Abstract
This study examined the effect of schisandrin, one of the major lignans isolated from Schisandra chinensis, on spontaneous contraction in rat colon and its possible mechanisms. Schisandrin produced a concentration-dependent inhibition (EC50 = 1.66 μM) on the colonic spontaneous contraction. The relaxant effect of schisandrin could be abolished by the neuronal Na+ channel blocker tetrodotoxin (1 μM) but not affected by propranolol (1 μM), phentolamine (1 μM), atropine (1 μM) or nicotine desensitization, suggesting possible involvement of non-adrenergic non-cholinergic (NANC) transmitters released from enteric nerves. Nω-nitro-L-arginine methyl ester (100-300 μM), a nitric oxide synthase inhibitor, attenuated the schisandrin response. The role of nitric oxide (NO) was confirmed by an increase in colonic NO production after schisandrin incubation, and the inhibition on the schisandrin responses by soluble guanylyl cyclase inhibitor 1H-[1,2,4] oxadiazolo[4,3-α]-quinoxalin-1-one (1-30 μM). Non-nitrergic NANC components may also be involved in the action of schisandrin, as suggested by the significant inhibition of apamin on the schisandrin-induced responses. Pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (100 μM), a selective P2 purinoceptor antagonist, markedly attenuated the responses to schisandrin. In contrast, neither 8-cyclopentyl-1,3-dipropylxanthine, an antagonist for adenosine A1 receptors, nor chymotrypsin, a serine endopeptidase, affected the responses. All available results have demonstrated that schisandrin produced NANC relaxation on the rat colon, with the involvement of NO and acting via cGMP-dependent pathways. ATP, but not adenosine and VIP, likely plays a role in the non-nitrergic, apamin-sensitive component of the response.
Keywords: Schisandrin, colonic smooth muscle, non-adrenergic non-cholinergic relaxation, enteric nervous system, nitric oxide
Introduction
Schisandra chinensis (Turcz.) Baill. (Schisandraceae) is a well-known medicinal herb in China and East Asia. The dried ripe fruits are used as a tonic and astringent in traditional Chinese medicine (Chinese Pharmacopoeia, 2005), and a number of Schisandra products or combinations are commercially available as dietary supplements in the Western countries. S. chinensis also found applications in the treatment of hepatitis due to its liver-protective effects (Ip et al., 1996; Pan et al., 2008). This herbal drug is rich in lignan compounds, and it has been suggested that they contribute to the anti-oxidant, hepatic-protective and cancer-preventive effects of the crude drug (Opletal et al., 2004; Panossian & Wikman, 2008). Schisandra lignans (such as gomisin J) also displayed relaxant effects on isolated smooth muscle preparations, including mesenteric artery, trachea, ileum and tenia coli (Suekawa et al., 1987). In recent studies, S. chinensis extracts and gomisin A were shown to produce vasorelaxant effect on rat thoracic aorta via endothelium-dependent and endothelium-independent pathways (Rhyu et al., 2006; Park et al., 2007; Park et al., 2009).
Coordination of smooth muscle contraction and relaxation, maintained by the enteric nervous system (ENS), is the basis of gut motility, which determines the transport of luminal contents. It has been reported that, in guinea pig colon, colonic propulsion was regulated by excitatory motor nerves (i.e. cholinergic nerve) and inhibitory motor nerves (i.e. non-adrenergic non cholinergic inhibitory nerve) of the ENS (Foxx-Orenstein and Grider, 1996). Thus, relaxing the spontaneously contracted colon may lead to attenuation of the enhanced colonic transit found in gastrointestinal disorders with diarrhoea symptoms. Our preliminary results have shown that the aqueous and ethanol extracts of S. chinensis caused concentration-dependent inhibition on the spontaneous contraction of isolated rat colon, and schisandrin is a major lignan constituent (Halstead et al., 2007). The present work was undertaken to examine the inhibitory effect of schisandrin on spontaneous contraction of isolated rat colon, and some of the relevant mediators were further identified using ion channel blockers, receptor antagonist and enzyme inhibitor, as well as quantitative determination of the mediators.
Materials and methods
Chemicals and reagents
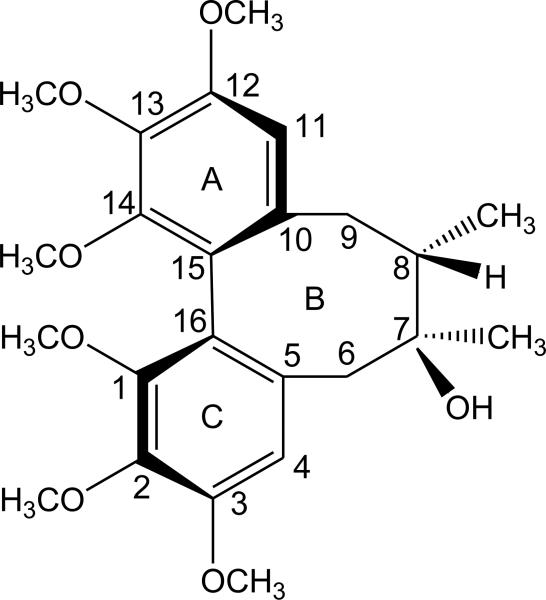
Schisandrin (Fig. 1, purity >98% by HPLC analysis) was obtained from the National Institute for Food and Drug Control (Beijing, China). Tetrodotoxin (TTX), atropine, propranolol, phentolamine, nicotine, phenylepherine, Nω-nitro-L-arginine methyl ester (L-NAME), 1H-[1,2,4] oxadiazolo[4,3-α]-quinoxalin-1-one (ODQ), SQ 22536, sodium nitroprusside (SNP) , cicaprost, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), adenosine, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS), adenosine triphosphate (ATP) and α-chymotrypsin were purchased from Sigma Co. (U.S.A.); Ro1138452 from Roche Co. (Swiss); apamin from Research Biochemicals International (U.S.A.). The chemicals for buffer solution were of analytical grade for common laboratory use. Schisandrin (10 mM), ODQ (50 mM) and Ro1138452 (10 mM) were dissolved in 100% DMSO as stock and diluted with DMSO. A stock solution of TTX (500 μM) was prepared in saline containing 0.01% acetic acid. Other stock solutions were prepared in distilled water and diluted with saline before use. Adenosine solutions were freshly prepared each day. All stock solutions were stored at -20 °C until use and diluted. The volume of administration was between 0.5 to 30 μl, and in each experiment, the total volume of sample solution (including DMSO) added to the bath was no more than 0.3% of the bath volume.
Fig. 1.
Chemical structure of schisandrin
Animals
Sprague-Dawley rats (250-300 g) were bred and housed by the Laboratory Animal Services Centre of the Chinese University of Hong Kong. All experiments were performed upon approval from the Animal Research Ethics Committee. The animals were kept in a temperature controlled room (23 ± 2°C) with a 12-hr light-dark cycle, with free access to food and water.
Isolation of colon preparations
Male rats were fasted overnight and sacrificed by cervical dislocation and exsanguination. An approximately 6 cm length of ascending colon from cecum was removed and placed in Krebs-Henseleit solution containing NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.18, NaHCO3 25, and glucose 10 mM. Subsequently, the colon was flushed with buffer solution, cleaned of mesenteric tissues, and cut into proximal and distal segments of 2 cm in length. The colon segments were suspended in a 10-ml organ bath filled with Krebs solution maintained at 37°C and aerated with 95%O2/5%CO2. Washout of the organ bath was performed by solution upward displacement and overflow. Contractions of the longitudinal muscle of isolated rat colon were measured with Grass FT03 isometric transducers connected to a Powerlab data acquisition system (AD Instruments Pty Ltd, Australia). Initially, a basal tension of 0.5 g was applied. On relaxation repeated tension was given until the basal tone remained steady at about 0.3 g.
Measurement of contractile activity
In all experiments, the proximal and distal segments of rat colon from the same rat were used as matched preparations. After about 1 h equilibration, with frequent washing, a sub-maximal dose of schisandrin (10 μM) was tested on each preparation to ensure stable and acceptable sensitivity had been reached. Concentration-response curve of schisandrin was obtained by non-cumulative incubation with the inhibitory agonist for 5 min. Washout for 3 min and resting for 10 min was allowed between each sample administration.
Spontaneous contractility of isolated rat colon was measured in the form of the average contractile force (in grams over baseline) in a 4-min period. Relaxant responses to inhibitory agonists were taken as the percentage change from the resting spontaneous contraction before priming with the inhibitory agonists (e.g. -100% corresponds to an abolition of contraction).
Effects of blockers/inhibitors on responses to inhibitory agonists
Reponses to single doses of inhibitory agonists, including schisandrin, cicaprost, SNP and ATP, were obtained on both proximal and distal colon segments from three different rats, and the effects of blockers or enzyme inhibitors were assessed against the agonist responses by pre-treatment of blockers or enzyme inhibitors for 10 – 15 min at subsequently increasing concentrations (n = 6). Responses to adenosine were obtained from four rats and individually analyzed on proximal and distal segments.
Measurement of nitric oxide (NO) production
Isolated colon from the same rat was cut into two proximal and two distal segments that were equilibrated in Krebs solution at 37°C with aeration of 95%O2/5%CO2. After 30 min equilibration, the segments were correspondingly transferred into aerated Krebs solution (2 ml) at 37°C containing schisandrin (100 μM) or DMSO (as vehicle blank). After incubation for 5 min, the colon segments were blotted dry, frozen in liquid nitrogen, and homogenized in 1 ml of distilled water. The supernatant was obtained by centrifugation at 10,000 × g for 20 min, and subsequently passed through a membrane filter (0.45 μm) prior to ultra-filtration using a 30 kDa molecular weight cut-off filter (Millipore, U.S.A.) at 2,000 × g for 60 min. The filtrate was used as assay sample for nitrite/nitrate measurement following the instructions of the colorimetric assay kit (Caymen, U.S.A). Generally, nitrate in the sample was converted to nitrite by the nitrate reductase mixture and the total nitrite was reacted with Griess reagent prior to absorbance measurement at 540 nm. Nitric oxide production was calculated by the total nitrite and normalized by the corresponding proximal or distal segment treated with vehicle blank.
Statistical analysis
All values were expressed as mean ± S.E.M.. Results from the controls and treatments were compared by unpaired Student's t test or one-way ANOVA followed by Bonferroni post test, as appropriate. All tests were two-tailed and the significance was set at P < 0.05.
Results
Effects of schisandrin on spontaneous colon contraction
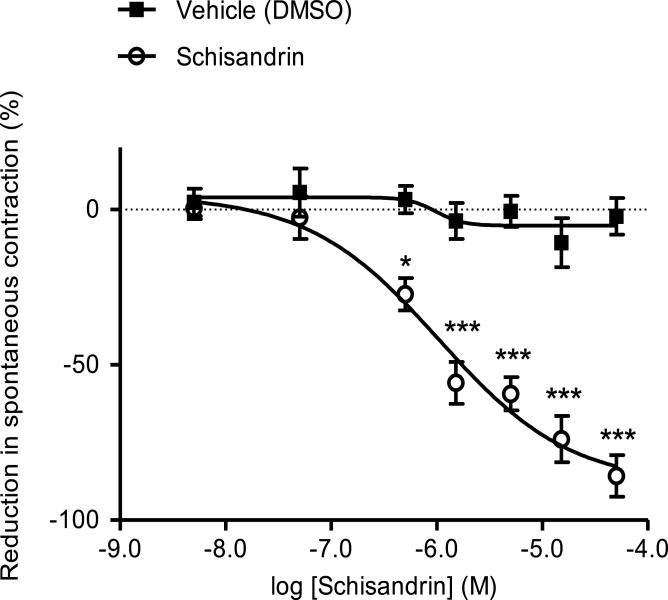
In all experiments, responses of both proximal and distal colons to the tested drugs were investigated. Schisandrin caused an acute relaxation within seconds upon sample administration, and the inhibitory responses on spontaneous contraction of proximal and distal colon segments were similar in magnitude. It was therefore decided to combine the data for statistic analysis and graph presentation. As shown in Fig. 2, schisandrin produced a concentration-dependent relaxation on isolated rat colon starting from a concentration as low as 0.5 μM. Non-cumulative dosing of schisandrin resulted in an EC50 value of 1.66 ± 0.31 μM and an Emax value of -85.8 ± 6.7 %, whereas the vehicle blank (DMSO) had no effect. In subsequent experiments, the effects of antagonists and enzyme inhibitor were assessed against the response of 10 μM schisandrin as a submaximal dose. The standard dosing elicited an approximately 60 % inhibition on the spontaneous contraction in isolated rat colon.
Fig. 2.
Effects of schisandrin on spontaneous contraction of isolated rat colon with DMSO as vehicle blank. Schisandrin produced a concentration-dependent relaxation on isolated rat colon with an EC50 value of 1.66 ± 0.31 μM. Values are expressed as mean ± S.E.M. (n = 6). *P < 0.05, ***P < 0.001 compared to vehicle using two-way ANOVA with Bonferroni post-tests.
Activation of non-adrenergic non-cholinergic (NANC) enteric nerves
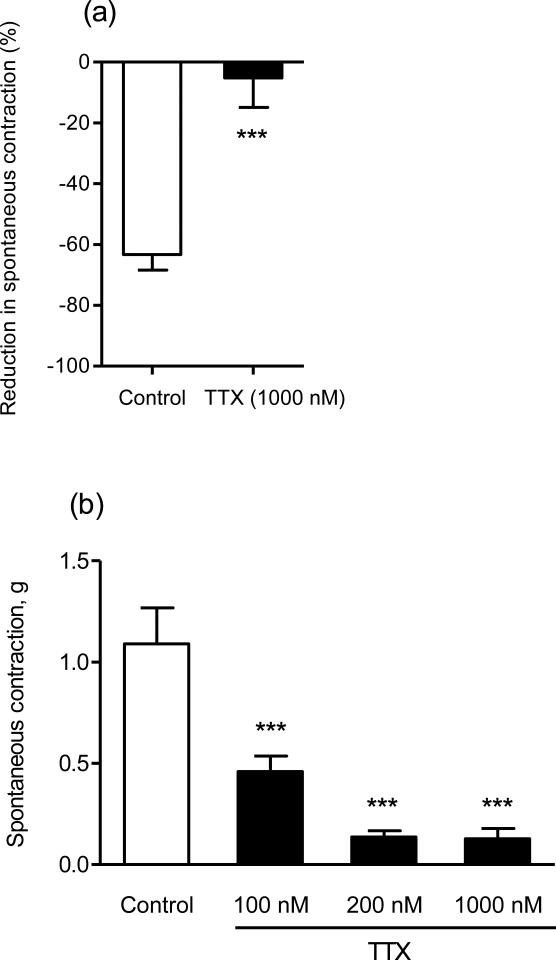
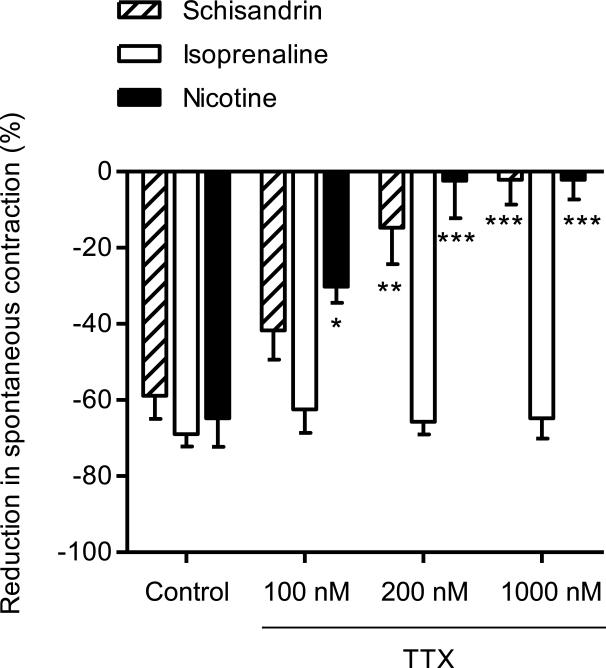
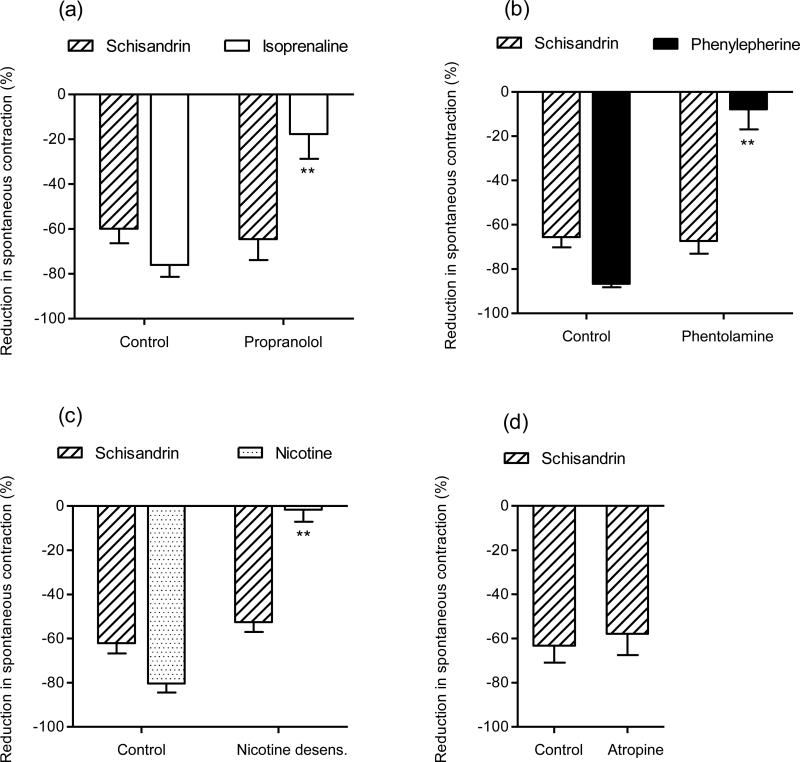
To determine the significance of neuronal mechanisms in the relaxant effects of schisandrin, rat colon segments were pretreated with tetrodotoxin (TTX), a neuronal Na+ channel blocker. TTX (0.1-1 μM) slightly increased the amplitude and frequency of spontaneous contractions, and tended to make the contraction pattern more regular. Aconitine, a neurotoxin that opens TTX-sensitive Na+ channels, was used to test the function of neuronal Na+ channels in our system. Addition of 1 μM aconitine produced inhibitory responses on the colonic spontaneous contractions in the first two minutes, while after the third minute, the spontaneous contractions tended to recover and were intensified. Treatment of TTX markedly inhibited both inhibitory and contractile responses to aconitine in rat colon (Fig. 3). As shown in Fig. 4, TTX (0.1-1 μM) dose-dependently reduced the inhibitory responses of schisandrin (10 μM) and nicotine (2 μM) and abolished the responses at a concentration of 1 μM (Fig. 5), but had no effect on isoprenaline (β-adrenoceptor agonist) responses (Fig. 4). Subsequently, propranolol, phentolamine, nicotine desensitization and atropine were used to examine the involvement of adrenergic and cholinergic systems. The β-adrenergic antagonist, propranolol (1 μM) and α-adrenergic antagonist, phentolamine (1 μM) markedly inhibited the responses of the respective agonists, isoprenaline (6 nM) and phenylepherine (10 μM), but did not affect schisandrin responses (P > 0.05) (Fig. 6(a) and Fig. 6(b)). Nicotine produced significant inhibitions (about -80% at 10 μM) on the colonic spontaneous contraction, but the responses were prone to tachyphylaxis. Desensitization with 3 subsequent applications of 10 μM nicotine abolished the inhibitory responses of nicotine, but had no effect on schisandrin responses (P > 0.05) (Fig. 6(c)). Since an initial dosing of atropine at 1 μM caused marked reduction in spontaneous contraction by 70-80 %, the colon preparations were initially exposed to 50 nM atropine with subsequent dosing of 0.1-1 μM. Atropine (1 μM) essentially blocked the acetylcholine-induced (10 μM) contraction in rat colon (>90% inhibition), but did not affect the inhibitory responses of schisandrin (P > 0.05) (Fig. 6(d)).
Fig. 3.
Spontaneous contractions in rat colon. Effects of tetrodotoxin (TTX) on (a) the inhibitory responses of aconitine (1 μM) in the first two minutes after addition (n = 4); (b) the aconitine-induced contractions at three minutes later after addition (n = 6).
Fig. 4.
Effects of tetrodotoxin (TTX) on the inhibitory responses of schisandrin (10 μM), isoprenaline (6 nM) and nicotine (2 μM) in isolated rat colon. The colon segments were pre-incubated with TTX for 10 min before the addition of inhibitory agonists. Values are expressed as mean ± S.E.M. (n = 6). *P<0.05, **P<0.01, ***P<0.001 as compared to appropriate control.
Fig. 5.
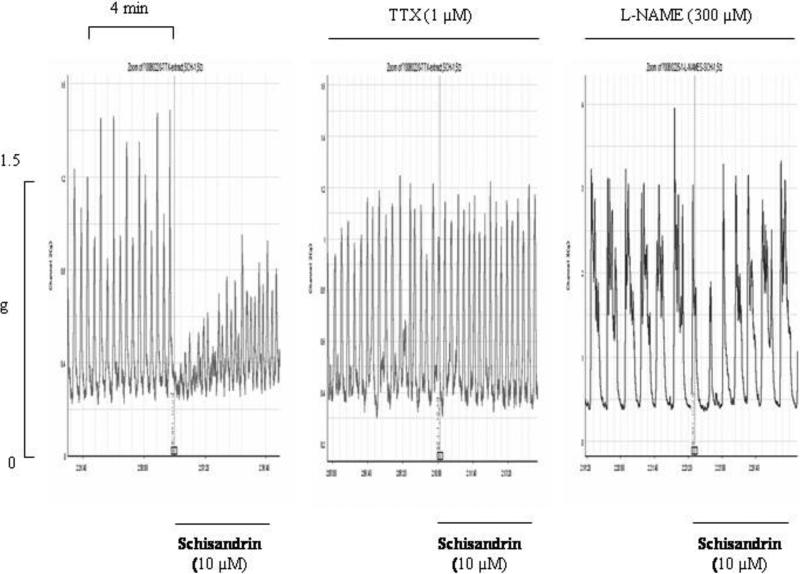
Representative experimental records of spontaneous contraction of rat colon showing the effects of tetrodotoxin and Nω-nitro-l-arginine methyl ester (l-NAME) on the inhibitory responses of schisandrin (10 μM). The colon segments were pre-incubated with the antagonists for 10 min before addition of schisandrin. TTX (1 μM) abolished the inhibitory response of schisandrin, whereas l-NAME (300 μM) attenuated but did not abolish the response of schisandrin.
Fig. 6.
Spontaneous contractions in isolated rat colon. Effects of (a) propranolol (1 μM) on the inhibitory responses of schisandrin (10 μM) and isoprenaline (6 nM) and (b) phentolamine (1 μM) on inhibitory responses of schisandrin (10 μM) and phenylepherine (10 μM) (n = 6). (c) Effects of desensitization with 3 subsequent applications of 10 μM nicotine (nicotine desens.) on the inhibitory responses of schisandrin (10 μM) and nicotine (10 μM) (n = 4). (d) Effects of atropine (1 μM) on the inhibitory response of schisandrin (10 μM) (n = 6). Values are expressed as mean ± S.E.M.. **P<0.01 compared to control.
Involvement of nitric oxide and cGMP pathway
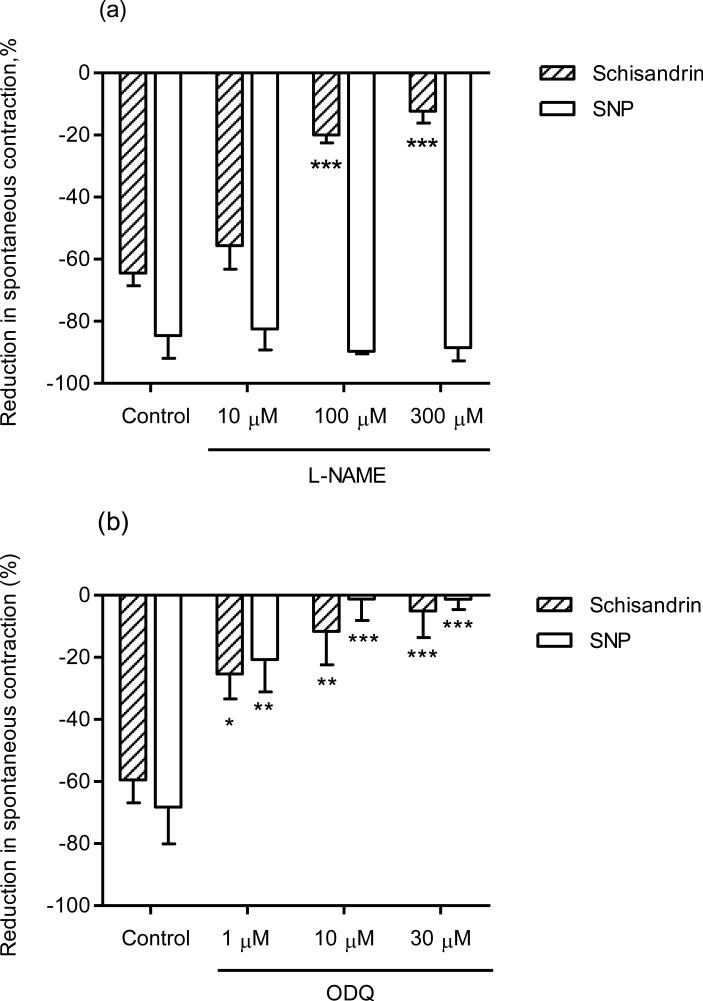
The nitric oxide synthase (NOS) inhibitor L-NAME slowed the rate of spontaneous contraction. As depicted in Fig. 5, 100 μM L-NAME attenuated, but did not abolish, the inhibitory response of schisandrin. Fig. 7(a) showed that the schisandrin responses (-64.5 ± 4.1%) were partially inhibited by L-NAME at the concentrations of 100 μM (-20.0 ± 2.6%) and 300 μM (-12.3 ± 3.8%), whereas the responses of the NO donor, sodium nitroprusside (SNP, 2 μM) were not affected by L-NAME. To further determine the involvement of nitric oxide, the contents of nitric oxide in isolated rat colon (proximal and distal) were measured using nitrite/nitrate colorimetric assays. The NO production in colon segments, expressed as percentage over vehicle control, markedly increased after incubation with 100 μM schisandrin for 5 min (schisandrin: 191.2 ± 23.3% v.s. vehicle control: 100 ± 17.7%).
Fig. 7.
Effects of (a) Nω-nitro-l-arginine methyl ester (l-NAME), at the concentrations of 10 μM, 100 μM and 300 μM, and (b) 1H-[1,2,4] oxadiazolo[4,3-α]-quinoxalin-1-one (ODQ), at the concentrations of 10 μM, 100 μM and 300 μM, on the inhibitory responses of schisandrin (10 μM) (n = 6) and sodium nitroprusside (SNP, 2 μM) (n = 4) in isolated rat colon. The colon segments were pre-incubated with the enzyme inhibitors for 15 min before the addition of schisandrin or SNP. Both l-NAME and ODQ attenuated the inhibitory response of schisandrin in a concentration-dependent manner (P < 0.001). Values are expressed as mean ± S.E.M.. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
A soluble guanylyl cyclase (GC) inhibitor ODQ was used to test the involvement of GC-dependent pathways in the relaxant effect of schisandrin. ODQ itself had no effect on the rate and amplitude of contraction. As shown in Fig. 7(b), ODQ (10 μM and 30 μM) essentially abolished the SNP responses, and the schisandrin responses (-59.5 ± 7.4%) were also inhibited by ODQ at the concentrations of 1 μM (-25.4 ± 8.0%), 10 μM (-11.6 ± 10.7%) and 30 μM (-5.0 ± 8.6%). In contrast to ODQ, pre-treatment of SQ22536 (100 μM), an adenylyl cyclase (AC) inhibitor, had no effect on the responses of schisandrin (unpublished observation)
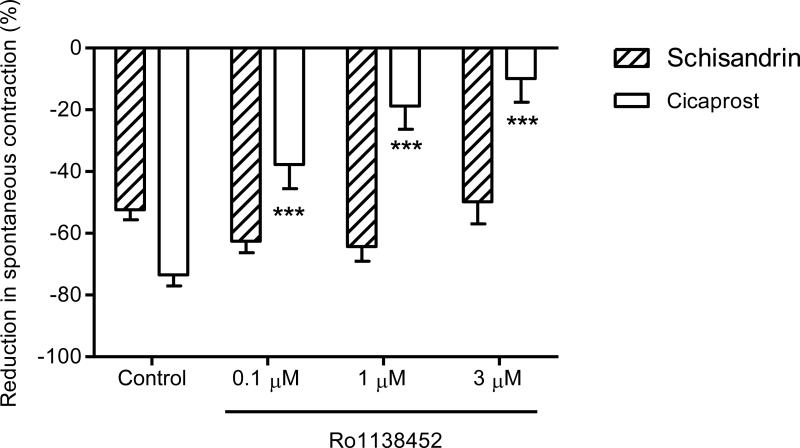
The effect of a selective prostacyclin receptor antagonist Ro1138452 was used to test the possible involvement of prostacyclin receptor in the schisandrin-induced response. Cicaprost, a prostacyclin receptor agonist with high selectivity and potency, caused a 73.4 ± 3.6 % inhibition on the spontaneous contraction and Ro1138452 markedly inhibited cicaprost responses in a concentration-dependent manner at concentrations of 0.1 μM (-37.7 ± 7.9%), 1 μM (-18.8 ± 7.5%), 3 μM (-9.9 ± 7.7%), as indicated in Fig. 8. In contrast, the inhibitory responses of schisandrin (10 μM) were not affected by Ro1138452 at all the concentrations used (P > 0.05).
Fig. 8.
Effects of Ro1138452 at the concentrations of 0.1 μM (n = 8), 1 μM (n = 8) and 3 μM (n = 5) on the inhibitory responses of schisandrin (10 μM) and cicaprost (10 nM) in isolated rat colon. The colon segments were pre-incubated with the prostacyclin receptor antagonist for 15 min before the addition of schisandrin or cicaprost. Ro1138452 attenuated the inhibitory response of cicaprost in a concentration-dependent manner (P < 0.001) while had no effect on schisandrin (P > 0.05). Values are expressed as mean ± S.E.M.. ***P < 0.001 compared to control.
Effects of other non-adrenergic, non-cholinergic (NANC) mediators
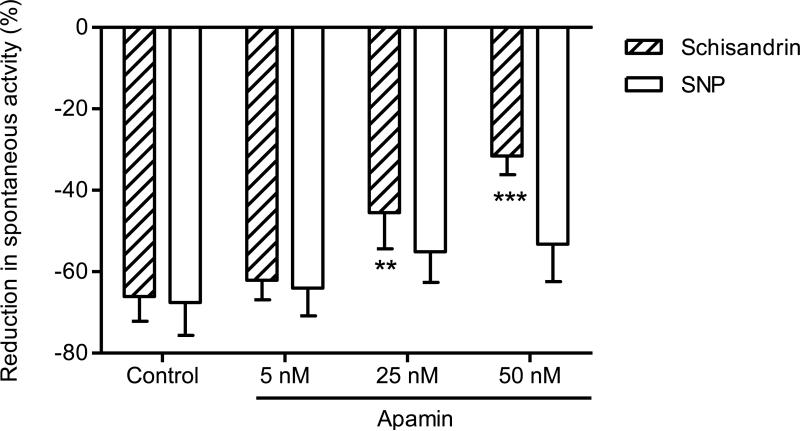
Experiments were performed to test whether other non-adrenergic, non-cholinergic transmitters apart from NO were involved in the schisandrin-induced relaxation on rat colon. As presented in Fig. 9, the response to 10 μM schisandrin (-66.1 ± 6.1%) was markedly inhibited by apamin (a specific blocker for calcium-activated K+ channels in smooth muscle cell) at the concentrations of 25 nM (-45.5 ± 8.9%) and 50 nM (-31.6 ± 4.6%). Sodium nitroprusside (SNP), a NO donor as negative control, caused a 67.6 ± 8.1% inhibition at a concentration of 1 μM, but the responses to SNP were not affected by apamin at the concentrations used (P > 0.05).
Fig. 9.
Effects of apamin at the concentrations of 5 nM, 25 nM and 50 nM on the inhibitory responses of schisandrin (10 μM) and sodium nitroprusside (SNP, 1 μM) in isolated rat colon. The colon segments were pre-incubated with apamin for 15 min before the addition of schisandrin or SNP. Apamin attenuated the inhibitory response of schisandrin in a concentration-dependent manner (P < 0.001) while had no effect on SNP (P > 0.05). Values are expressed as mean ± S.E.M. (n = 6). **P < 0.01, ***P < 0.001 compared to control.
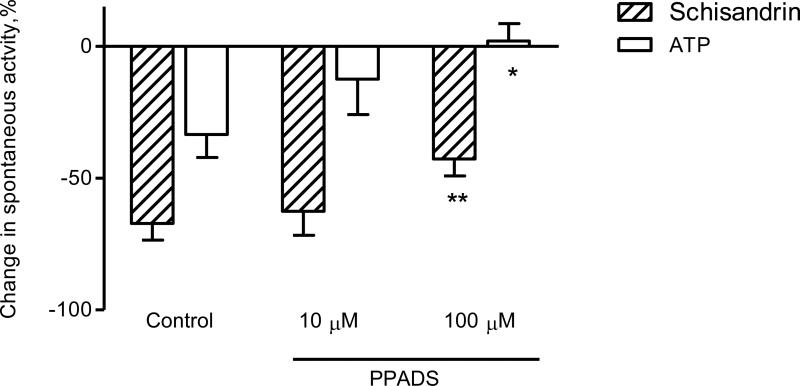
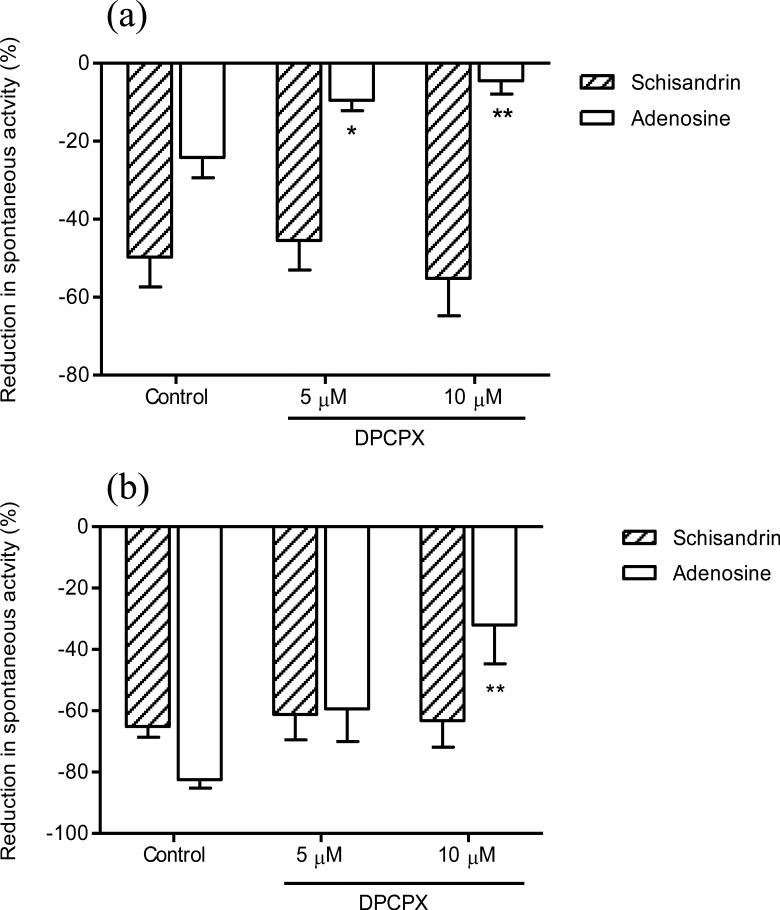
Effects of several treatments intended to inhibit purinergic and VIP systems were investigated on the response of schisandrin. Pre-treatment with 100 μM of PPADS, a selective P2 purinoceptor antagonist, abolished the response of ATP (2 μM), and markedly attenuated the response of schisandrin (Fig. 10). To determine the possible involvement of adenosine, DPCPX (a potent antagonist for adenosine A1 receptors) was used. As shown in Fig. 11, although adenosine (100 μM) produced different inhibitory responses in proximal colon preparations (-24.2 ± 5.2 %) and distal colon preparations (-82.4 ± 2.8%), the adenosine responses in both preparations were markedly inhibited by DPCPX (10 μM). In contrast, DPCPX did not affect the responses to schisandrin (10 μM). Chymotrypsin, a serine endopeptidase, was used to test the interference with the action of vasoactive intestinal peptide (VIP). The schisandrin responses (-60.0 ± 10.2%) were unaffected by chymotrypsin at a sufficient concentration of 2 u/ml (-62.2 ± 4.9%).
Fig. 10.
Effects of pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS) at the concentrations of 10 μM and 100 μM on the inhibitory responses of schisandrin (10 μM) and ATP (2 μM) in isolated rat colon. The colon segments were pre-incubated with PPADS for 15 min before the addition of schisandrin or ATP. PPADS (100 μM) markedly attenuated the inhibitory responses of schisandrin (P < 0.01) and ATP (P < 0.05). Values are expressed as mean ± S.E.M. (n = 6). *P < 0.05, **P < 0.01 compared to control.
Fig. 11.
Effects of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) at the concentrations of 5 μM and 10 μM on the inhibitory responses of schisandrin (10 μM) and adenosine (100 μM) in (a) proximal (n = 4) and (b) distal isolated rat colon (n = 4). Pre-incubation of DPCPX for 15 min concentration-dependently attenuated the responses of adenosine in both proximal and distal colon (P < 0.05), whereas did not affect the responses of schisandrin (P > 0.05). Values are expressed as mean ± S.E.M.. *P < 0.05, **P < 0.01, compared to control.
Discussion
Schisandra chinensis is a well-known tonic and astringent agent and it has been widely used to control excessive loss of essential energy and body fluid in Chinese medicine. This herb is often prescribed for the treatment of night sweating and diarrhea. The present study is an attempt to study the in vitro modulatory effects of a major ingredient of the herbal drug, schisandrin, on the motility of rat colon. Schisandrin produced concentration-dependent relaxation on spontaneous contraction of rat colon with an EC50 value of 1.66 μM, which was more potent than three other Schisandra lignans, gomisin A, deoxyschisandrin and γ-schisandrin (unpublished results). The mechanisms of the acute response to schisandrin in spontaneously contracting rat colon were thus investigated to identify the involvement of receptors, enzymes, ion channels and/or mediators.
Gastrointestinal tract is regulated by the innervation of enteric nervous systems that control the rhythemic activity of gut smooth muscle cells (Schemann, 2005). In rat colon, the cyclic episodes of myenteric potential oscillations of circular muscles are associated with spontaneous contractions (Pluja et al., 1999). Anatomical and electrophysiological investigations on contractility of rat and dog colon preparations have shown that three components are involved in the colonic spontaneous contractions, i.e. the smooth muscle cells, a network of interstitial cells of Cajal (which generate pacemaker potentials) located near myenteric plexus, and a largely inhibitory innervation which is concentrated on the interstitial cells of Cajal (Huizinga et al., 1990; Faussone-Pellegrini, 1992.). In this study, the neuronal Na+ channel blocker TTX essentially abolished the inhibitory responses of schisandrin, suggesting the involvement of neuronal action in schisandrin-mediated colonic relaxation. Furthermore, the inability of propranolol, phentolamine, atropine and nicotine desensitization to inhibit the schisandrin responses suggested that the relaxant effect was mediated by inhibitory non-adrenergic non-cholinergic (NANC) transmitters released from the enteric nerves. In spite of the regional difference along intestine and the variance between species, vasoactive intestinal polypeptide (VIP), nitric oxide and ATP have been recognized as the main NANC inhibitory neurotransmitters. Generally speaking, the inhibitory NANC response involves a combination of two or more messengers, but one of them could play a predominant role in a particular section of gastrointestinal tract of some species (Maggi et al., 1993; Benkó et al., 2007).
Nitric oxide is an important NANC inhibitory transmitter in gastrointestinal tract. It plays an important role in colon relaxation elicited by activation of enteric nerves. In rat colon, relaxation of circular muscle to distension and longitudinal muscle to electrical field stimulation was blocked by L-NOARG (Suthamnatpong et al., 1994). In this study, pre-treatment of a NOS inhibitor L-NAME markedly inhibited schisandrin-mediated colonic relaxation (60-80% at 100-300 μM), suggesting the involvement of NO in the action of schisandrin. This was further demonstrated by the colorimetric measurement showing that NO production in the colon segments was elevated after incubation with schisandrin. Thus NO appeared to play an important role in schisandrin-induced relaxation on spontaneous contraction of rat colon. In previous studies, neuronal NOS (nNOS) has been identified in enteric nerve terminals and concentrated in the longitudinal muscle layer with attached myenteric plexus (Huber et al., 1998). It is therefore conceivable that schisandrin may trigger the Ca2+ influx via voltage-dependent Ca2+ channels in colonic NANC nerve, thereby activates nNOS that can catalyze the conversion of L-arginine to NO (Kurjak et al., 2002). The neuronally produced NO could subsequently diffuse to the adjacent colonic smooth muscle cells to take actions. Nevertheless, it remains unclear at this time whether the biosynthesis of NO within the enteric nerves is directly activated by schisandrin or it happens via induction of other transmitters such as VIP interplaying with NO in NANC inhibition (Grider et al., 1992). In addition, colonic prostacyclin (IP) was unlikely involved in the schisandrin-mediated NANC relaxation, as Ro1138452, a potent IP receptor antagonist (Bley et al., 2006), markedly inhibited the responses of an IP-receptor agonist cicaprost, but did not affect the schisandrin response.
In the gastrointestinal tract, the neuronally generated NO diffuses to smooth muscle cells containing soluble guanylyl cyclase (sGC). sGC is an important NO-sensitive mediator in the signal transduction of smooth muscle relaxation. The activated sGC catalyzes the conversion of GTP to cyclic GMP (cGMP), which in turn activates the cGMP-dependent protein kinase (PKG). PKG subsequently stimulates the extrusion of intracellular Ca2+ by phosphorylating a number of important target proteins including ion channels, ion pumps, receptors and enzymes, thereby decreases the sensitivity of contractile apparatus to Ca2+, leading to smooth muscle relaxation (Friebe et al., 2003). In this study, the sGC inhibitor ODQ strongly inhibited the schisandrin-mediated relaxation. The inhibitory responses of ODQ displayed comparable inhibitory extent as L-NAME (80-90%) at the highest concentration, implying NO could mainly act through sGC to elicit relaxation on the rat colon. The results agreed with a number of reports demonstrating that NO-sGC coupled pathways mediates colonic relaxation in different species including rodent, canine and human (Ward et al., 1992; Benkó et al., 2007; Dhaese et al., 2008). In contrast, adenylyl cyclase (AC)-dependent pathway was unlikely involved in the schisandrin-induced relaxation, as indicated by the inability of SQ22536 to inhibit the colonic response to schisandrin.
It has been recognized that more than one transmitter can be involved in NANC relaxation in response to stimulation in most regions of gastrointestinal tract (Belai and Burnstock, 1994; Mule and Serio, 2003). Apamin, a small conductance Ca2+-activated K+ channel blocker, is widely used to study the non-nitrergic components of NANC relaxation (Kishi et al., 1996). In this study, apamin concentration-dependently inhibited schisandrin-mediated colonic relaxation but did not affect the responses of a NO donor sodium nitroprusside (SNP), indicating the participation of non-nitrergic, apamin-sensitive transmitter(s). Generally, purinergic mediators such as ATP and adenosine (Burnstock G., 2008), as well as neuropeptides such as pituitary adenylate cyclase activating peptide (PACAP) and VIP (Kishi et al., 1996), are recognized as apamin-sensitive candidates. In the present study, ATP appeared to play a role in the action of schisandrin, as a purinoceptor antagonist PPADS at adequate concentrations significantly attenuated the schisandrin-induced relaxation. The results were in agreement with previous studies reporting the participation of ATP and purinoceptor in apamin-sensitive NANC relaxation in colon preparations (Rózsai et al., 2001; Benkó et al., 2007). In contrast, a potent adenosine A1 receptor antagonist, DPCPX, markedly attenuated the adenosine-induced relaxation, but did not influence the schisandrin responses, suggesting the activators of adenosine receptors unlikely participated in the response. Although VIP-like peptides are not likely involved in mediating NANC inhibition in colon preparations (Rózsai et al., 2001), the interplay between VIP and NO should be noted. The inability of α-chymotrypsin to affect the schisandrin response implied that a mediating role of VIP and other susceptible peptides appeared to be excluded in the action of schisandrin.
NANC inhibitory neurotransmitters, particularly NO, play important roles in regulating peristaltic reflex in colon (Foxx-Orenstein and Grider, 1996; Mizuta et al., 1999). The schisandrin-mediated colonic NANC relaxation is thus speculated to increase the accommodation of colon, reduce the velocity of colonic propulsion, and thereby moderate the colonic transit, which could be possibly beneficial for releasing diarrhea symptoms in gastrointestinal diseases. Since schisandrin is known to be one of the major ingredients isolated from Schisandra chinensis, the relaxant activity of schisandrin could be an important component for the applications of this herbal drug in treating gastrointestinal diseases. Given the significance of NANC innervation in various parts of the gastrointestinal tract, schisandrin is also speculated to be potentially useful in regulating esophageal motility (Konturek et al, 1997), gastrin motility and emptying (Sarna, 1996), although the selectivity of drug action must be systematically addressed.
In conclusion, schisandrin has been demonstrated to induce NANC relaxation on the rat colon and the response appeared to involve two or more mediators. NO appears to be one of the inhibitory transmitters that acts via cGMP-dependent pathways, and ATP likely plays a role in the non-nitrergic, apamin-sensitive component of the response. On the other hand, neither adenosine nor VIP seems to participate in the intestinal relaxation process.
Acknowledgement
This study was partially supported by grant 1-U19-AT003266 (PI: Brian Berman, University of Maryland) from the National Center for Complementary and Alternative Medicine (NCCAM), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM. All members of this international collaborative project are acknowledged for their collaborative efforts. The authors are also grateful to Mr. Kam Ming Chan for his kind assistance in the tissue isolation experiments, Ms. Penelope Mei Yu Or and Dr. Sam Hip Tsai for their technical support. Dr. Jia Ming Yang received a postgraduate studentship from the Chinese University of Hong Kong during the course of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belai A, Burnstock G. Evidence for coexistence of ATP and nitric oxide in non-adrenergic, non-cholinergic (NANC) inhibitory neurons in the rat ileum, colon and anococcygeus muscle. Cell Tissue Res. 1994;278:197–200. doi: 10.1007/BF00305792. [DOI] [PubMed] [Google Scholar]
- Benkó R, Undi S, Wolf M, Vereczkei A, Illényi L, Kassai M, Cseke L, Kelemen D, Horvath ÖP, Antal A, Magyar K, Barthó L. P2 purinoceptor antagonists inhibit the non-adrenergic, non-cholinergic relaxation of the human colon in vitro. Neuroscience. 2007;147:146–152. doi: 10.1016/j.neuroscience.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Bley KR, Bhattacharya A, Daniels DV, Gever J, Jahangir A, O'Yang C, Smith S, Srinivasan D, Ford Anthony PDW, Jett MF. RO1138452 and RO3244794: characterization of structurally distinct, potent and selective prostacyclin receptor antagonists. Br. J. Pharmacol. 2006;147:335–345. doi: 10.1038/sj.bjp.0706554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterology and Motility. Eur. J. Pharmacol. 2008;20:8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Vanneste G, Sips P, Buys E, Brouckaert P, Lefebvre RA. Involvement of soluble guanylate cyclas α1 and α2, and SKCa channels in NANC relaxation of mouse distal colon. Eur. J. Pharmacol. 2008;589:251–259. doi: 10.1016/j.ejphar.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS. Histogenesis, structure and relationships of interstitial cells of Cajal (ICC): from morphology to functional interpretation. Eur. J. Morphol. 1992;30:137–148. [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Grider JR. Regulation of colonic propulsion by enteric excitatory and inhibitory neurotransmitters. Am. J. Physiol. Gastrointest. Liver Physiol. 1996;271:433–437. doi: 10.1152/ajpgi.1996.271.3.G433. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Grider JR, Murthy KS, Jin JG, Makhlouf GM. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am. J. Physiol. Gastrointest. Liver Physiol. 1992;262:774–778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Halstead CW, Lee S, Khoo CS, Hennell JR, Bensoussan A. Validation of a method for the simultaneous determination of four schisandra lignans in the raw herb and commercial dried aqueous extracts of Schisandra chinensis (Wu Wei Zi) by RP-LC with DAD. J. Pharm. Biomed. Anal. 2007;45:30–37. doi: 10.1016/j.jpba.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Huber A, Saur D, Kurjak M, Schusdziarra V, Allescher HD. Characterization and splice variants of neuronal nitric oxide synthase in rat small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;275:1146–1156. doi: 10.1152/ajpgi.1998.275.5.G1146. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Berezin I, Daniel EE, Chow E. Inhibitory innervation of colonic smooth muscle cells and interstitial cells of Cajal. Can. J. Physiol. Pharmacol. 1990;68:447–454. doi: 10.1139/y90-063. [DOI] [PubMed] [Google Scholar]
- Ip SP, Mak DHE, Li PC, Poon MKT, Ko KM. Effect of a lignan-enriched extract of Schisandra chinensis on aflatoxin b1 and cadmium chloride-induced hepatotoxicity in rats. Pharmacol. Toxicol. 1996;78:413–416. doi: 10.1111/j.1600-0773.1996.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, Takewaki T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br. J. Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek JW, Thor P, Lukaszyk A, Gabryelewicz A, Konturek SJ, Domschke W. Endogenous nitric oxide in the control of esophageal motility in humans. J. Physiol. Pharmacol. 1997;48:201–209. [PubMed] [Google Scholar]
- Kurjak M, Sennefelder A, Aigner M, Schusdziarra V, Allescher HD. Characterizing voltage-dependent Ca2+ channels coupled to VIP release and NO synthesis in enteric synaptosomes. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:1027–1034. doi: 10.1152/ajpgi.00400.2001. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giulani S. Multiple inhibitory mechanisms mediate non-adrenergic noncholinergic relaxation in the circular muscle of the guinea-pig colon. Naunyn Schmiedebergs Arch. Pharmacol. 1993;347:630–634. doi: 10.1007/BF00166946. [DOI] [PubMed] [Google Scholar]
- Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277:275–279. doi: 10.1152/ajpgi.1999.277.2.G275. [DOI] [PubMed] [Google Scholar]
- Mule F, Serio R. NANC inhibitory neurotransmission in mouse isolated stomach: Involvement of nitric oxide, ATP and vasoactive intestinal polypeptide. Br. J. Pharmacol. 2003;140:31–437. doi: 10.1038/sj.bjp.0705431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Committee of Chinese Pharmacopoeia . Pharmacopoeia of the People's Republic of China. Chemical Industry Press; Beijing: 2005. pp. 44–45. [Google Scholar]
- Opletal L, Sovova H, Bartlova M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra importance, isolation and determination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812:357–371. doi: 10.1016/j.jchromb.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Pan SY, Dong H, Zhao XY, Xiang CJ, Fang HY, Fong WF, Yu ZL, Ko KM. Schisandrin B from Schisandra chinensis reduces hepatic lipid contents in hypercholesterolaemic mice. J. Pharm. Pharmacol. 2008;60:399–403. doi: 10.1211/jpp.60.3.0017. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Park JY, Lee SJ, Yun MR, Seo KW, Bae SS, Park JW, Lee YJ, Shin WJ, Choi YW, Kim CD. Gomisin A from Schisandra chinensis induces endothelium-dependent and direct relaxation in rat thoracic aorta. Planta Med. 2007;73:1537–1542. doi: 10.1055/s-2007-993757. [DOI] [PubMed] [Google Scholar]
- Park JY, Shin HK, Lee YJ, Choi YW, Bae SS, Kim CD. The mechanism of vasorelaxation induced by Schisandra chinensis extract in rat thoracic aorta. J. Ethnopharmacol. 2009;121:69–73. doi: 10.1016/j.jep.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Pluja L, Fernandez E, Jimenez M. Neural modulation of the cyclic electrical and mechanical activity in the rat colonic circular muscle: putative role of ATP and NO. Br. J. Pharmacol. 1999;126:883–892. doi: 10.1038/sj.bjp.0702363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu MR, Kim E-Y, Yoon B-K, Lee YJ, Chen S-N. Aqueous extract of Schizandra chinensis fruit causes endothelium-dependent and -independent relaxation of isolated rat thoracic aorta. Phytomedicine. 2006;13:651–657. doi: 10.1016/j.phymed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Rózsai B, Lázár Z, Benkó R, Barthó L. Inhibition of the NANC relaxation of the Guinea-pig proximal colon longitudinal muscle by the purinoceptor antagonist PPADS, inhibition of nitric oxide synthase, but not by a PACAP/VIP antagonist. Pharmacol. Res. 2001;43:83–87. doi: 10.1006/phrs.2000.0742. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Nitronergic regulation of gastric motility and emptying. Curr. Opin. Gastroenterol. 1996;12:512–516. [Google Scholar]
- Schemann M. Control of gastrointestinal motility by the “gut brain” - the enteric nervous system. J. Pediatr. Gastroenterol. Nutr. 2005;41:4–6. doi: 10.1097/01.scs.0000180285.51365.55. [DOI] [PubMed] [Google Scholar]
- Suekawa M, Shiga T, Sone H, Ikeya Y, Taguchi H, Aburada M, Hosoya E. Effects of gomisin J and analogous lignan compounds in schisandra fruits on isolated smooth muscles. Yakugaku Zasshi. 1987;107:720–726. doi: 10.1248/yakushi1947.107.9_720. [DOI] [PubMed] [Google Scholar]
- Suthamnatpong N, Hosokawa M, Takeuchi T, Tata F, Takewaki T. Nitric oxide-mediated inhibitory response of rat proximal colon: independence from changes in membrane potential. Br. J. Pharmacol. 1994;112:676–682. doi: 10.1111/j.1476-5381.1994.tb13129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Dalziel HH, Bradley ME, Buxton ILO, Keef K, Westfall DP, Sanders KM. Involvement of cyclic GMP in non-adrenergic, non-cholinergic inhibitory neurotransmission in dog proximal colon. Br. J. Pharmacol. 1992;107:1075–1082. doi: 10.1111/j.1476-5381.1992.tb13409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]