/IL-10/IFN-γ increased % PD-1 expressing CD8+ T cells (Fig. 1C). M-CSF and IL-10, in the absence of DCs, did not affect % PD-1 expressing CD8+ T cells (data not shown). This suggests that contact with M-DC/IL-10 was responsible for induction of PD-1 expression on IFN-γ producing CD8+ T cells. This indicates that IL-10 is important for inducing PD-1 on CD8+ T cells, but the effect is mediated through M-DC/IL-10.
Figure 1.
M-DC/IL-10 possess a unique ability to induce PD-1 expression on CD8+ T cells and IFN-γ enhances effect of M-DC/IL-10. (A) CD8+ T cells were cultured for 5 days in presence of IL-15 and co-cultured in IL-15 for 4 days with DCs generated from peripheral CD14+ monocytes with M-CSF in the absence (M-DCs), in the presence of IL-10 (M-DC/IL-10) plus IFN-γ (M-DC/IL-10/IFN-γ), IL-4 (M-DC/IL-10/IL-4) and TNF-α (M-DC/IL-10/TNF-α) at a ratio of 1 to 1. Cells were stained for CD11c, CD8 and PD-1. Cells negative for CD11c were analyzed for PD-1 expression in CD8+ T cells. Percent of PD-1 expressing CD8+ T cells are displayed in the second quadrant of dot plots. Dot plots are representative of results from four independent experiments. (B) Bar histograms are expressed as mean % PD-1+CD8+ T cells +/− STD from four independent experiments. (C) CD8+ T cells were cultured for 4 days in the presence of IL-15 with conditioned media collected from corresponding DCs instead of by coculture with DCs. Percent PD-1 expressing cells in a gated CD8+ T cell population were measured by flow cytometry and are displayed with standard deviations (STD). Histograms are representative of averages of three independent experiments with each experiment carried out in triplicate.
IFN-γ induces PD-L1 expression at high level on M-DC only after IL-10 stimulation
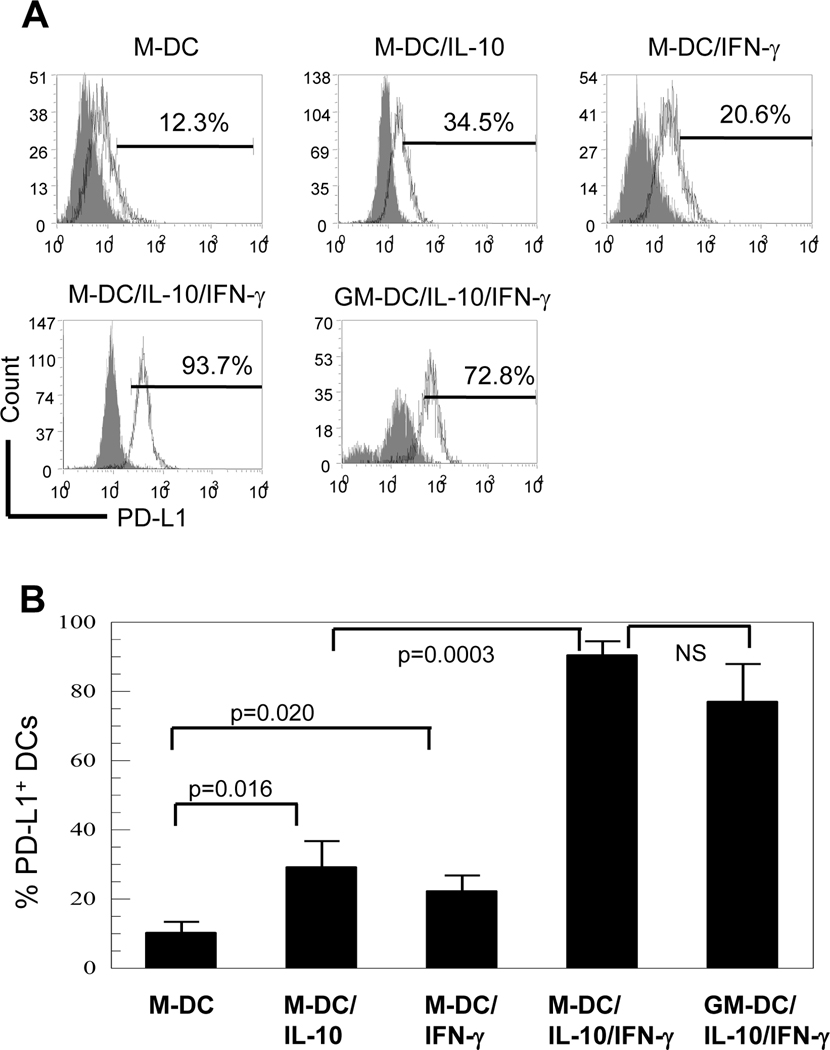
Because M-DC/IL-10 are unique in ability to enhance PD-1 expression on CD8+ T cells, we investigated whether M-DC/IL-10 in comparison with M-DC expressed PD-L1, a ligand for PD-1, (Fig. 2A; 2B). DCs generally express costimulatory or coinhibitory molecules after DCs mature. We found that M-DC/IL-10 expressed higher levels of PD-L1 (34.5%) on the cell surface compared to M-DC (12.3%). PD-L1 expression levels were remarkably increased to 93.7% when M-DC/IL-10 were stimulated with IFN-γ for an additional 4 days. In contrast to M-DC/IL-10, M-DC failed to respond to IFN-γ as much as M-DC/IL-10 with only a slight increase in PD-L1 expression from 12.3% to 20.6%. GM-DC/IL-10/ IFN-γ expressed a high level of PD-L1 expression (72.8%), but it was slightly lower than for M-DC/IL-10/IFN-γ. The results suggest that IFN-γ is a potent inducer of PD-L1, and IL-10 acts as a key mediator for a maximal response to IFN-γ.
Figure 2.
M-DC/IL-10/IFN-γ are characterized by high level expression of inhibitory PD-L1 and ability to reduce numbers of CD8+ T cells upon coculture. (A) M-CSF- and GM-CSF-derived DCs without (M-DC and GM-DC) or with IL-10 (M-DC/IL-10 and GM-DC/IL-10) plus or minus IFN-γ were analyzed for surface expression of PD-L1 using anti-PD-L1-PE-Cy7. Open curves in each plot represent % PD-L1+ cells and shaded curves represent isotype controls. It was noted that IL-10 treated DCs showed extraordinarily high staining backgrounds. Dot plots are representative of six independent experiments. (B) The histograms are representative of three independent experiments and each experiment was carried with two independent blood samples. Results are expressed as mean +/− STD from six experiments.
M-DC/IL-10 are unique for low CD86 and high CD14 and CD200R expression
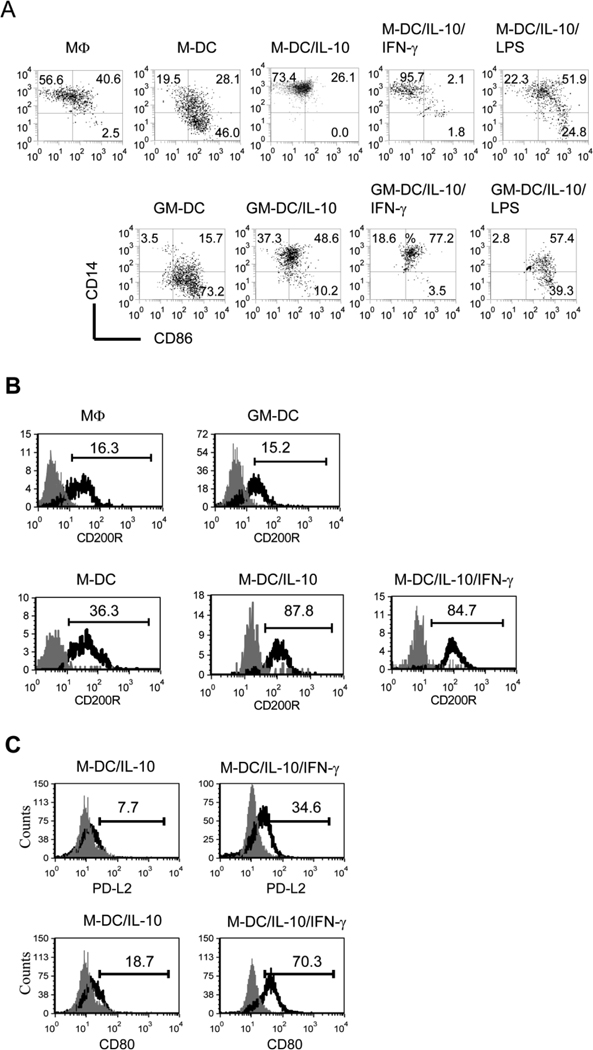
As M-DC/IL-10 induced PD-1, a coinhibitory receptor on cocultured CD8+ T cells, we investigated whether M-DC/IL-10 differed from M-DC in expression of costimulatory receptors such as CD86 which is important for T cell activation. We found that M-DC/IL-10 expressed CD86 at much lower levels (26.1%) compared to M-DC (74.1%) (Fig. 3A). In contrast, M-DC/IL-10 maintained higher levels of CD14 (96.5%) than M-DC (47.6%). The expression levels of CD14 on M-DC/IL-10 levels are comparable to those on macrophages (MΦs) developed by M-CSF without IL-4 from CD14+ monocytes. IFN-γ lowered levels of CD86 expression on M-DC/IL-10 (3.9%), while maintaining high levels of CD14 expression. In contrast to IFN-γ, LPS, known to promote maturation of macrophages and DCs, increased expression of CD86 on M-DC/IL-10 (M-DC/IL-10/LPS) from 26.1% to 76.7%, reducing CD14 expression. This indicates that although both IFN-γ and LPS are considered to be proinflammatory, IFN-γ is unique in its inhibitory effects on CD86 expression on M-DC/IL-10. Therefore, a phenotype of M-DC/IL-10/IFN-γ with little expression of costimulatory CD86 but high expression of coinhibitory PD-L1 as seen in Fig. 2 may relate to their tolerogenic effect on CD8+ T cells. Like M-DC/IL-10, GM-DC/IL-10 expressed lower levels of CD86 (58.8%) compared to GM-DC (88.9%). Similar to M-DC/IL-10, GM-DC/IL-10 expressed higher levels of CD14 (85.9%) compared to GM-DC (19.2%), indicating that IL-10 is equally inhibitory for M-DC and GM-DC with regards to differentiating into a mature form of DC. In contrast to the inhibitory effect of IFN-γ on lowering CD86 expression on M-DC/IL-10, IFN-γ increased CD86 expression on GM-DC/IL-10. This suggests that IFN-γ may have dual functions as an immune suppressor for M-DC/IL-10 as well as an immune activator for GM-DC/IL-10.
Figure 3.
M-DC/IL-10/IFN-γ cells are characterized by low CD86 and high CD14 and CD200R expression. (A) M-DC, M-DC/IL-10, M-DC/IL-10 stimulated without or with IFN-γ (M-DC/IL-10/IFN-γ) or LPS (M-DC/IL-10/LPS) and counterpart GM-DCs were compared for expression of CD86 and CD14 using anti-CD86-PE-Cy5 and anti-CD14-FITC. MΦ denotes a prototype macrophage developed with M-CSF in the absence of IL-4. Dot plots are representatives of six independent experiments. Numbers in dot plots represent % CD86+ and % CD14+ cells. (B) Expression levels of CD200R on a prototype MΦ, GM-DC, M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ cells were displayed as mean fluorescence intensities in histograms. Open curves in each histogram represent CD200R+ cells, and shaded curves represent isotype controls. Flow cytometry histograms are representative of at least four independent experiments. (C) M-DC/IL-10 and M-DC/IL-10/IFN-γ cells were compared for the expression of PD-L2 and CD80 using anti-PD-L2-PE and anti-CD80-PE. Open curves in each histogram represent PD-L2+ and CD80+ cells, and shaded curves represent isotype controls. Flow cytometry histograms are representatives of four independent experiments.
High expression of CD14 on M-DC/IL-10 and M-DC/IL-10/IFN-γ cells implies that they may represent a unique subset of myeloid derived DCs or MΦs. To better define M-DC/IL-10 and M-DC/IL-10/IFN-γ cells, we assessed expression of CD200R on M-DC/IL-10 and M-DC/IL-10/IFN-γ because CD200R is expressed almost exclusively by myeloid cells, including MΦs and delivers an inhibitory signal to myeloid cells to suppress CD8+ T cell immune responses and thus acts as an inhibitory receptor (43). Consistent with this, CD200 expression on malignant cells is correlated with poor prognosis (44). Thus, we compared expression levels of CD200R on M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ with that on prototype MΦs and conventional DCs (referred to as GM-DC in this paper) developed in vitro by M-CSF and GM-CSF plus IL-4, respectively. Modest levels of CD200R were detected on MΦs and GM-DCs at 16.3% and 15.2% respectively, when estimated by mean fluorescence intensity (Fig. 3B). Relatively, higher levels of CD200R were expressed on M-DCs (at 36.3%) compared to MΦs and GM-DC. Even higher levels of CD200R expression were observed in M-DC/IL-10 and M-DC/IL-10/IFN-γ (at 87.8% and 84.7%, respectively). As CD200R is involved in tolerogenic activities by specific subsets of MΦs and DCs (45–46), high expression of CD200R on M-DC/IL-10 and especially on M-DC/IL-10/IFN-γ may relate to the tolerogenic nature of these cells. There are additional ligands for PD-1, such as PD-L2 and CD80 (4). While PD-L2 and CD80 were expressed only at marginal levels, 7.7% and 18.7%, respectively on M-DC/IL-10, they were increased to 34.6% and 70.3%, respectively on M-DC/IL-10/IFN-γ, as shown in Fig. 3C, indicating that IFN-γ acts as an important factor for inducing PD-L2 and CD80 as well as PD-L1.
Viable CD8+ T cell numbers are decreased in presence of M-DC/IL-10/IFN-γ
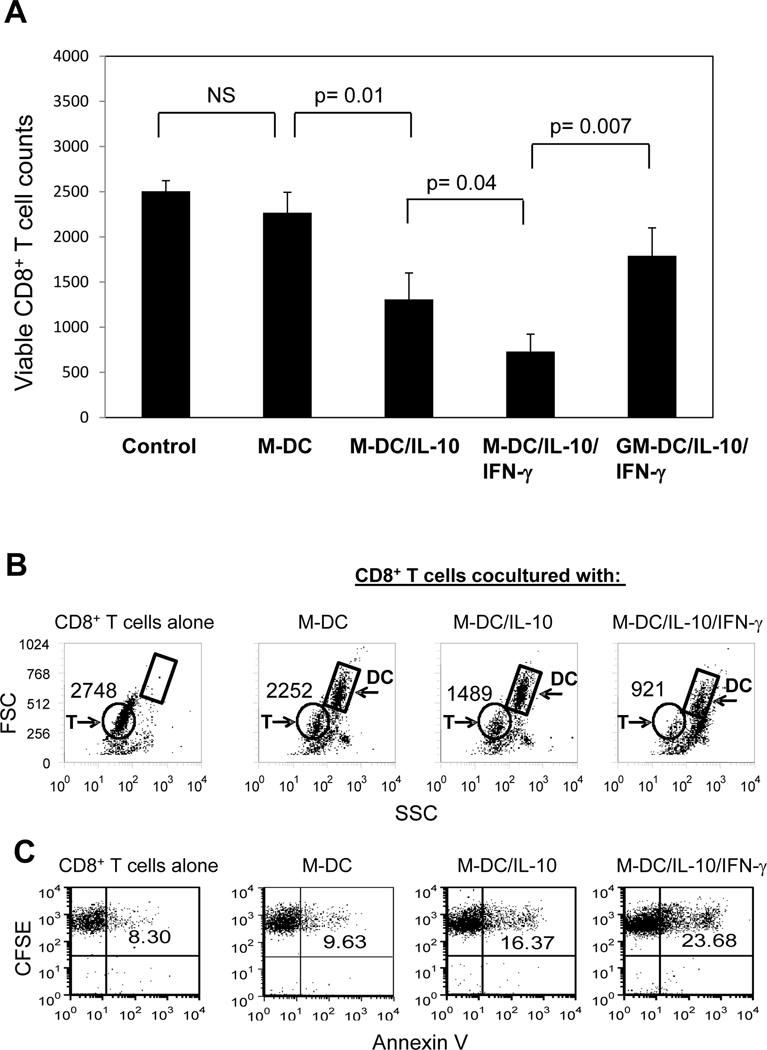
Because M-DC/IL-10 are intrinsically different from M-DC in high expression of cell surface PD-L1 and CD200R and in ability to induce PD-1 expression on CD8+ T cells, we evaluated whether M-DC/IL-10 have a greater negative impact on survival of CD8+ T cells compared to M-DC, especially in response to IFN-γ. To test this possibility, numbers of viable CD8+ T cells were counted by flow cytometry, using gating for CD8+ T cells following 4 day coculture with M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ (Fig. 4A). CD8+ T cells cocultured with M-DC/IL-10 were lower in numbers than those cocultured with M-DC. CD8+ T cell numbers were further reduced when they were instead cocultured with M-DC/IL-10/IFN-γ. Numbers of viable CD8+ T cells cocultured with M-DC/IL-10 or M-DC/IL-10/IFN-γ were below numbers of CD8+ T cells cultured with or without M-DC. In contrast to M-DC/IL-10, GM-DC/IL-10/IFN-γ had a minimal effect on CD8+ T cell numbers. M-DC or IFN-γ itself did not result in decreased CD8+ T cells (data not shown). We also evaluated the status of CD8+ T cells cocultured without or with M-DC, M-DC/IL-10 or M-DC/IL-10/IFN-γ by plotting the cells against forward scatter (FSC) and slide scatter (SSC). The dot plots allowed us to distinguish viable CD8+ T cells from dead cells and DCs (Fig. 4B). Significant decreases of CD8+ T cell numbers with apparent dead cells were noticed after coculture with M-DC/IL-10. Even more cell loss was observed in CD8+ T cells cocultured with M-DC/IL-10/IFN-γ. The ability of M-DC/IL-10/IFN-γ to cause CD8+ T cell apoptosis was further substantiated by measuring Annexin V+CD8+ T cells following their coculture with M-DC/IL-10/IFN-γ (Fig. 4C). CD8+ T cells were dyed with CFSE prior to coculture to use CFSE as a marker for CD8+ T cells to avoid surface markers staining steps after coculture, which might affect the following Annexin V staining. CD8+ T cells cocultured without or with M-DC showed 8.3% and 9.6% Annexin+, respectively. However, increased Annexin+ cells were found in CD8+ T cells (16.4%) when cocultured with M-DC/IL-10, and to even higher levels (23.7%) with M-DC/IL-10/IFN-γ. This suggests that M-DC/IL-10 induce apoptosis of CD8+ T cells and this effect is more severe in the presence of IFN-γ.
Figure 4.
Viable CD8+ T cell numbers are decreased in presence of M-DC/IL-10/IFN-γ. (A) CD8+ T cells were cocultured without or with M-DC, M-DC/IL-10, M-DC/IL-10/IFN-γ or GM-DC/IL-10/IFN-γ at a ratio of 1 to 1 for 4 days. The cells were stained with anti-CD8-PE-Cy 5.5 and anti-CD11c–APC and analyzed for the numbers of viable CD8+ cells by flow cytometry. Viable cells were gated based on FSC and SSC, and CD8+ CD11c− cells gated from the viable cells were electronically counted. The relative cell numbers are displayed in the histogram. Data from six independent experiments are shown and values of p indicate a significant difference in numbers of CD8+ T cells cocultured with M-DC, M-DC/IL10 or M-DC/IL-10/IFN-γ. (B) CD8+ T cells cocultured without or with M-DC, M-DC/IL-10 or M-DC/IL-10/IFN-γ as described above were displayed in dot plots against FSC and SSC to identify viable CD8+ T cells (cells in circles) and DCs (cells in boxes) which are distinguished from dead cells (cells with low FSC and high SSC). Numbers depicted in the plots represent counts of viable CD8+ T cells, as representative results of six independent experiments. (C) Apoptotic status of the cocultured CD8+ T cell populations was assessed by Annexin V staining. CD8+ T cells were dyed with CFSE prior to coculture, and CD8+ T cells were identified as CFSEbright cells after gating based on FSC and SSC as shown in (B). The numbers depicted in the plots represent % Annexin V+ cells in the CFSE bright CD8+ T cell populations, as representative results of four experiments.
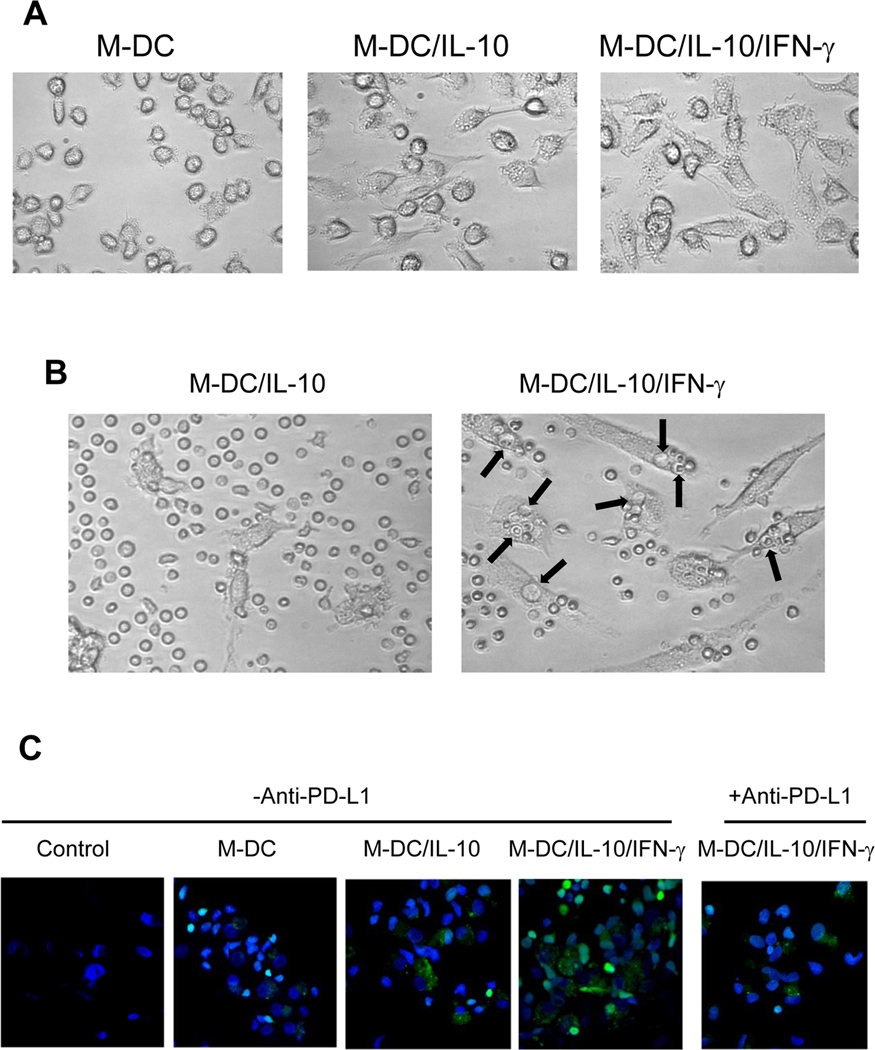
Microscopic images indicate phagocytic activity by M-DC/IL-10/IFN-γ
M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ are morphologically distinct as shown in Fig. 5A. M-DC/IL-10 are larger and irregular in their shape with many granules in the cytoplasm compared to M-DC. M-DC/IL-10/IFN-γ showed even greater morphological changes expressing more granules with many thin filaments extending from the cell surface. We noticed numerous vacuoles in the M-DC/IL-10/ IFN-γ cells when they were co-cultured with CD8+ T cells under contrast microscopic observations, as indicated by arrows (Fig. 5B). Interestingly, a large portion of the vacuoles were occupied by small round cells, most likely CD8+ T cells. Such phenomena were rarely detected with M-DC/IL-10. Interestingly, a large portion of the vacuoles were occupied by CD8+ T cells. These observations suggest possible phagocytic activity of M-DC/IL-10/IFN-γ.
Figure 5.
M-DC/IL-10/IFN-γ cells show signs of phagocytic activity. (A) Pictures of M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ were observed under contrast light microscopy at a magnification of 200 ×. M-DC/IL-10/IFN-γ were unique with many granules and filaments. (B) M-DC/IL-10 and M-DC/IL-10/IFN-γ were cocultured with the CD8+ T cells for 4 days. Cells were observed under contrast light microscope at a magnitude of 200 ×. Arrows indicate apparent vacuoles on the surface of M-DC/IL-10/IFN-γ. Some of the vacuoles are occupied by small round cells, presumably CD8+ T cells. (C) M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ were cocultured with CFSE stained CD8+ T cells on glass slides for 4 days without or with neutralizing anti-PD-L1. Control slide contained CD8+ T cells without M-DC. The glass plates were washed with PBS to remove CD8+ T cells loosely bound to adherent DCs. The remaining cells were fixed with 4% paraformaldehyde for 10 min at room temperature, stained with DAPI, mounted and observed under confocal microscope to detect fluorescence in DCs at a magnitude of 400x. Small bright green round cells represent CFSE stained CD8+ T cells
The many vacuoles occupied by CD8+ T cell-like cells in M-DC/IL-10/IFN-γ cells led us to speculate that loss of the CD8+ T cells as noted in Fig. 4A might result from phagocytic events. To verify this possibility, CFSE stained CD8+ T cells were cocultured with M-DC, M-DC/IL-10 or M-DC/IL-10/IFN-γ for 4 days on glass slides. Before confocal microscopic observations, we removed loosely bound CD8+ T cells from adherent DCs by washing the glass slides with PBS. Following cell fixation, staining with DAPI and mounting on glass plates, we assessed the possibility that DCs acquired fluorescence due to engulfment of CFSE stained CD8+ T cells under confocal microscopy (Fig. 5C). CD8+ T cells were distinguished from DCs by their green fluorescence, shape and size. Indeed, we detected many M-DC/IL-10 cells fluorescent with speckles, presumably originating from CFSE stained CD8+ T cells. M-DC/IL-10/IFN-γ became even more fluorescent than M-DC/IL-10 upon coculture with CFSE stained CD8+ T cells. We also noticed that many more CD8+ T cells remained associated with M-DC/IL-10/IFN-γ after washing, compared to those cultured with M-DC and M-DC/IL-10. This suggests that M-DC/IL-10/IFN-γ had stronger interactions with CD8+ T cells compared to M-DC and M-DC/IL-10. Importantly, addition of neutralizing anti-PD-L1 to the cocultures inhibited the detection of fluorescence in M-DC/IL-10/IFN-γ, as well as numbers of CD8+ T cells associated with M-DC/IL-10/IFN-γ (Fig. 5C). Based on these results, we suggest that M-DC/IL-10/IFN-γ are able to phagocytose CD8+ T cells and that PD-L1 induced by IFN-γ may be involved at least in part in this phagocytic activity. These phenomena were unique for M-DC/IL-10/IFN-γ cells because GM-DC/IL-10/IFN-γ cells showed little signs of fluorescent speckles upon co-culture with CFSE stained CD8+ T cells. Taking all microscopic observations into consideration, we suggest that M-DC/IL-10/IFN-γ possess phagocytic activity for CD8+ T cells, and IFN-γ acts as a driving force for the M-DC/IL-10/IFN-γ-mediated phagocytic activity through PD-L1/PD-1 interaction.
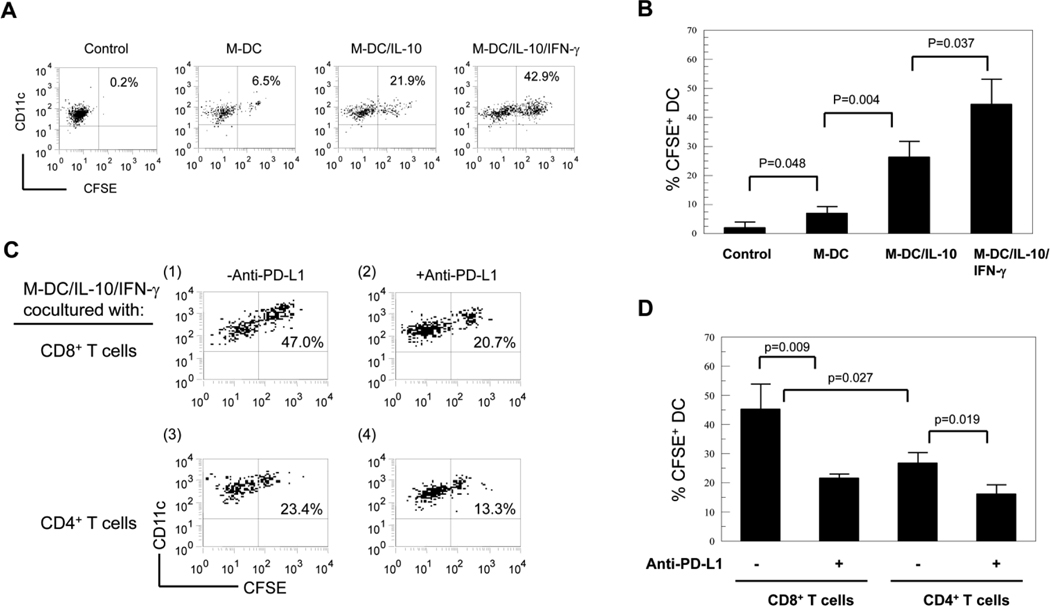
Quantification of phagocytic activities by flow cytometry
We undertook flow cytometric analysis for M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ co-cultured with CFSE stained CD8+ T cells to quantitatively confirm an IFN-γ inducing effect on phagocytic activity. Cells were stained with CD11c and CD8, and CFSE intensities of CD11c positive DCs was assessed by flow cytometry (Fig. 6A). Low levels of CFSE were detected in M-DC and M-DC/IL-10 co-cultured with CFSE stained CD8+ T cells (Fig. 6A, 6.5% and 21.9%, respectively). In agreement with our previous microscopic observations, significantly increased levels of CFSE were found in the M-DC/IL-10/IFN-γ cells cocultured with CFSE stained CD8+ T cells (42.9%). Controls represent M-DC cultured with CFSE stained CD8+ T cells. Results were statistically evaluated as shown in Fig. 6B. We assessed whether CD8+ and CD4+ T cells were equivalently subjected to phagocytic activity by M-DC/IL-10/IFN-γ (Fig. 6C). The M-DC/IL-10/IFN-γ cells cocultured with CFSE stained CD4+ T cells showed significantly lower levels of CFSE compared to those with CD8+ T cells (23.4% versus 47.0%). In agreement with our previous results, neutralizing anti-PD-L1 markedly reduced CFSE in M-DC/IL-10/IFN-γ cocultured with CFSE stained CD8+ T cells from 47.0% to 20.7% (Fig. 6C; 6D). Neutralizing anti-PD-L1 also reduced CFSE in M-DC/IL-10/IFN-γ cocultured with CFSE stained CD4+ T cells from 23.4% to 13.3%.
Figure 6.
IFN-γ increases phagocytic activity of M-DC/IL-10 preferentially for CD8+ T cells. (A) CFSE stained CD8+ T cells were co-cultured without or with M-DC, M-DC/IL-10 and M-DC/IL-10/IFN-γ for 4 days. The cells were stained with anti-CD11c-APC and anti-CD8-PE-Cy5.5 and analyzed for CFSE in the CD11c+ DCs by flow cytometry. Control represents M-DCs cultured without CFSE stained CD8+ T cells. Dot plots are represent of three independent experiments. Numbers in dot plots represent % CFSE+ cells in CD11c+ cells. (B) Results are expressed as mean +/− STD from three experiments. (C) M-DC/IL-10/IFN-γ were cocultured with CFSE stained CD4+ T cells or CD8+ T cells for 4 days with or without neutralizing anti-PD-L1(clone MIH1) at 5 µg/ml. Cells were stained with anti-CD11c-APC and anti-CD8-PE-Cy5.5 and CD8 negative cells gated were analyzed for CFSE by flow cytometry. Numbers represent percentages of CFSE+ cells in CD11c+ cells. (D) Results are expressed as mean % +/− STD from three independent experiments.
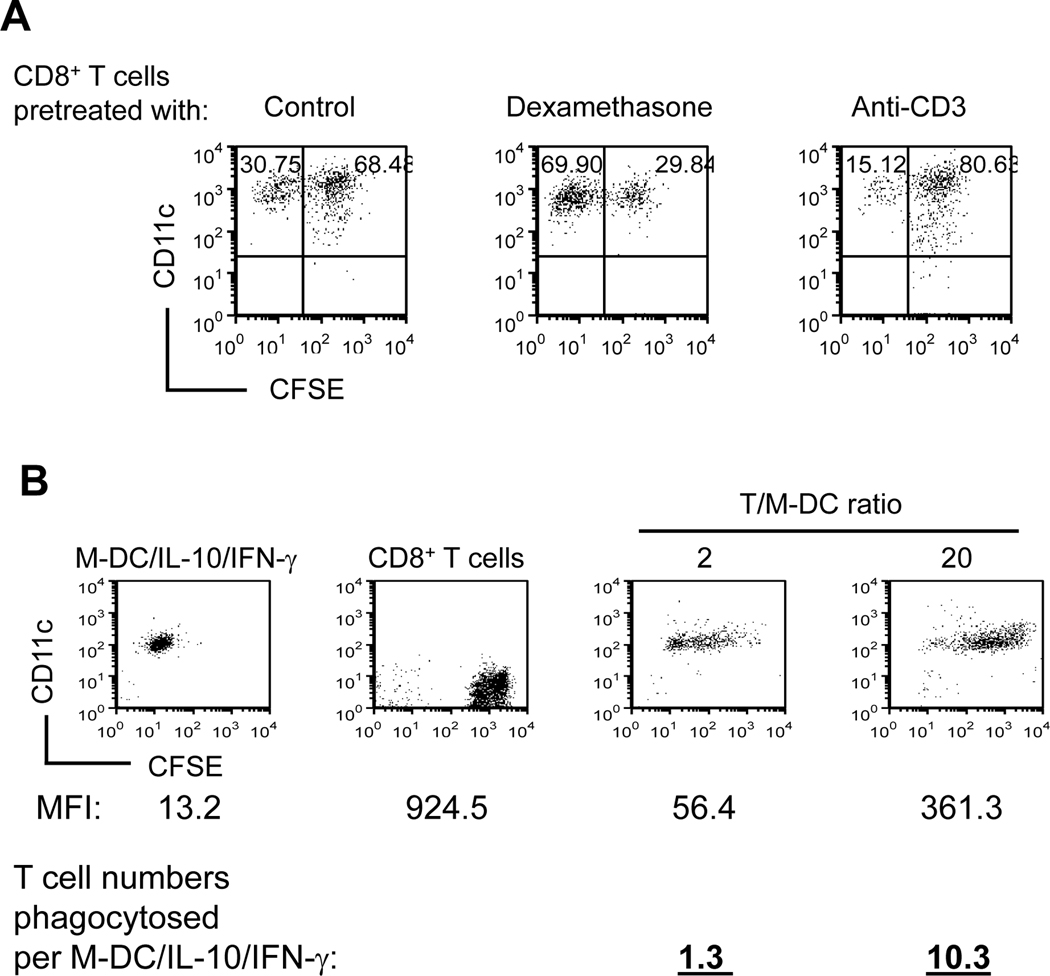
Effects of apoptotic and activation status of CD8+ T cells on their responsiveness to phagocyotsis, and estimated number of CD8+ T cells phagocytosed by M-DC/IL-10/INF-γ cells
To examine whether apoptosis of CD8+ T cells is sufficient for their phagocytosis, we pretreated CD8+ T cells with dexamethasone to induce apoptosis prior to coculture with M-DC/IL-10 in the presence of INF-γ. The degree of apoptosis of CD8+ T cells correlated with their phagocytosis (Fig. 4B). However, CD8+ T cells pretreated with dexamethasone, which would induce apoptosis and possibly attenuate INF-γ production, were rendered less efficiently phagocytosed by M-DC/IL-10 compared to non-treated CD8+ T cells. In contrast, anti-CD3 pretreated CD8+ T cells were more efficiently phagocytosed by M-DC/IL-10 than control CD8+ T cells (Fig. 7A), indicating the importance of pre-activation of CD8+ T cells for them to be phagocytosed. These two lines of evidence suggest that timely apoptosis of previously activated CD8+ T cells upon encounter with M-DC/IL-10 is necessary, but CD8+ T cells already predestinated for apoptosis may not be critical for their phagocytosis by M-DC/IL-10. High level expression of PD-1 is detected on chronically activated CD8+ T cells (42). Thus, our view may explain a potential mechanism for PD-1 mediated exhaustion of previously activated CD8+ T cells.
Figure 7.
Pretreatment of CD8+ T cells with dexamethasone or anti-CD3 affects responsiveness to phagocytosis by M-DC/IL-10. (A) CD8+ T cells were incubated in IL-15 (20ng/ml) with or without dexamethasone (10−7 M) or anti-CD3 coated to culture plates for 5 days. Following CFSE staining, the CD8+ T cells were cocultured with M-DC/IL-10 in the presence of INF-γ for 4 days. Cells were stained with anti-CD8 and anti-CD11c, and CD11c+ cells were analyzed for levels of CFSE by flow cytometry. Numbers in second quadrants represent % CFSE+ CD11c+ cells. This result is a representative of four independent experiments. (B) CD8+ T cells were incubated in IL-15 (20 ng/ml) for 5 days and subsequently stained with CFSE. CFSE+CD8+ T cells were cultured in the presence of IFN-γ for additional 4 days without or with M-DC/IL-10 at T cell to M-DC/IL-10 ratios of 2 and 20. M-DC/IL-10 and CD8+ T cells cultured separately or cocultured for 4 days were analyzed for MFI of CFSE by flow cytometry. Numbers of CD8+ T cells phagocytosed by M-DC/IL-10 were estimated from MFIs using the methods described in the Materials and Methods. This result is a representative of eight experiments.
It was not easy to estimate exact numbers of accumulated CD8+ T cells taken up by M-DC/IL-10 during a 5-day co-culture, mainly because the phagocytosed CD8+ T cells did not remain intact enough to be counted. Since M-DC/IL-10 gained MFI as a result of phagocytosis of green fluorescent CFSE+ CD8+ T cells, we attempted to estimate numbers of CD8+ T cells taken up by M-DC/IL-10 in the presence of INF-γ using cytometric analysis for mean fluorescence intensity (MFI). Based on the assumption that CD8+ T cell volume was about 1/27 of M-DC cell based from our microscopic observation of the diameter of CD8+ T cell being approximately 1/3 of that of M-DC, we estimated the numbers of CD8+ T cells phagocytosed by using methods described in the Materials and Methods. Our results showed that approximately 1.3 and 10.3 CD8+ T cells were phagocytosed by M-DC/IL-10/INF-γ, respectively at CD8+ T cell to M-DC ratios of 2 and 20.
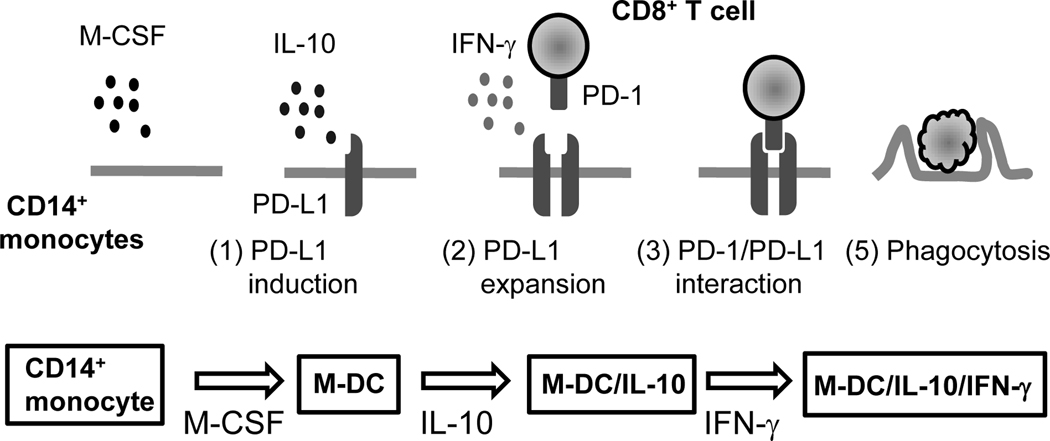
In summary, we demonstrate that PD-1 expression is induced on CD8+ T cells upon coculture with M-DC/IL-10 or M-DC/IL-10/IFN-γ cells that were developed from human CD14+ monocytes in vitro by a combination of IL-4, M-CSF, IL-10 and IFN-γ. We also found that M-DC/IL-10/IFN-γ, which express high levels of PD-L1, act as phagocytic cells for CD8+ T cells, and to a lesser extent for CD4+ T cells. Inhibition of phagocytosis by neutralizing anti-PD-L1 indicates that PD-1/PD-L1 interaction mediates this phagocytic activity. This study suggests a role for M-DC/IL-10 cells in down-modulating CD8+ T cell function in response to IFN-γ by phagocytosis, as modeled in Fig. 8.
Figure 8.
A model for phagocytic response of exhausted CD8+ T cells to M-DC/IL-10/IFN-γ. M-CSF is a key cytokine for the development of phagocytic DC against CD8+ T cells. IL-10 changes characteristics of M-CSF-derived DC (M-DC) with an enhanced responsiveness to IFN-γ for an ability to induce PD-1 on CD8+ T cells. Subsequent IFN-γ stimulation further modifies IL-10-derived M-DC (M-DC/IL-10) with induction of elevated PD-L1 expression on M-DC/IL-10 (M-DC/IL-10/IFN-γ). CD8+ T cells able to produce IFN-γ may further stabilize expression of PD-L1 on M-DC/IL-10/IFN-γ. Tight interactions between CD8+ T cells and M-DC/IL-10/IFN-γ cells through PD-1 and PD-L1 may induce apoptosis of CD8+ T cells, which are eventually phagocytosed by M-DC/IL-10/IFN-γ.
Discussion
It is well accepted that DCs can induce tolerance as well as immunity, but underlying mechanisms for induction or tolerance of immunity by DCs remain unclear. Immature, or alternatively differentiated, DCs are probably primary cells for tolerance responses. T cell exhaustion is defined as gradual loss of effector functions, finally resulting in deletion of virus-specific T cells (39, 42). Loss of functional CD8+ T cell response to persistent viral infection and tumors results from active inhibitory DCs (47–49). One type of inhibitory DC for inducing T cell tolerance is a MDSC (22, 50) and another is an immature macrophages (21). PD-L1 on MDSC has been implicated in induction of exhaustion of PD-1 expressing virus-specific T cells (9, 21). An important question yet to be answered was what factors modulate respective induction of PD-L1 and PD-1 on MDSC and CD8+ T cells.
Our study provides evidence that orchestrated serial stimulation processes are required for induction of PD-L1 and PD-1, respectively on tolerogenic DCs and CD8+ T cells. M-CSF-derived DCs are characterized by a skewed balance toward high IL-10 and low IL-12 secretion (40). The anti-inflammatory environment facilitated by IL-10 may be critical for inducing PD-L1 on the cell surface and an alternative differentiation process towards tolerance-inducing DCs (Fig. 2). Upon interaction with the PD-L1 expressing DCs, CD8+ T cells enhanced PD-1 expression levels as demonstrated in Fig. 1A. IFN-γ secreted by encountering CD8+ T cells may further elevate PD-L1 levels on DCs primed with M-CSF and IL-10 (Fig. 2). The high level of PD-L1 expressed on the DCs may allow tight interactions between DCs and CD8+ T cells and induce growth arrest or cell death of CD8+ T cells. The PD-l/PD-L1 signaling pathway may then trigger IFN-γ producing CD8+ T cells to initiate apoptosis and consequently phagocytic response. Phagocytosis was significantly decreased by neutralizing anti-PD-L1. This indicates that elevated PD-L1 expression on M-DC/IL-10 by IFN-γ plays an important role in eliciting phagocytic activity (Fig. 5 and Fig. 6). Thus, the local cytokine micromilieu, enriched with M-CSF and IL-10 may control functional differentiation of DCs from immunostimulatory to tolerogenic cells targeting IFN-γ producing CD8+ T cells for phagocytosis. Although PD-1 acts on both CD4+ and CD8+ T cell populations as a co-inhibitory receptor (51), CD8+ T cells were more susceptible, compared to CD4+ T cells, in response to phagocytosis (Fig. 6C; 6D). This may be because CD8+ T cells express PD-1 more readily or respond to PD-L1 more sensitively compared to CD4+ T cells.
Our results emphasize the importance of IFN-γ, a pro-inflammatory cytokine, for phagocytic clearance of CD8+ T cells as an additional level of CD8+ T cell tolerance. IFN-γ is a pro-inflammatory cytokine produced mainly by type 1 effector T cells and is required for CD8+ T cell cytotoxic functions. Thus, it seems paradoxical that phagocytic deletion of CD8+ T cells depends on IFN-γ. We believe that IFN-γ is important not only for type 1 effector T cell immune responses, but also for clearing effector CD8+ T cells by MDSC. Therefore, the phagocytic activity we observed in this study may take place in vivo to keep IFN-γ-producing CD8+ T cells in check to prevent excessive or continuous immune responses. TNF-α failed to develop phagocytic activity in M-DC/IL-10 cells, but instead induced cell death (data not shown). In mouse models, IFN-γ has been reported to negatively regulate CD8+ T cell response by promoting cell death mediated by PD-1, and blockade of IFN-γ augments the severity of graft-versus-host disease (GVHD) in allogeneic hematopoietic cell transplantation (52–53). Furthermore, other mouse systems show that IFN-γ plays immune suppressive roles in conjunction with MDSCs (24, 54).
Our study does not determine whether or not M-DC/IL-10 are relevant to certain DC subtypes in mice that show suppressive effects on CD8+ T cell. In a mouse model, treatment of animals with neutralizing anti-PD-L1 did not eliminate or reduce Gr-1+CD11b+ MDSC induced CD8+ T cell tolerance (28), suggesting that PD-L1 is not required for MDSC-mediated CD8+ T cell immune suppression. Therefore, the MDSC identified as Gr-1+CD11b+ cells in the mouse may not be relevant to the M-DC we generated from human CD14+ monocytes in vitro. In another mouse study (29), it was reported that IFN-γ-stimulated monocyte-derived cells could suppress T cell immune responses by killing activated CD4+ T cells. However, this suppressive activity was accomplished by a caspase-dependent, but PD-1/PD-L1-independent mechanism. However, phenotypes of the mouse IFN-γ-stimulated monocyte-derived cells were similar to those of our M-DC/IL-10/IFN-γ cells with high expression of PD-L1 and CD14. Thus it would be of interest in the future to explore the possibility that M-DC/IL-10/IFN-γ cells may utilize caspase to promote apoptosis and/or phagocytosis of CD8+ T cells as seen in our study. The mouse IFN-γ-stimulated monocyte-derived cells were also shown to convert co-cultured T cell populations to CD4+CD25+Foxp3+ regulatory cells (29). We have previously demonstrated that M-DC were able to suppress CD4+ T cell proliferation (40), but it remains unclear whether M-DC have a capacity to generate CD4+FOXP3 regulatory T cells.
Prior IL-10 stimulation was critical for IFN-γ to be effective for developing phagocytic activity in M-DC/IL-10. In fact, IL-10 and IFN-γ are considered to play opposite roles, as anti- and pro-inflammatory cytokines, but they may cooperate to elicit phagocytic activity against CD8+ T cells via M-DC, as long as IL-10 precedes IFN-γ stimulation. An important role of IL-10 in these processes is to block maturation of DCs (25) as seen in Fig. 3A. Importantly, immaturity of M-DC/IL-10 resulting from exposure to IL-10 may be an important conditioning event for subsequent IFN-γ to elicit phagocytic activity in M-DC/IL-10, indicating that strict sequential pathways starting from anti-inflammatory to inflammatory cytokine stimulation may be crucial for development of phagocytic DCs. The IL-10 effect depended on M-CSF for developing phagocytic activity. GM-CSF-derived DCs after IL-10 and IFN-γ stimulation showed little phagocytic activity even though they expressed relatively high levels of PD-L1.
Thus, M-CSF and IL-10 are required for guiding the direction of DC maturation from peripheral CD14+ monocytes to phagocytic DCs rather than to immunostimulatory DCs. M-DC/IL-10 cells may be locked-in as tolerogenic DCs at this stage and may be unable to convert to immunogenic DCs even in the presence of IFN-γ. This idea is supported by high expression of CD200R on macrophages, which delivers negative regulatory signals to induce tolerogenic macrophages and DCs (55), on M-DC/IL-10 and M-DC/IL-10/IFN-γ. As IL-10 inhibits production of IFN-γ by CD8+ T cells, IL-10 and IFN-γ may rarely coexist at the same local sites. Thus, M-DC/IL-10 and IFN-γ producing CD8+ T cells may be derived from supposedly distinct sites, enriched respectively with anti-inflammatory IL-10 or pro-inflammatory IL-12. Phagocytosis may arise in vivo when M-DC/IL-10 are recruited to pro-inflammatory sites where IFN-γ producing CD8+ T cells prevail. Alternatively, this may happen as well when IFN-γ producing CD8+ T cells migrate to the immune evasive sites such as tumor microenvironments enriched with M-DC/IL-10. In any event, PD-1 and PD-L1, respectively expressed on target and phagocytic cell surfaces may be important cognate receptors for phagocytosis.
M-DC/IL-10 and M-DC/IL-10/IFN-γ cells show high expression levels of CD14 and CD200R as well as CD11c, a marker for myeloid DCs. While CD14 is expressed largely on macrophages, CD200R is expressed on both activated macrophages and DCs (55). M-DC/IL-10 and M-DC/IL-10/IFN-γ cells were less adherent cells, compared with prototype macrophages generated by M-CSF in the absence of IL-4 which favors DC differentiation. Therefore, although we initially used the term, 'DCs' for M-DC generated in vitro from CD14+ monocytes by M-CSF and IL-4, which shares many typical DC surface markers upon LPS stimulation (40), M-DC/IL-10 and M-DC/IL-10/IFN-γ cells may represent unique macrophage-like DCs or possibly an alternatively activated macrophage or an anti-inflammatory M2 macrophage subset (55). M-DC/IL-10 cells may be relevant to decidual macrophages, which are involved in maternal immunosuppression against fetus, because decidual macrophages produce high levels of IL-10 with cell surface expression of PD-L1 and CD200R, but low levels of costimulatory molecule CD86 (56–59).
Our confocal microscopic images indicate that many CD8+ T cells remain associated with M-DC/IL-10 even after washing the glass slides with PBS, especially in the presence of IFN-γ during the coculture (Fig. 5C). Moreover, IFN-γ increased many fluorescent speckles in M-DC/IL-10 cells cocultured with CFSE stained CD8+ T cells, presumably derived from CFSE stained CD8+ T cells. We noticed many vacuoles created on the surface of M-DC/IL-10, only when both CD8+ T cells and IFN-γ were present (Fig. 5B). Many these vacuoles appeared to be occupied by CD8+ T cells. This suggests that CD8+ T cells may be directly involved in creating the vacuoles. Exhausted CD8+ T cells may continue to produce IFN-γ (3), and the final fate of the IFN-γ producing CD8+ T cells expanded by chronic virus infection is unclear. The symptomatic CD8+ T cell exhaustion by HIV, as well as other infections, are mainly attributed to PD-1 and IL-10 (32). In a mouse model, IFN-γ is known to attenuate graft-versus-host disease (GVHD), promoting CD8+ T cell exhaustion (52). Our results suggest that M-DC/IL-10/IFN-γ may be involved in causing CD8+ T cell exhaustion, as seen in HIV as well as other virus infections, perhaps by phagocytosing exhausted CD8+ T cells.
Acknowledgements
We thank Sue Rice at the Flow Cytometry Facility of IU Simon Cancer Center and Jeff Clendenon at the Indiana Center for Microscopy, Division of Nephrology for technical assistance.
This work was supported by U.S. Public Health Service Grants NIH R01 HL056416 and HL067384 from the National Institutes of Health to H.E.B.
Abbreviations used in this paper
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-1 ligand
- DC
Dendritic cell
- M-DC
M-CSF-derived dendritic cell
- M-DC/IL-10
IL-10 stimulated M-DC
- M-DC/IL-10/IFN-γ
IFN-γ stimulated M-DC/IL-10
- GM-DCs
GM-CSF-derived DCs
- GM-DC/IL-10
IL-10 stimulated GM-DC
- GM-DC/IL-10/IFN-γ
IFN-γ stimulated GM-DC/IL-10/IFN-γ
- MDSC
Myeloid derived suppressive cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 7.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 10.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 11.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 12.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Zhang Z, Zhang S, Fu J, Yao J, Jiao Y, Wu H, Wang FS. B7-H1 up-regulation impairs myeloid DC and correlates with disease progression in chronic HIV-1 infection. Eur. J. Immunol. 2008;38:3226–3236. doi: 10.1002/eji.200838285. [DOI] [PubMed] [Google Scholar]
- 15.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, Dong H, Maserati R, Shearer GM, Chen L, Clerici M. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 16.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 17.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 18.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 20.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 24.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-γ treatment impairs DC development. Eur. J. Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 25.Steinbrink K, Mahnke K, Grabbe S, Enk AH, Jonuleit H. Myeloid dendritic cell: From sentinel of immunity to key player of peripheral tolerance? Hum. Immunol. 2009;70:289–293. doi: 10.1016/j.humimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 27.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 28.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brem-Exner BG, Sattler C, Hutchinson JA, Koehl GE, Kronenberg K, Farkas S, Inoue S, Blank C, Knechtle SJ, Schlitt HJ, Fandrich F, Geissler EK. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J. Immunol. 2008;180:335–349. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin. Immunol. 2008;129:471–481. doi: 10.1016/j.clim.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sc.i USA. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi CM, von Herrath MG. IL-10 and the resolution of infections. J. Pathol. 2008;214:224–230. doi: 10.1002/path.2272. [DOI] [PubMed] [Google Scholar]
- 34.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin..Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin. Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Geng L, Jiang G, Fang Y, Dong S, Xie H, Chen Y, Shen M, Zheng S. B7-H1 expression is upregulated in peripheral blood CD14+ monocytes of patients with chronic hepatitis B virus infection, which correlates with higher serum IL-10 levels. J. Viral. Hepat. 2006;13:725–733. doi: 10.1111/j.1365-2893.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 38.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 39.Martinic MM, von Herrath MG. Novel strategies to eliminate persistent viral infections. Trends Immunol. 2008;29:116–124. doi: 10.1016/j.it.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Kim YJ, Broxmeyer HE. Macrophage colony-stimulating factor drives cord blood monocyte differentiation into IL-10highIL-12absent dendritic cells with tolerogenic potential. J. Immunol. 2005;174:4706–4717. doi: 10.4049/jimmunol.174.8.4706. [DOI] [PubMed] [Google Scholar]
- 41.Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 42.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J. Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Curr. Opin. Investig. Drugs. 2005;6:483–488. [PubMed] [Google Scholar]
- 44.Wong KK, Khatri I, Shaha S, Spaner DE, Gorczynski RM. The role of CD200 in immunity to B cell lymphoma. J. Leukoc. Biol. 2010 doi: 10.1189/jlb.1009686. [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Eizumi K, Fukaya T, Fujita S, Sato Y, Takagi H, Yamamoto M, Yamashita N, Hijikata A, Kitamura H, Ohara O, Yamasaki S, Saito T. Naturally occurring regulatory dendritic cells regulate murine cutaneous chronic graft-versus-host disease. Blood. 2009;113:4780–4789. doi: 10.1182/blood-2008-10-183145. [DOI] [PubMed] [Google Scholar]
- 46.Gorczynski RM, Chen Z, He W, Khatri I, Sun Y, Yu K, Boudakov I. Expression of a CD200 transgene is necessary for induction but not maintenance of tolerance to cardiac and skin allografts. J. Immunol. 2009;183:1560–1568. doi: 10.4049/jimmunol.0900200. [DOI] [PubMed] [Google Scholar]
- 47.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RAD, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. USA. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J. Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 50.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur. J. Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 51.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 52.Asavaroengchai W, Wang H, Wang S, Wang L, Bronson R, Sykes M, Yang YG. An essential role for IFN-γ in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow. Transplant. 2007;13:46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 54.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koning N, van Eijk M, Pouwels W, Brouwer MS, Voehringer D, Huitinga I, Hoek RM, Raes G, Hamann J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J. Innate Immunol. 2010;2:195–200. doi: 10.1159/000252803. [DOI] [PubMed] [Google Scholar]
- 56.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am. J. Reprod. Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 57.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. Gene Expression Profiling of Human Decidual Macrophages: Evidence for Immunosuppressive Phenotype. PLoS ONE. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, Ragni N, Moretta L, Mingari MC. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc. Natl. Acad. Sci. USA. 2010;107:11918–11923. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]