Abstract
Background
Cystatin C has been proposed as an alternative marker of kidney function among HIV-infected persons in whom serum creatinine is affected by extra-renal factors.
Methods
In this cross-sectional study, we compared estimated glomerular filtration rates (eGFR) using serum creatinine versus cystatin C between 150 HIV-uninfected and 783 HIV-infected men. We evaluated the prevalence of chronic kidney disease (CKD; eGFR<60 mL/min/1.73 m2) and examined the influence of extra-renal factors on GFR-estimates among HIV-infected men.
Results
Estimated GFRSCR was similar by HIV serostatus, but eGFRCYSC was lower in HIV-infected men. A higher proportion of HIV-infected men were classified as having CKD when using eGFRCYSC versus eGFRSCR (7% vs. 5%, P<0.01). In HIV-infected individuals without CKD, eGFRSCR was higher than eGFRCYSC while it was lower than eGFRCYSC in persons with CKD. In HIV-infected men, older age, proteinuria, and prior clinical AIDS were inversely associated with both GFR-estimates. Higher serum albumin levels and ACE-inhibitor/ARB use were associated with lower eGFRSCR. HIV viral load, hepatitis C co-infection, and serum alkaline phosphatase were inversely associated with eGFRCYSC.
Conclusion
Among HIV-uninfected and HIV-infected men of similar social risk behaviors, GFR estimates differed by biomarker and kidney function level. Estimated GFRCYSC classified a larger proportion of HIV-infected men with CKD compared to eGFRSCR. Differences between these GFR-estimating methods may be due to the effects of extra-renal factors on serum creatinine and cystatin C. Until GFR-estimating equations are validated among HIV-infected individuals, current GFR estimates based on these biomarkers should be interpreted with care in this patient population.
Keywords: HIV, kidney disease, serum creatinine, cystatin C, glomerular filtration rate, Multicenter AIDS Cohort Study
INTRODUCTION
Since the introduction of highly active antiretroviral therapy (HAART) in 1996, a growing number of HIV-infected individuals are now developing non immunodeficiency related chronic medical illnesses such as chronic kidney disease (CKD) (1). In the context of HIV infection, CKD develops in response to several, often simultaneous risk factors including genetic predisposition (2,3), severity of HIV infection,(4) hepatitis C virus co-infection (5), HAART-related metabolic changes (6–8), and aging (9). As a result, nearly one-third of HIV-infected individuals have renal abnormalities (10).
Among HIV-infected persons, impaired kidney function is associated with faster progression to AIDS and death (11,12), and similar to the general population, severity of kidney dysfunction is positively associated with cardiovascular disease and mortality risk. HIV-infected persons who have estimated glomerular filtration rates (eGFRs) <15 mL/min/1.73 m2 experience a 6-fold greater risk of developing cardiovascular disease (13) and death compared to persons with eGFRs ≥60 mL/min/1.73 m2 (12).
Given these adverse associations, current guidelines recommend that all HIV-infected persons undergo screening for CKD by serum creatinine-based eGFR (eGFRSCR) (14). However, serum creatinine-based eGFRs have not been rigorously validated among HIV-infected persons and are limited by their propensity to either under- or over-estimate true GFR across the full range of kidney function. These estimates are also particularly biased in HIV-infected individuals due to abnormalities in muscle mass and nutrition (15,16), liver disease (17), and exposure to several medications that alter urinary creatinine handling or serum creatinine measurement (18,19). Therefore, interest has focused on an alternative indicator of kidney function, serum cystatin C. Cystatin C is produced constantly by almost all human cells, is freely filtered by renal glomeruli and nearly completely metabolized by renal tubules. In addition, serum cystatin C is generally thought to be unaffected by lean muscle mass and thus a more accurate marker of kidney disease (20). Recent studies conducted in the general population, however, demonstrate that serum cystatin C is also dependent on age, sex, and race like creatinine (21). To account for these extra-renal influences, the CKD-Epidemiology Collaboration developed a cystatin C-based eGFR equation adjusted for age, sex, and race (22).
Identification of impaired kidney function in the HIV-infected population often mandates changes to antiretroviral dosing, work-up for the etiology of kidney dysfunction, and initiation of CKD management strategies. Therefore, understanding additional factors which alter serum creatinine and cystatin C and subsequently the estimates of kidney function is necessary for the clinical interpretation of GFR estimates in this high-risk population. In this study, we compared serum creatinine and cystatin C-based estimates of kidney function among HIV-uninfected and HIV-infected men with similar lifestyle and risk behaviors in the Multicenter AIDS Cohort Study (MACS). In addition, we sought to compare the prevalence of CKD among HIV-infected participants using the serum creatinine versus cystatin C-based estimates of GFR and evaluate the impact of extra-renal factors on these GFR estimates.
METHODS
Study Population
The MACS is an ongoing prospective cohort study of HIV-uninfected and HIV-infected men in four U.S. urban areas (23). A total of 6,972 men have been enrolled. Participants underwent semi-annual standardized interviews, physical examinations, and collection of blood and urine for laboratory testing and storage in repositories The Institutional Review Board of each center approved the study protocols.
Our investigation was a cross-sectional study of HIV-uninfected and HIV-infected men. All participants had to have serum creatinine and urine protein excretion measured and stored serum available for cystatin C measurements at visits 45 (n=194) or 46 (n=739), corresponding with the calendar periods of March 20, 2006 to November 3, 2006 and September 12, 2006 to April 14 2007, respectively. HIV-uninfected were chosen randomly among those who had the above three components. A total of 150 HIV-uninfected and 783 HIV-infected men were included. Our sub-study was approved by the Johns Hopkins Institutional Review Board.
Estimation of Kidney Function
To standardize serum creatinine values which were previously measured by the modified Jaffe method across study sites, we re-measured serum creatinine on frozen samples in a separate, representative subset of 150 participants from corresponding visits. Secondary serum creatinine was analyzed enzymatically on the Roche ModP instrument (Roche Diagnostics Corp, Indianapolis, IN) at the University of Minnesota (Minneapolis, MN) in conjunction with National Institute of Standards and Technology SRM 967 reference materials traceable to isotope dilution mass spectrometry analysis. The original values were calibrated to standardized serum creatinine using errors-in-variables regression. The original serum creatinine values highly correlated with standardized values (r=0.96). Calibrated serum creatinine was used for this study. Serum cystatin C was measured on frozen samples using an automated particle-enhanced nephelometric assay (N Latex Cystatin C, Dade Behring, Deerfield, IL) run on the Dade Behring BN 100 Nephelometer. Serum creatinine-based eGFR was calculated using the CKD-EPI equation which adjusts for age and race according to the serum creatinine level (24). Serum cystatin C-based eGFR was calculated using the equation developed by the CKD-Epidemiology Collaboration (eGFRCYSC=127.7 × serum cystatin C[mg/L]−1.17 × age[y]−0.13 ×1.06 if black) (22). An eGFR <60 mL/min/1.73m2 was used to define CKD (25). Urine protein and creatinine were measured from untimed samples, and their ratio was used to estimate proteinuria. A ratio >200 mg/g defined proteinuria (25).
Extra-Renal Factors
Age, race, and history of injection drug use were self-reported. Interviews included questions regarding AIDS-defining illnesses and were confirmed through medical record review. Weight was measured using a standardized protocol and calibrated scales. Body mass index (BMI) was calculated from weight and height measurements. Diabetes mellitus was defined as a fasting glucose concentration ≥126 mg/dL or a self-reported diabetes diagnosis with self-reported use of anti-diabetic medication. Hypertension was defined by a measured systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg, or self-reported hypertension diagnosis with self-reported use of anti-hypertensive medication. Dyslipidemia was defined as a fasting total cholesterol >200 mg/dL, triglyceride >150 mg/dL, high-density lipoprotein <50 mg/dL, or self-reported use of lipid-lowering medication.
HIV infection was determined by positive serum antibody tests. Use of antiretroviral drugs, co-trimoxazole, angiotensin-converting enzyme inhibitor (ACEi), and angiotensin receptor blocker (ARB) were assessed at each visit along with medications taken since the last visit. Antiretroviral drugs were subdivided into three classes: 1) nucleoside/nucleotide reverse transcriptase inhibitors (NRTI); 2) non-nucleoside reverse transcriptase inhibitors (NNRTI); and 3) protease inhibitors (PI). The definition of HAART was guided by the U.S. Department of Health and Human Services guidelines (26). The duration of HAART exposure was calculated for individuals exposed to HAART.
CD4+ cell counts were measured using standardized flow cytometry. Plasma HIV-1 RNA levels were measured using an ultrasensitive RNA polymerase chain reaction assay. Hepatitis C virus infection was based on a positive hepatitis C virus antibody serostatus and a detectable RNA level. Serum albumin levels were measured using a kinetic colorometric method while liver enzymes were measured enzymatically. CRP levels were measured using a high-sensitivity nephelometric assay, and were carried forward from the prior visits over a median period of 1.4 years (interquartile range [IQR]: 1.2 – 1.5 years) if they were missing (n=164).
Statistical methods
Statistical analyses were performed using Stata/MP 11 (StataCorp LP, College Station, TX). Variables with skewed distributions were log10-transformed. Variables were compared using Student’s t-test or Wilcoxon rank-sum test and chi-square or Fisher exact test, as appropriate. Bland-Altman plots depicting the average of log10-eGFRSCR (X) and log10-eGFRCYSC (Y) versus their difference were used to evaluate the agreement between the two methods among HIV-infected men. We calculated the slope and intercept of the linear regression of the difference (X−Y) on the average of the two methods ([X+Y]/2) to evaluate the ratio of the standard deviations (SD) of X and Y and assess bias, respectively (27). The analyses were stratified by eGFRSCR <60 and ≥60 mL/min/1.73 m2. Influential points were determined by evaluating both their leverages and residuals and were excluded. The agreement between eGFRSCR and eGFRCYSC in classifying individuals with CKD was evaluated using the κ-statistic with 95% confidence intervals (CI) determined by bootstrapping. Unadjusted and adjusted linear regression models were constructed to evaluate the association of extra-renal factors with each GFR-estimating method, presented as percent change in eGFR. Factors which were significant at P-values <0.10 in unadjusted analysis were included in the adjusted model.
RESULTS
Clinical characteristics of HIV-uninfected and HIV-infected men are presented in Table 1. Compared with HIV-uninfected participants, a greater proportion of HIV-infected men were co-infected with hepatitis C and had elevated liver enzymes. The two groups were similar with regard to other risk factors for CKD such as diabetes and hypertension. HIV-infected men had a lower median BMI and a higher median CRP level. Co-trimoxazole use was noted only among HIV-infected men. Among HIV-infected participants, 15% previously received HAART; 73% were currently receiving HAART. The median duration of HAART receipt was 4.03 years (interquartile range [IQR]: 2.90–13.71 years) and 8.21 years (IQR: 4.42–9.79 years) among prior and current HAART-users, respectively. Although both HIV-uninfected and HIV-infected men had similar levels of kidney function by eGFRSCR, HIV-infected men had lower eGFRCYSC. A greater proportion of HIV-infected participants had proteinuria compared to HIV-uninfected men (17% versus 2%, respectively; P<0.01).
Table 1.
Demographic and clinical characteristics of HIV-uninfected and infected men
| Characteristics | HIV Serostatus
|
P-value | |
|---|---|---|---|
| Uninfected (n=150) | Infected (n=783) | ||
| Black, n (%) | 52 (35) | 261 (33) | 0.77 |
| Median age, y (IQR) | 47(41–57) | 47 (42 – 53) | 0.46 |
| Injection drug use, n (%) | 1 (<1) | 18 (2) | 0.34 |
| Hepatitis C co-infection, n (%) | 6 (4.0) | 75 (10) | 0.03 |
| Diabetes, n (%) | 11 (10) | 75 (13) | 0.53 |
| Hypertension, n (%) | 52 (38) | 270 (37) | 0.85 |
| Hypercholesterolemia, n (%) | 113 (75) | 564 (72) | 0.43 |
| ACEi or ARB use, n(%) | 20 (13) | 108 (14) | 0.88 |
| Co-trimoxazole, n(%) | 0 (0) | 58 (7) | <0.01 |
| Median BMI, kg/m2 (IQR) | 26.9 (23.9 – 29.6) | 24.9 (22.7 – 27.8) | <0.01 |
| Median CD4+ cell count, cells/mm3 (IQR) | --- | 511 (348 – 695) | --- |
| Median HIV-1 RNA level, copies/mL (IQR) | --- | <50 (<50 – 1830) | --- |
| Median CRP level, mg/L (IQR) | 0.9 (0.5 – 2.5) | 1.5 (0.7 – 3.6) | 0.01 |
| Median serum albumin, g/dL (IQR) | 4.5 (4.3 – 4.7) | 4.4 (4.2 – 4.6) | 0.02 |
| Median AST, U/L (IQR) | 22 (18 – 26) | 25 (21 – 33) | <0.01 |
| Median ALT, U/L (IQR) | 22 (17–19) | 25 (19–37) | <0.01 |
| Medianalkaline phosphatase, U/L (IQR) | 68 (57 – 80) | 80 (64 – 102) | <0.01 |
| Median serum creatinine, mg/dL (IQR) | 1.0 (0.9 – 1.1) | 0.9 (0.7 – 1.0) | 0.41 |
| Median serum cystatin C, mg/L (IQR) | 0.76 (0.70 – 0.85) | 0.85 (0.75 – 0.98) | <0.01 |
| Median eGFRSCR, mL/min/1.73m2 (IQR) | 103.1 (91.0 – 117.9) | 105.2 (91.3 – 118.8) | 0.78 |
| Median eGFRCYSC, mL/min/1.73m2 (IQR) | 108.0 (92.5 – 124.0) | 94.6 (80.2 – 110.3) | <0.01 |
| Median urine PCR, mg/g (IQR) | 60 (50–83) | 98 (68 – 159) | <0.01 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine transaminase; eGFRSCR, serum creatinine-based estimated glomerular filtration rate; eGFRCYSC, cystatin C-based estimated glomerular filtration rate, PCR, protein-to-creatinine ratio
Comparison of kidney function estimates
The agreement between the two GFR estimates for CKD classification was only moderate (κ=0.53, 95% CI: 0.40, 0.65). The proportion of HIV-infected men with CKD was higher by eGFRCYSC compared with eGFRSCR (7% versus 5%, respectively; P<0.01). When we defined CKD based on eGFRSCR, eGFRSCR was generally 9% higher than eGFRCYSC among HIV-infected men without CKD (P<0.01) (Figure 1A); the degree of discordance between the two GFR estimates varied by level of kidney function (P for equality of SDs<0.01). Conversely, eGFRSCR was generally 7% lower than eGFRCYSC among HIV-infected men with kidney dysfunction (P<0.01) (Figure 1B).
Figure 1.
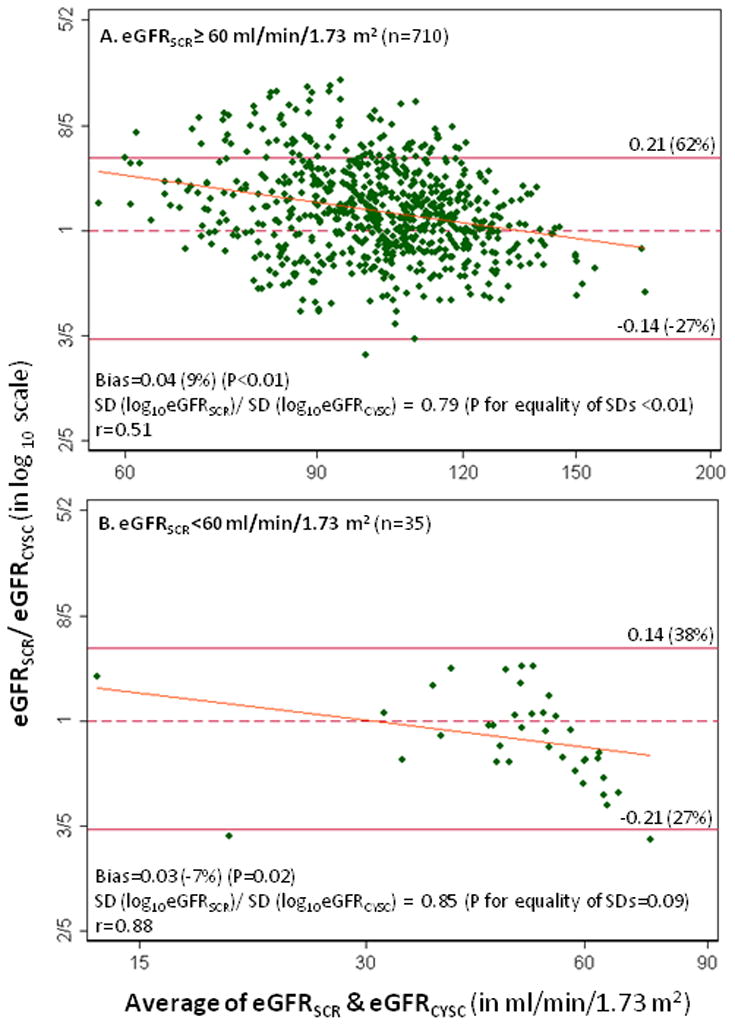
Bland-Altman plots of the average vs. the difference between log10-transformed eGFRSCR and eGFRCYSC among HIV-infected men with eGFRSCR≥60 (A) and eGFRSCR<60 mL/min/1.73 m2 (B). The diagonal lines are the slope of the linear regression of the difference on the mean of eGFRSCR and eGFRCYSC. Thirty-six and two influential points were excluded in Panels A and B, respectively.
Clinical factors which influence eGFRSCR and eGFRCYSC and therefore CKD classification in the context of HIV infection are displayed in Tables 2 and 3, respectively. In general, fewer clinical factors were associated with eGFRSCR than eGFRCYSC in unadjusted analyses. In adjusted analyses, older age, history of clinical AIDS and ACEi or ARB use, higher serum albumin, and proteinuria were associated with lower eGFRSCR. With the exception of ACEi or ARB use and serum albumin, eGFRCYSC was associated with the same factors. In addition, eGFRCYSC was lower with hepatitis C co-infection, higher HIV viral loads, and higher serum alkaline phosphatase levels. In contrast to eGFRSCR, cystatin C-based GFR estimates were affected by race, with African Americans having nearly 3% higher eGFRCYSC levels compared with persons of other ethnicities.
Table 2.
Unadjusted and adjusted associations of serum creatinine-based eGFR with extra-renal factors
| Variable | Unadjusted % change in eGFRSCR (95% CI) | P-value | Adjusted % change in eGFRSCR (95% CI) | P-value |
|---|---|---|---|---|
| Age, per 10-y increase | −4.71 (−5.37, −4.06) | <0.001 | −4.11 (−4.92, −3.29) | <0.001 |
| Black vs. non-black | 3.94 (2.42, 5.47) | <0.001 | 1.05 (−0.55, 2.67) | 0.20 |
| History of clinical AIDS | −8.02 (−10.17, −5.82) | <0.001 | −5.44 (−7.46, −3.38) | <0.001 |
| History of hypertension | −5.07 (−6.46, −3.67) | <0.001 | −1.21 (−2.88, 0.48) | 0.16 |
| History of hypercholesterolemia | −3.00 (−4.50, −1.47) | <0.001 | 0.67 (−0.98, 2.37) | 0.42 |
| Use of ACEi or ARB vs. neither | −8.51 (−10.53, −6.44) | <0.001 | −2.82 (−5.08, −0.51) | 0.02 |
| HIV-1 RNA, per 1-log increase | 1.20 (0.55, 1.85) | <0.001 | 3.47 (−0.25, 9.56) | 0.26 |
| C-reactive protein, per 1 mg/L increase | −0.22 (−0.35, −0.08) | 0.002 | −0.08 (−0.20, 0.04) | 0.20 |
| Serum albumin, per 1 g/dL increase | −1.81 (−3.66, 0.07) | 0.06 | −4.79 (−6.73, −2.82) | <0.001 |
| Proteinuria, per 100 mg/g increase | −1.18 (−0.13, −0.10) | <0.001 | −1.03 (−1.25, −0.81) | <0.001 |
Abbreviations: eGFRSCR, serum creatinine-based estimated glomerular filtration rate; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker
Table 3.
Unadjusted and adjusted associations of serum cystatin C-based GFR with extra-renal factors
| Variable | Unadjusted % change in eGFRCYSC (95% CI) | P-value | Unadjusted % change in eGFRCYSC (95% CI) | P-value |
|---|---|---|---|---|
| Age, per 10-y increase | −4.23 (−4.99, −3.47) | <0.001 | −3.76 (−4.69, −2.82) | <0.001 |
| Black vs. non-black | 2.57 (0.89, 4.28) | 0.003 | 2.62 (0.76, 4.51) | 0.006 |
| History of clinical AIDS | −7.55 (−10.12, −5.33) | <0.001 | −3.50 (−5.88, −1.05) | 0.005 |
| History of hypertension | −3.98 (−5.73, −2.22) | <0.001 | −1.01 (−2.91, 0.92) | 0.30 |
| Hepatitis C infected vs. uninfected | −3.37 (−4.72, −2.00) | <0.001 | −2.22 (−3.62, −0.84) | 0.002 |
| Use of ACEi or ARB vs. neither | −8.51 (−10.53, −6.44) | <0.001 | −2.22 (−4.81, 0.45) | 0.10 |
| CD4 cell count, per 100 cells/mm3 | 0.57 (0.25, 0.89) | <0.001 | 0.14 (−0.18, 0.46) | 0.40 |
| HIV-1 RNA, per 1-log increase | −1.53 (−2.22, −0.84) | <0.001 | −1.84 (−2.56, −1.11) | <0.001 |
| C-reactive protein, per 1mg/L increase | −0.25 (−0.40, −0.09) | 0.001 | −0.09 (−0.22, 0.04) | 0.21 |
| Serum albumin, per 1 g/dL increase | 5.70 (3.35, 8.10) | <0.001 | 1.69 (−0.73, 4.19) | 0.17 |
| AST, per 100 U/L increase | −0.03 (−0.06, −0.01) | 0.005 | 3.54 (−0.50, 7.75) | 0.09 |
| ALT, per 100 U/L increase | −0.03 (−0.05, <0.01) | 0.04 | −2.71 (−6.61, 1.36) | 0.19 |
| Alkaline phosphatase, per 100 U/L increase | −0.07 (−0.09, −0.04) | <0.001 | −3.92 (−6.38, −1.39) | 0.003 |
| Proteinuria, per 100 mg/g increase | −0.01 (−0.02, −0.01) | <0.001 | −0.92 (−1.18, −0.65) | <0.001 |
Abbreviations: eGFRCYSC, serum cystatin C-based estimated glomerular filtration rate; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; ALT, alanine transaminase
DISCUSSION
In a large population of HIV-uninfected and HIV-infected men of similar social risk behaviors, we demonstrated that GFR estimates differed by the biomarker used and by kidney function level. Estimates of GFR based on serum cystatin C differed by HIV status. Among HIV-infected men, estimates of GFR based on serum cystatin C classified a larger proportion of individuals with moderate to severe CKD compared with GFR estimates based on serum creatinine. These discordant classifications are clinically relevant as drug dosing and the institution of a multitude of CKD management strategies are advocated at the threshold of eGFR below 50 to 60 mL/min/1.73m2 by national guidelines (25).
The differences between these two GFR-estimating methods and therefore CKD classification reflect the differential factors associated with the respective biomarkers. We observed that more clinical factors correlated with eGFRCYSC than eGFRSCR, supporting ongoing concerns of cystatin C’s utility as an uninfluenced biomarker of kidney function. Since HIV infection can be accompanied by systemic inflammation and multiple non-AIDS co-morbidities that may alter markers of kidney function independently of kidney function, our study is an important step in understanding the relationship of these concurrent factors with indicators of renal function among HIV-infected persons and may help with interpretation of GFR estimates in this patient population.
Similar to prior studies, we demonstrated that HIV-infected individuals have higher serum cystatin C levels compared with social and demographically similar HIV-uninfected individuals (28,29). Whether these elevated serum cystatin C levels in HIV-infected persons necessarily reflect decrements in kidney function, however, remains unclear. In the Study of Fat Redistribution and Metabolic Change (FRAM) study, HIV-infected individuals who had traditional CKD risk factors (older age, higher baseline or greater increase in HIV RNA levels, higher serum glucose levels, and dyslipidemia) were more likely to have a decline in eGFRCYSC (30). Conversely, individuals with improved HIV RNA levels had improvement in eGFRCYSC. While these findings by Longenecker and colleagues suggests that elevated serum cystatin C levels seen in HIV-infected individuals may be explained by subclinical kidney disease, our data suggest that additional factors occurring in HIV-infected persons are differentially associated with serum creatinine and cystatin C, and thus their corresponding estimates of kidney function. While some clinical factors, such age and proteinuria, were consistent in their influence across GFR-estimating methods, others factors affected only one or the other method. For example, ACEi or ARB use and higher serum albumin levels were inversely associated with eGFRSCR but not eGFRCYSC; in this context, ACEi or ARB use may reflect a drug-related reduction in true GFR via vasodilatation of the renal efferent arteriole or may serve as a surrogate for hypertension, a risk factor for CKD. On the other hand, serum albumin’s effect on serum creatinine may be reflective of one’s nutritional state and protein stores rather than kidney function. Prior studies comparing various GFR-estimating equations to an exogenous indicator of GFR have shown that both eGFRSCR and eGFRCYSC are considerably biased (−10 to −29 mL/min/1.72 m2) in HIV-infected persons (31). Moreover, Barraclough and colleagues demonstrated that cystatin C-based estimates of kidney function were less accurate than those based on serum creatinine, with 41% versus 89% falling within 30% of the measured GFR, respectively (31,32). These studies, however, consisted of no more the 30 participants, limiting their generalizability to the HIV-infected population at large. While our study also underscores the need for large studies which rigorously validate GFR-estimating equations in the HIV-infected population, clinicians currently rely on available GFR-estimating equations (which were developed in studies excluding HIV-infected persons) to inform management of their HIV-infected patients. Therefore, recognition and understanding of extra-renal factors which affect these estimates are important in the application of these GFR-estimating equations to HIV-infected persons.
Our study has several limitations to consider. Results of the agreement between eGFRSCR and eGFRCYSC should be interpreted cautiously at the extremes of eGFR since we had few individuals with either very high or low eGFRs; therefore, these results may have been unduly influenced by the few participants at the eGFR extremes. In addition, we relied upon only one time point for GFR estimation. Our calibration of the original serum creatinine values to standardized serum creatinine across sites may have introduced bias or additional measurement error which may have affected our results; however, the high correlation and excellent agreement between the original and repeated serum creatinine values across sites indicate that these potential effects were minimal. A systematic downward drift in cystatin C values due to changes in the assay has been noted since the development of the CKD-EPI eGFRCYSC equation (33). This may have led to an underestimation of the CKD prevalence based on eGFRCYSC in our study. Direct comparisons of cystatin C concentrations from our study to previous studies of HIV-infected populations should account for the change in the assay over time.
Kidney function estimates based on serum creatinine versus cystatin C differentially classified individuals with CKD. This discordance in identifying some individuals with CKD may be due to the effects of extra-renal factors on both serum creatinine and cystatin C. Until GFR-estimating equations are rigorously validated among HIV-infected individuals, current estimates of kidney function based on these biomarkers should be interpreted cautiously in this patient population in light of these potential extra-renal influences.
Acknowledgments
Support:
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041. Website located at http://www.statepi.jhsph.edu/macs/macs.html. M.M.E is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 1K23DK081317 and the Young Investigator Grant from the National Kidney Foundation of Maryland. R.S.P is supported by the NIDDK grant 5R01DK072367. B.C.A is supported by the NIDDK grants 1R21DK078218and 1R01 DK076770.
Footnotes
Results of this study have not been previously presented in part or whole.
Competing interests
All authors declare that they have no competing interests.
Authors’ Contributions
All authors played a role in editing the manuscript and approved the text. M.M.E, R.S.P. and L.P.J. designed the study. M.M.E. wrote the manuscript. M.M.E. performed the data analysis, and L.P.J. and R.S.P. assisted in the interpretation of statistical data. R.S.P, B.C.A., R.B., R.W.E., F.J.P., and L.P.J. reviewed and edited the manuscript.
References
- 1.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16(8):2412–20. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 2.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40(10):1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS Family Investigation of Nephropathy and Diabetes Research Group. MYH9 is associated with nondiabetic end-stage renal disease in african americans. Nat Genet. 2008;40(10):1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmel PL. HIV-associated nephropathy: Virologic issues related to renal sclerosis. Nephrol Dial Transplant. 2003;18(Suppl 6):vi59, 63. doi: 10.1093/ndt/gfg1062. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: A systematic review and meta-analysis. AIDS. 2008;22(14):1799–1807. doi: 10.1097/QAD.0b013e32830e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Multicenter AIDS Cohort Study. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19(9):953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 7.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 8.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 9.Mack KA, Ory MG. AIDS and older americans at the end of the twentieth century. J Acquir Immune Defic Syndr. 2003;33 (Suppl 2):S68–75. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dube MP. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61(1):1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 11.Szczech LA, Hoover DR, Feldman JG, Cohen MH, Gange SJ, Gooze L, Rubin NR, Young MA, Cai X, Shi Q, Gao W, Anastos K. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39(8):1199–206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 12.Choi AI, Rodriguez RA, Bacchetti P, Volberding PA, Havlir D, Bertenthal D, Bostrom A, O’Hare AM. Low rates of antiretroviral therapy among HIV-infected patients with chronic kidney disease. Clin Infect Dis. 2007;45(12):1633–9. doi: 10.1086/523729. [DOI] [PubMed] [Google Scholar]
- 13.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121(5):651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta SK, Eustace JA, Winston JA, IIB, Ahuja TS, Rodriguez RA, Tashima KT, Roland M, Franceschini N, Palella FJ, Lennox JL, Klotman PE, Nachman SA, Hall SD, Szczech LA. Guidelines for the management of chronic kidney disease in HIV-infected patients: Recommendations of the HIV medicine association of the infectious diseases society of america. Clin Infect Dis. 2005;40(11):1559–85. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 15.Salomon J, de Truchis P, Melchior JC. Body composition and nutritional parameters in HIV and AIDS patients. Clin Chem Lab Med. 2002;40(12):1329–1333. doi: 10.1515/CCLM.2002.229. [DOI] [PubMed] [Google Scholar]
- 16.McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. Am J Clin Nutr. 2001;74(5):679–686. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- 17.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl):S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Myre SA, McCann J, First MR, JCR Effect of trimethoprim on serum creatinine in healthy and chronic renal failure volunteers. Ther Drug Monit. 1987;9(2):161–5. doi: 10.1097/00007691-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell EK. Flucytosine and false elevation of serum creatinine level. Ann Intern Med. 1984;101(2):278. doi: 10.7326/0003-4819-101-2-278_1. [DOI] [PubMed] [Google Scholar]
- 20.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the united states: The third national health and nutrition examination survey (NHANES III) Am J Kidney Dis. 2008;51(3):385–94. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The multicenter AIDS cohort study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-ifnected adults and adolescents. Department of Health and Human Services; 2009. pp. 1–161. [Google Scholar]
- 27.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 28.Jones CY, Jones CA, Wilson IB, Knox TA, Levey AS, Spiegelman D, Gorbach SL, Van Lente F, Stevens LA. Cystatin C and creatinine in an HIV cohort: The nutrition for healthy living study. Am J Kidney Dis. 2008;51(6):914–24. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: The FRAM study. Arch Intern Med. 2007;167(20):2213–9. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. HIV viremia and changes in kidney function. AIDS. 2009;23(9):1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barraclough K, Er L, Ng F, Harris M, Montaner J, Levin A. A comparison of the predictive performance of different methods of kidney function estimation in a well-characterized HIV-infected population. Nephron Clin Pract. 2009;111(1):c39–48. doi: 10.1159/000178978. [DOI] [PubMed] [Google Scholar]
- 32.Beringer PM, Owens H, Nguyen A, Mordwinkin N, Louie S, Mak M, Sattler F. Estimation of glomerular filtration rate by using serum cystatin C and serum creatinine concentrations in patients with human immunodeficiency virus. Pharmacotherapy. 2010;30(10):1004–1010. doi: 10.1592/phco.30.10.1004. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Manzi J, Levey AS, Eckfeldt JH, Van Lente F, Coresh J. Drift in dade behring cystatin C assay 2003 to 2009. 43rd Annual Meeting and Scientific Exposition of the American Society of Nephrology; Denver, CO. October 2010. [Google Scholar]


