Abstract
Development of asthma and allergic inflammation involves innate immunity but the environmental contributions remain incompletely defined. Analysis of dust collected from the homes of asthmatic individuals revealed that the polysaccharide chitin is environmentally widespread, and associated with β-glucans, possibly from ubiquitous fungi. Cell wall preparations of Aspergillus isolated from house dust induced robust recruitment of eosinophils into mouse lung, an effect that was attenuated by enzymatic degradation of cell wall chitinand β-glucans. Mice expressing constitutively active acidic mammalian chitinase (AMCase) in the lungs demonstrated a significant reduction in eosinophil infiltration after fungal challenge. Conversely, chitinase inhibition prolonged the duration of tissue eosinophilia. Thus, fungal chitin derived from home environments associated with asthma induces eosinophilic allergic inflammation in the lung, and mammalian chitinases, including AMCase, limit this process.
INTRODUCTION
The rise in asthma and allergic disorders appears to involve intersecting genetic and environmental factors, such that genetically susceptible individuals develop disease that can be triggered or exacerbated by one or more of the myriad insults encountered by the airway mucosa (1). This external environment comprises a diverse mix of potentially allergenic materials; however, certain common components have emerged as candidates underlying the persistent immune activation associated with these chronic conditions. Among them, fungi and fungal-derived bioactive agents, prominent as ubiquitous constituents of airborne particulate matter and inhaled house dust, have been linked to severe asthma and allergic conditions, and pose an additional systemic threat to immunocompromised individuals (2, 3).
Inhaled conidia from Aspergillus species and other fungi are normally immunologically silent due to the presence of a surface hydrophobin layer that prevents recognition (4), together with rapid phagocyte-oxidase-mediated killing by alveolar macrophages (5). Impairment of this process, however, can result in conidial germination, leading to the recruitment of inflammatory cells such as eosinophils and neutrophils, which are able to inhibit hyphal growth (3, 6). Interestingly, immune triggering only occurs with concomitant conidial swelling and hyphal growth, a process linked to exposure and recognition of fungal cell wall β-glucan polymers (7, 8, 9).
In addition to β-glucans, the fungal cell wall contains chitin, a polysaccharide consisting of linear β-1,4-N-acetyl-glucosamine, which is intimately interconnected with β-glucans, galactomannans and mannoproteins to form the structural foundation of the hyphal cell wall (10). Recently, chitin has been identified as a recognition element capable of initiating innate immune responses associated with allergy and asthma (11), but whether chitin induces immunologic activity as a constituent of natural environments or organisms relevant to these diseases is unknown. Here, we assess the presence of chitin in house dust samples from asthmatic individuals and characterize its role in the innate immune response to an environmentally-derived fungal preparation.
MATERIALS AND METHODS
Mice
BALB/c IL-4 reporter (4get) and 4get x SPAM mice(11, 12), 8–12 weeks of age, were maintained under specific pathogen-free conditions and used in compliance with polices and procedures approved by the UCSF Institutional Animal Care and Use Committee.
House dust collection / fungal preparation
Settled house dust used for chitin, endotoxin, and β-1,3-glucan analyses was collected from subjects’ living rooms with a Shark vacuum cleaner (Euro-Pro Turbo Hand Vacuum, Model EP033). Air was drawn (250–550 L/min) through a pre-filter that removed large particles measuring 356 microns or more, and a final filter, which retained small particles. After one minute, excess dust was removed from the pre-filter. This process was repeated 3 more times (4 minutes total). Samples were collected similarly for dust mite and cockroach antigen analysis, but included 4 additional minutes of living room sampling and 4 minutes of kitchen sampling. Dust samples used for chitin, endotoxin and β-1,3-glucan analyses were stored at −20ºC, and those used for dust mite and cockroach antigen assays were stored at 4ºC.
Hyphae from a single Aspergillus niger isolate derived from house dust(3)(Asp) were lyophilized, ground with mortar and pestle, lyophilized again, sterilized by cesium irradiation, and reconstituted in phosphate-buffered saline (PBS). The resulting suspension was sonicated and incubated at 65ºC for 30 minutes to inactivate endogenous proteinases, or maintained on ice for experiments examining the effect of heat treatment. For enzyme treatments, Asp (1 mg) was incubated at room temperature in PBS containing chitinases (20U B. malayi, New England Biolabs, NEB; 0.167U S. marcescens, Sigma), or recombinant β-1,3-glucanase (200U Quantazyme, MP Biomedicals), separately or in combination, in a total volume of 100 μl for 16 hours with agitation, followed by heat-inactivation at 65ºC for 30 minutes. Control Asp preparations contained equivalent amounts of heat-inactivated chitinases and/or β-glucanase.
Analysis of house dust and fungal samples
Detection of chitin was performed by dot-blotting 1 μl of sonicated Asp or house dust (1 mg/ml) onto nitrocellulose membranes (GE Healthcare). Crab shell chitin (Sigma) and curdlan (Wako) were used as positive controls. The membranes were dried at room temperature, rinsed in tris-buffered saline containing 0.05% Tween-20 (TBST), blocked with 5% bovine serum albumin (BSA) in TBST, and incubated with FITC-chitin-binding probe (CBD; NEB) in 1% BSA/TBST for 16 hours at 4ºC with gentle rocking. After washing with TBST, the membranes were incubated with HRP-anti-fluorescein (Invitrogen) for 45 minutes at room temperature, washed and developed with ECL (GE Healthcare). Densitometry was performed using Adobe Photoshop (Adobe Systems Inc.). Detection of β-1,3-glucans in Asp was performed similarly, except that monoclonal β-1,3-glucan antibody (Biosupplies Ltd.) was substituted for FITC-CBD, and HRP-anti-mouse IgG (BioRad) was used instead of HRP-anti-fluorescein.
Sonicated, heat-treated dust samples were extracted with 0.05% Tween-20 in PBS; supernatants were assayed for β-1,3-glucans by ELISA, using recombinant mouse Dectin-1 (R&D Systems) as a capture reagent and β-1,3-glucan antibody (Biosupplies) for detection. Endotoxin levels were determined by kinetic LAL assay with Glucashield buffer (Associates of Cape Cod, Inc.). Dust mite (Der p 1, Der f 1) and cockroach (Bla g 2) antigens were extracted and quantified by a commercial laboratory using standard ELISA technology (WM Labs, San Mateo CA).
Enzyme assays
Proteinase activity was measured by production of fluorescence upon cleavage of a FITC-casein substrate (Sigma), as compared to trypsin control after 1 or 24 hours of incubation at 37ºC. Some samples were heat-treated at 65ºC for 30 minutes prior to the assay. Chitinase assays were performed using 4-nitrophenyl-N,N′-diacetyl-β-chitobioside as a substrate per the manufacturer’s protocol (Sigma).
Intranasal challenges
Mice were anesthetized with isofluorane before aspirating 50 μl Asp (1 mg/ml) or PBS into the nostrils on two consecutive days, followed by euthanasia and analysis 24 hours after the second dose. Allosamidin (2 μg/40 μl PBS; Industrial Research Ltd.) was administered intranasally in some experiments 1 hour prior to Asp and subsequently daily for 10 days after the initial dose. In other experiments, intranasal Asp was administered 3 times per week for 3 weeks, with analysis 48 hours after the final dose. For ovalbumin challenge (OVA), mice were injected intraperitoneally with either 50 μg OVA emulsified in 1 mg alum or PBS on days 0, 7 and 14. On days 21, 22, and 23, anesthetized mice were instilled intranasally with 100 μg OVA or PBS; euthanasia and analysis followed on day 24.
Flow cytometry
Minced lung lobes were dispersed through 70-μm nylon filters, washed, and resuspended in PBS/2% fetal calf serum, then incubated with PE-anti-Siglec-F, APC-anti-DX5, PerCP-Cy5.5-anti-Gr-1 and APC-AlexaFluor-750-anti-CD4 (BD Biosciences). DAPI exclusion identified live cells, which were enumerated with counting beads (Invitrogen). Sample data were acquired with a LSRII (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.).
Airway responsiveness / bronchoalveolar lavage
Mice were anesthetized, tracheae cannulated and attached to flexiVent (SCIREQ), then paralyzed with pancuronium (0.1 mg/kg) and ventilated at a tidal volume of 9 ml/kg, 150 breaths/minute, and 2 cm H2O positive end-expiratory pressure. Measurements used the linear single compartment model, and acetylcholine was administered via tail vein. Lungs were lavaged with 3 × 1 ml PBS. After centrifugation and red blood cell lysis, cells were resuspended in saline and counted via hemacytometer. Cytospin preparations were stained with HEMA 3 (Fisher), and differential percentages were determined upon evaluation of >300 cells/slide.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad, Inc.) and SAS (Cary, NC).
RESULTS
Chitin is a common constituent of house dust samples associated with asthma and levels correlate with β-1,3-glucans and lung eosinophilia
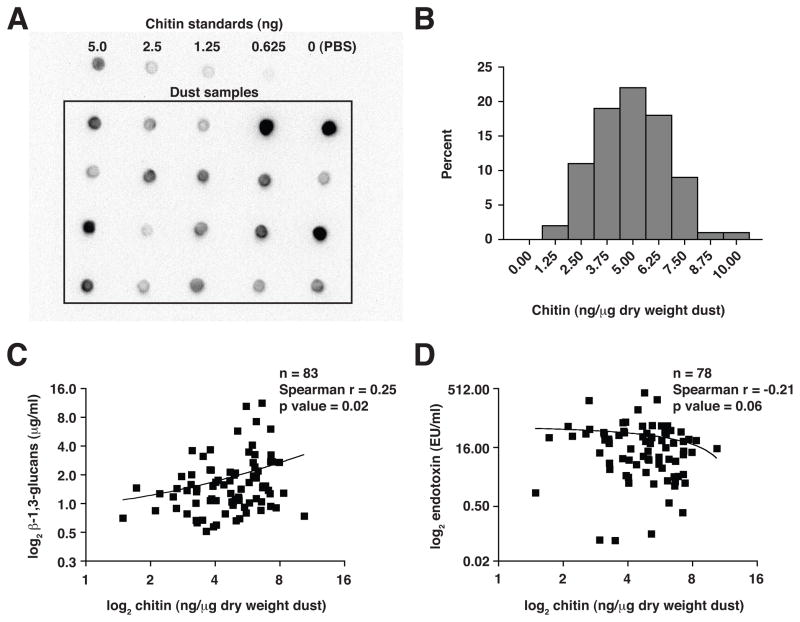
In marine environments, massive amounts of chitin derived from phytoplankton and crustaceans are efficiently catabolized by chitinolytic bacteria(13); however, the distribution of chitin and its turnover within terrestrial environments is unclear. Chitin can be found in arthropods and fungi, which not only represent a source of recycled biomass, but also are significant elements of indoor environments linked to the triggering of asthma and allergic diseases. Thus, we assayed for the presence of chitin in settled dust samples collected from the homes of 83 individuals who are part of an ongoing cohort study of adults with physician-diagnosed asthma and/or rhinitis. Chitin was detected using a high-affinity probe with specificity for intact chitin(14), and concentrations were determined by comparison to known standards (Fig. 1A).
Figure 1. Chitin is a common constituent of house dust samples associated with asthma.
(A) Blot images of house dust samples and chitin standards probed with chitin-binding domain protein. (B) Frequency distribution of chitin content in 83 house dust samples quantified by densitometry. Relationships between chitin and (C) β-1,3-glucan or (D) endotoxin levels in house dust.
Chitin was detected in all dust samples, with a mean concentration of 4.95±1.7 ng/μg dust (dry weight), ranging from 1.49 to 10.40 ng/μg dust (Fig. 1B). Chitin levels were significantly higher in the homes with detectable dust mite (Der p 1, Der f 1) or cockroach (Bla g 1) antigen as compared to the remainder (Wilcoxon rank-sum p=0.016), and chitin concentrations positively correlated with dust mite antigen concentrations (Spearman r=0.31; p=0.004). Either dust mite or cockroach antigen was detected in only 44 (53%) of 83 samples, however, suggesting the presence of a more widespread source of chitin. In this respect, we observed the ubiquitous presence of fungal-associated β-1,3-glucans, levels of which also correlated significantly with chitin (Spearman r=0.25; p=0.02; Fig. 1C). In contrast, endotoxin levels in dust were inversely correlated with chitin, albeit not significantly so (Spearman r=−0.21; p=0.06; Fig. 1D), possibly reflecting activity of chitinolytic bacteria. Nevertheless, these findings implicated ubiquitous fungi as a widespread source of environmental chitin, consistent with observations that fungi also represent the main source of active proteinases in household dust(3).
We tested the in vivo effects of select dust samples that were chosen to maximize divergent relative concentrations of chitin and endotoxin by administering them intranasally to mice. Chitin concentrations in dust were significantly related to percentages of eosinophils recruited to the lung, whereas endotoxin was not (Table I). Total lung cell counts, which included both eosinophil and other cell recruitment, were strongly related to endotoxin, with chitin manifesting this association as well.
Table 1.
Lung cellular constituents following intranasal administration of household dust samples containing both chitin and endotoxin.
| Percent Eosinophils Mean (range) 0.4% (0.1–1.1%) p value |
Eosinophils/μL Mean (range) 9.1 (0.1–17.7) p value |
Total cells Mean (range) 7.9 X106 (1.8–15.5X106) p value |
|
|---|---|---|---|
| Chitin (ng/μg) | 0.018 | 0.013 | 0.016 |
| Endotoxin (EU/ml) | 0.40 | 0.044 | 0.004 |
P values for mixed multiple linear regression models including chitin and endotoxin, exposure sample treated as a random effects variable (4 dust samples tested in 2 animals; 4 tested solely; 2 PBS controls). All variables log transformed.
Insoluble fungal cell wall components induce recruitment of innate effector cells, independent of proteinase activity
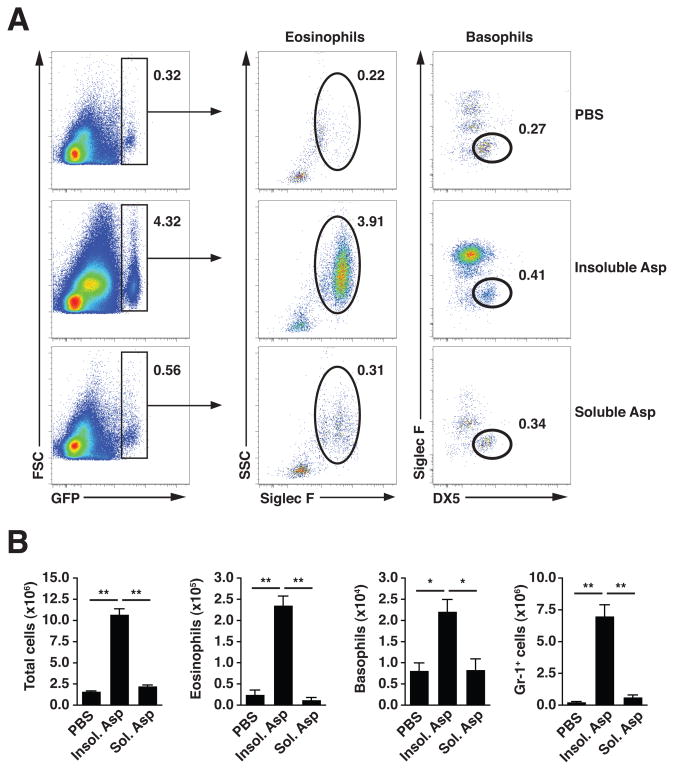
We subsequently challenged mice with a preparation of whole Aspergillus niger hyphae (Asp) derived from an environmental house dust isolate(3). As fungal antigenic extracts are often subjected to removal of insoluble cell wall elements, we compared the in vivo immunostimulatory potential present in different fractions prepared from Asp. One day after 2 consecutive challenges with the insoluble fraction, a substantial population of GFP-expressing cells, comprised mainly of eosinophils and basophils, was recruited to the lungs of IL-4 reporter mice (4get; 12), whereas the soluble fraction failed to elicit an appreciable response as compared to saline (Fig. 2A). This cell infiltrate consisted of significant increases in total numbers of lung tissue eosinophils and basophils, and was accompanied by an influx of Gr-1+ cells with the characteristics of neutrophils and monocytes (Fig. 2B; and data not shown). This innate response to insoluble hyphal fungal fragments, which, along with conidia, can become airborne and inhaled in the indoor environment (15), suggested a key role for exposed cell wall elements, consistent with prior studies demonstrating the requirement for conidial swelling or subsequent hyphal branching to induce innate immune cell recruitment (3, 4, 7).
Figure 2. Intranasal challenge with insoluble components of fungal preparation induces accumulation of innate effector cells.
(A) Flow cytometric analysis of GFP-expressing lung eosinophils (GFP+SiglecF+SSC+) and basophils (GFP+SiglecF−DX5+) in 4get mouse lung tissue 1 day after 2 intranasal challenges with soluble or insoluble fractions from A. niger preparation (Asp). Numbers indicate the percentage of total live cells (DAPI−, not shown), and are representative of 3 independent experiments. (B) Total live cell numbers, with eosinophil and basophil subsets calculated from flow cytometry percentages as shown in (A), or percentage Gr-1+ cells (not shown). Mean±SEM, n=3/group; *p<0.01, **p<0.001, unpaired t-test.
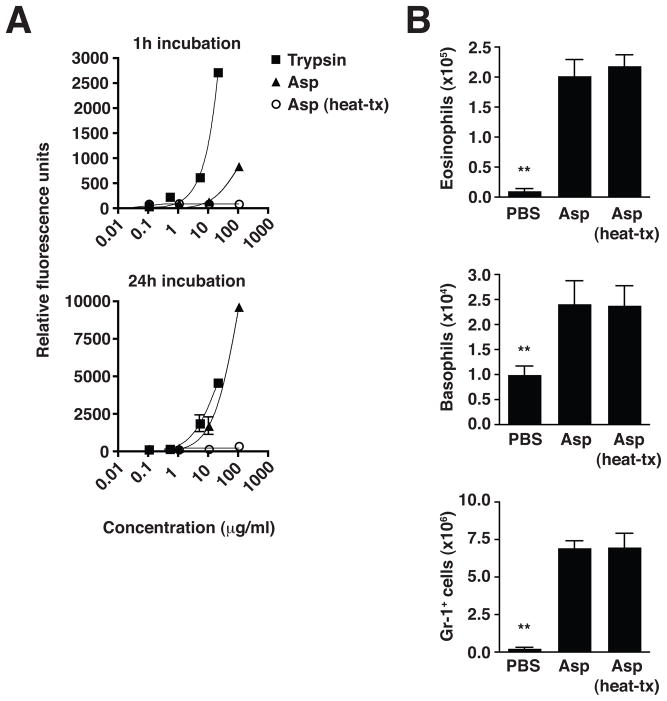
Proteinase allergens have been proposed as initiators of basophil-mediated Th2 immune responses (16) and fungal-derived proteinases have been implicated as adjuvants in allergic inflammatory responses (17). We tested whether intrinsic proteinase activity was responsible for the Asp-induced innate effector cell recruitment into the lungs of 4get mice. The Asp preparation contained significant proteinase activity, as measured by cleavage of a sensitive casein-FITC substrate, which could be completely abolished by heat-treatment (Fig. 3A). This proteinase activity, however, was dispensable for the recruitment of innate effector cells, including eosinophils, basophils and neutrophils/monocytes, as both Asp and heat-treated Asp induced equivalent recruitment of these cells into the lungs (Fig. 3B). Thus, elements of the insoluble fungal cell wall trigger activation of innate immunity via pathways that are not dependent on associated proteinase activity.
Figure 3. Heat-sensitive proteinase activity in fungal preparation is not required for innate effector cell accumulation.
(A) Proteinase activity in A. niger preparation (Asp), with or without heat treatment (heat-tx), compared to trypsin control. Fluorescence produced upon cleavage of FITC-casein substrate was measured after indicated incubation times. (B) Numbers of total live eosinophils, basophils, and Gr-1+ neutrophils/monocytes in the lung 1 day after 2 intranasal Asp challenges, with or without prior heat treatment. Total live cell subset numbers were calculated from flow cytometry percentages as described in Figure 2. Mean±SEM, n=3/group; **p<0.001, significantly different from both Asp and Asp (heat-tx) groups, unpaired t-test.
Degradation of fungal cell wall polysaccharides attenuates eosinophilia
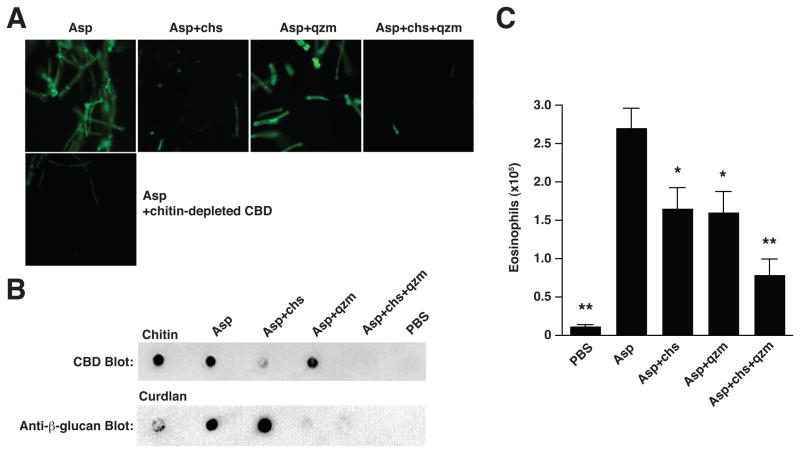
Chitin and β-glucans are major polysaccharides present in the alkali-insoluble fraction of Aspergillus fumigatus hyphae, where they form the structural basis underlying cell wall integrity (10). We verified the presence of chitin in the Asp cell wall preparation, most prominently in septae and hyphal tips (Fig. 4A). This chitin component was susceptible to partial degradation by chitinase, and was digested further by combined chitinase/β-glucanase treatment, likely resulting from disruption of chitin and β-1,3-glucan inter-strand linkages(10). In contrast, the chitin component remained intact after treatment with β-glucanase alone, whereas β-1,3-glucans were digested to nearly undetectable levels, regardless of additional chitinase treatment (Fig. 4A, B).
Figure 4. Fungal-induced eosinophil accumulation is attenuated by enzymatic degradation of chitin and β-glucans.
(A) Reactivity of FITC-conjugated chitin-binding domain (CBD) with A. niger preparation (Asp) before and after digestion with chitinase (chs) and/or β-glucanase (Quantazyme; qzm). Lower panel, Asp reactivity with CBD-FITC that was pre-incubated with chitin. Magnification, 20x. (B) Dot-blot assay indicating the presence of chitin (top) or β-glucans (bottom) before and after enzymatic treatment. (C) Numbers of total lung eosinophils 1 day after 2 intranasal Asp challenges, with or without prior enzymatic treatment as indicated. Total live eosinophil numbers were calculated from flow cytometry percentages as described in Figure 2. Mean±SEM, n=8–10/group; *p<0.01, **p<0.001, significantly different from Asp, unpaired t-test.
We next tested the ability of these polysaccharide-depleted Asp preparations to stimulate in vivo immune responses. After intranasal administration, we observed significant reductions in the number of lung tissue eosinophils in 4get mice treated with polysaccharide-depleted Asp preparations as compared to mice treated with Asp containing intact chitin and β-glucans; specifically, chitinase or β-glucanase treatment alone resulted in approximately 40% fewer eosinophils, and a 71% reduction was observed in response to Asp digested with both enzymes (Fig. 4C). Interestingly, the numbers of other cell types induced by Asp treatment, such as basophils and neutrophils/monocytes, were not significantly altered by polysaccharide depletion but remained elevated (Supplemental Fig. 1), indicating that additional chitinase- and β-glucanase-resistant immunostimulatory elements recruit these cell types by mechanisms not involved in eosinophil influx. A reproducible decrease, albeit not statistically significant, in the number of lung neutrophils/monocytes, however, was noted in response to β-glucan digestion, possibly reflecting diminished activation of dectin-mediated β-glucan recognition pathways (Supplemental Fig. 1).
Chitinase overexpression attenuates the magnitude of eosinophil influx
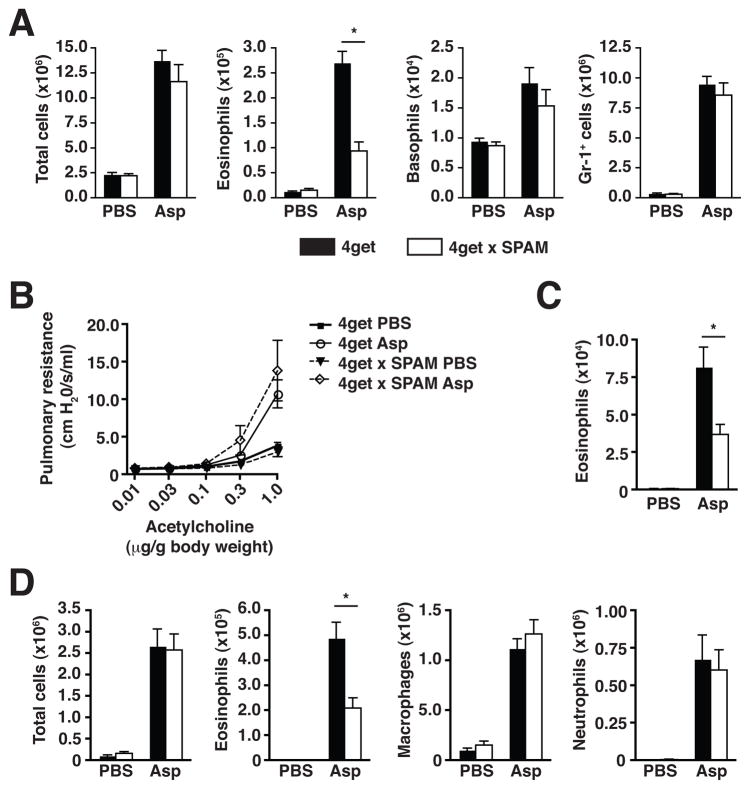
The contribution of chitin was examined more specifically using 4get x SPAM mice, which express elevated levels of acidic mammalian chitinase (AMCase) in the lungs and thus possess enhanced ability to degrade chitin in vivo (11). One day after consecutive intranasal doses of Asp, lung tissue eosinophils in 4get x SPAM mice were significantly diminished in comparison to wild-type 4get littermates, whereas basophil and neutrophil/monocyte numbers remained unaltered (Fig. 5A), consistent with polysaccharide depletion experiments (Fig. 4C), and suggesting a specific link between eosinophil recruitment and chitin content in the fungal cell wall.
Figure 5. Constitutive pulmonary expression of acidic mammalian chitinase attenuates fungal-induced accumulation of eosinophils.
(A) Numbers of total live cells, eosinophils, basophils, and Gr-1+ neutrophils/monocytes in the lungs of 4get x SPAM or wild-type 4get mice 1 day after 2 intranasal challenges with A. niger preparation (Asp). Total live cell subset numbers were calculated from flow cytometry percentages as described in Figure 2. 4get x SPAM or wild-type 4get mice exposed to an extended intranasal Asp dosing regimen (3 doses/week for 3 weeks) were assessed for (B) airway hyperresponsiveness, (C) left lung lobe tissue eosinophil numbers, and (D) numbers of total cells, eosinophils, macrophages, and neutrophils in bronchoalveolar lavage fluid. Mean±SEM, n=6–10/group; *p<0.01, unpaired t-test.
We further tested the effects of AMCase overexpression using a model of extended challenge with the fungal cell wall constituents to induce an asthma-like allergic lung disease in mice. Intranasal doses of Asp were administered 3 times per week for 3 consecutive weeks, followed by tissue collection and analysis 48 hours after the final dose. This regimen was sufficient to induce significant airway hyperresponsiveness in Asp-treated 4get mice as compared to PBS-treated controls, although there was no difference in the Asp-induced pulmonary resistance between wild-type 4get and 4get x SPAM mice (Fig. 5B). In contrast, the level of eosinophilia was significantly lower in the 4get x SPAM mice in both lung tissue (Fig. 5C) and bronchoalveolar lavage (BAL), while the levels of other inflammatory cell types remained unaltered as compared to wild-type control mice (Fig. 5D, and data not shown), in agreement with the results from the short-term exposures and polysaccharide depletion experiments (Fig. 4C and Supplemental Fig. 1). Notably, a substantial CD4 T cell response developed in response to the extended Asp exposure, and, although not quite statistically significant, was negatively influenced by AMCase overexpression (Supplemental Fig. 2). Further, there were no marked differences in serum IgE levels or epithelial goblet cell hyperplasia (Supplemental Fig. 2 and data not shown). To test whether the defect in eosinophil recruitment after Asp challenge was related to its chitin content, 4get x SPAM mice were challenged with a non-chitin-containing protein, ovalbumin, in a well-characterized model of allergic lung disease; under these circumstances, the magnitude of eosinophil recruitment to the lungs was identical to that in wild-type 4get mice, suggesting that the AMCase-mediated defect in eosinophil recruitment observed in response to fungal challenge was dependent on the presence of chitin (Supplemental Fig. 3).
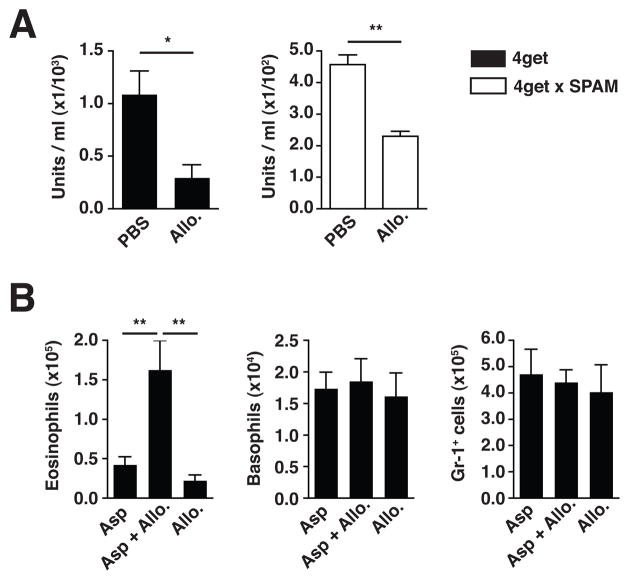
Chitinase inhibition in vivo prolongs eosinophil infiltration after fungal challenge
To test whether this process could also be reciprocally regulated, we conducted in vivo inhibition experiments. Allosamidin, a pseudotrisaccharide inhibitor of family 18 chitinases, was administered to wild-type 4get and 4get x SPAM mice, resulting in significantly reduced chitinase activity in the BAL (Fig. 6A). Strikingly, when this inhibitor was co-administered during and after Asp challenge, eosinophil numbers in the lungs remained significantly increased 10 days after initial challenge, when eosinophil numbers had returned to near-baseline levels in the absence of allosamidin (Fig. 6B). These results were consistent with the effects observed in response to increased chitinase activity (Figs. 4C, 5A, C, D), and indicated that chitinase blockade resulted in an inability to diminish the chitin-containing fungal stimulus and associated eosinophilia. This finding contrasts with the prior report that allosamidin administration during ovalbumin challenge suppressed pulmonary eosinophilia(18), suggesting that some eosinophilic responses formed in the absence of a chitin-containing organism are induced by distinct physiologic pathways, although the exact function of chitinases in such settings is unclear. Nevertheless, these data indicate that when chitin is present within a relevant environmentally-derived organism, mammalian chitinases act to restrict the magnitude and duration of eosinophil influx into the tissue effector site.
Figure 6. Chitinase inhibition in vivo prolongs eosinophil infiltration after fungal challenge.
(A) Chitinase activity present in BAL from mice treated with a single dose of PBS or allosamidin (Allo). (B) Total eosinophil, basophil, and Gr-1+ neutrophil/monocyte numbers in lung tissue from mice treated daily with PBS or allosamidin 10 days after receiving A. niger hyphal preparation (Asp) intranasally. Total cell numbers were calculated from flow cytometry percentages as described in Figure 2. Mean±SEM, n=5–9/group; *p<0.05, **p<0.01, unpaired t-test.
DISCUSSION
Recent work demonstrating that purified chitin induces eosinophilic infiltration of tissues, along with the increasing realization of the varied asthma-associated inflammatory components and potential exacerbating role of fungal exposure in asthma severity, led us to examine whether chitin is present in home environments associated with asthma and, in the context of a relevant biologic organism, whether environmentally-derived chitin possesses an innate eosinophil-recruiting capacity in vivo. Here, we provide evidence that chitin is widespread in house dust (Fig. 1), with concentrations that correlated with fungal-associated β-1,3-glucans and tissue eosinophilia. Further, a fungal preparation isolated from house dust induced lung eosinophilia in a manner dependent on accompanying chitinase activity, thereby supporting a model in which AMCase plays a protective role in the immune response to environmental fungal challenge by degrading cell wall chitin and consequently removing an ongoing stimulus for eosinophil recruitment and/or retention.
As such, in vitro and in vivo modulation of fungal chitin levels or chitinase activity had a direct influence on the magnitude of eosinophil influx at the pulmonary effector site, contrasting with consistently elevated levels of recruited basophils and neutrophils/monocytes (Figs. 4–6). Intriguingly, similar effects were observed upon depletion of β-glucans from Asp, yielding a maximal decrease in eosinophilia when both polysaccharides were depleted (Fig. 4C). In this context, targeting fungal cell wall β-glucans delayed mortality in a mouse model of invasive pulmonary aspergillosis, but did not afford complete protection(19), suggesting an additional potential benefit by targeting chitin. Addition of exogenous AMCase can inhibit fungal growth in vitro (20), and may also represent a viable means to attack fungal cell wall chitin in vivo, along with chitin synthase inhibitors, which have shown in vitro fungicidal activity, particularly in combination with inhibitors of β-glucan synthases(21).
The relationship between chitinases and fungal exposure in humans has not been fully explored, but persistent fungal burden in the airways, which is elevated in conditions such as asthma in allergic bronchopulmonary aspergillosis, may directly induce ongoing neutrophil and eosinophil recruitment. Targeting eosinophils has proven efficacious in some patients with severe asthma(22). Eosinophils, frequently associated with chronic allergic conditions and asthma, can kill fungi (3, 23) and are recruited in response to chitin by a mechanism dependent on the high-affinity leukotriene B4 receptor, BLT1(11). Several molecular receptors have been implicated in the recognition of A. fumigatus, including dectin-1 (7, 8, 9), pentraxin-3 (24), and TLRs (25); however, receptors that bind fungal chitin have yet to be defined. Notably, binding of Aspergillus conidia to alveolar macrophages can be inhibited in vitro by N-acetylglucosamine or chitotriose(26), suggesting the existence of chitin recognition elements in the conidial cell wall as well as the expression of chitin-specific binding receptors on alveolar macrophages. Moreover, polymorphisms in human AMCase have been associated with asthma risk(27, 28), and a haplotype encoding an isoform of AMCase with heightened enzyme activity was associated with protection from asthma in humans(29). Thus, modulation of chitin recognition or content may have dual benefits, by both attenuating potential for invasive disease and reducing allergic inflammation.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (AI030663, AI077439, R.M.L.; R01 ES10906, P.J.Q. and P.D.B.) HHMI, and the SABRE Center at UCSF
We thank N. Flores and M. Consengco for animal care, and Miriam Cisternas for statistical assistance.
References
- 1.Holgate ST, Davies DE. Rethinking the pathogenesis of asthma. Immunity. 2009;31:362–367. doi: 10.1016/j.immuni.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Frohlich-Nowoisky J, Pickersgill DA, Despres VR, Poschl U. High diversity of fungi in air particulate matter. Proc Natl Acad Sci U S A. 2009;106:12814–12819. doi: 10.1073/pnas.0811003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, Delclos GL, Abramson S, Kheradmand F, Corry DB. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 5.Philippe B, Ibrahim-Granet O, Prevost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latge JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol. 2006;176:3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem. 2000;275:27594–27607. doi: 10.1074/jbc.M909975199. [DOI] [PubMed] [Google Scholar]
- 11.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 13.Keyhani NO, Roseman S. Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta. 1999;1473:108–122. doi: 10.1016/s0304-4165(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Ito Y, Yamada T, Hashimoto M, Sekine S, Tanaka H. The roles of the C-terminal domain and type III domains of chitinase A1 from Bacillus circulans WL-12 in chitin degradation. J Bacteriol. 1994;176:4465–4472. doi: 10.1128/jb.176.15.4465-4472.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, Grinshpun SA. Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol. 2002;68:3522–3531. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 19.Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52:1171–1172. doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Shen Z, Wu J. Expression, purification and in vitro antifungal activity of acidic mammalian chitinase against Candida albicans, Aspergillus fumigatus and Trichophyton rubrum strains. Clin Exp Dermatol. 2009;34:55–60. doi: 10.1111/j.1365-2230.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 21.Ganesan LT, Manavathu EK, Cutright JL, Alangaden GJ, Chandrasekar PH. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin Microbiol Infect. 2004;10:961–966. doi: 10.1111/j.1469-0691.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 22.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 25.Chignard M, Balloy V, Sallenave JM, Si-Tahar M. Role of Toll-like receptors in lung innate defense against invasive aspergillosis. Distinct impact in immunocompetent and immunocompromized hosts. Clin Immunol. 2007;124:238–243. doi: 10.1016/j.clim.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Kan VL, Bennett JE. Lectin-like attachment sites on murine pulmonary alveolar macrophages bind Aspergillus fumigatus conidia. J Infect Dis. 1988;158:407–414. doi: 10.1093/infdis/158.2.407. [DOI] [PubMed] [Google Scholar]
- 27.Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, Superti-Furga A, Heinzmann A. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005;172:1505–1509. doi: 10.1164/rccm.200506-890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee R, Batra J, Das S, Sharma SK, Ghosh B. Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J Allergy Clin Immunol. 2008;122:202–208. 208, e201–207. doi: 10.1016/j.jaci.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Seibold MA, Reese TA, Choudhry S, Salam MT, Beckman K, Eng C, Atakilit A, Meade K, Lenoir M, Watson HG, Thyne S, Kumar R, Weiss KB, Grammer LC, Avila P, Schleimer RP, Fahy JV, Rodriguez-Santana J, Rodriguez-Cintron W, Boot RG, Sheppard D, Gilliland FD, Locksley RM, Burchard EG. Differential enzymatic activity of common haplotypic versions of the human acidic Mammalian chitinase protein. J Biol Chem. 2009;284:19650–19658. doi: 10.1074/jbc.M109.012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.