Abstract
Clinical hallmarks of mucormycosis infections include the unique susceptibility of patients with increased available serum iron, the propensity of the organism to invade blood vessels, and defective phagocytic function. These hallmarks underscore the critical roles of iron metabolism, phagocyte function, and interactions with endothelial cells lining blood vessels, in the organism’s virulence strategy. In an attempt to understand how Mucorales invade the host, we will review the current knowledge about interactions between Mucorales and the host while evading phagocyte-mediated killing. Additionally, since iron is an important determinant of the disease, we will focus on the role of iron on these interactions. Ultimately, a superior understanding of the pathogenesis of mucormycosis will enable development of novel therapies for this disease.
Introduction
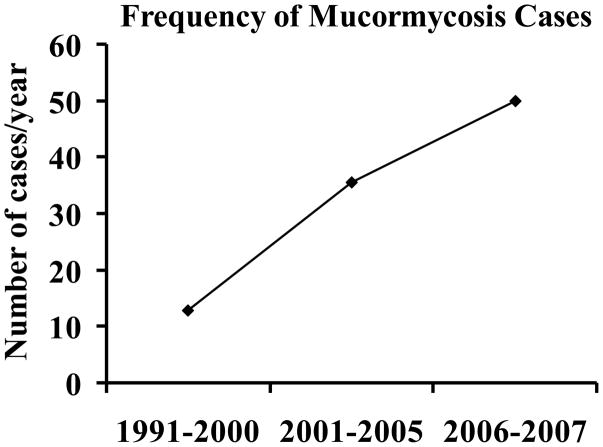
Mucormycosis (zygomycosis) is caused by fungi belong to the order Mucorales.[1–3] This life-threatening infection occurs in patients with: 1) increased available serum iron (e.g. from diabetic ketoacidosis [DKA]); 2) weakened immune system due to neutropenia or steroid treatment; 3) and/or trauma.[2] Recent data have demonstrated a striking increase in the number of reported cases of mucormycosis, possibly due to the rising prevalence of risk factors including diabetes, cancer, and organ transplantation in the ageing population of developed countries.[4], [5] For example, there has been an alarming rise in the incidence of mucormycosis at major transplant centers and the number of cases over a 15 year period has more than doubled.[5], [6] In fact, the prevalence of mucormycosis is up to 8% in autopsied patients with leukemia.[7] Additionally, a recently published population based study demonstrated a 70% increase in mucormycosis cases between 1997 and 2006.[8] Further, data from a tertiary care center demonstrated ≥ 400% increase in the incidence of mucormycosis, mainly among DKA patients between 1991 and 2007 (Fig. 1).[9,10] These studies suggest that the incidence of mucormycosis is increasing in both immunocompromised and DKA patients alike.
Figure 1.
Increasing incidence of mucormycosis at a tertiary care center compiled from Chakrabarti et al. 2008 and 2009.[9,10]
Despite disfiguring surgical debridement and adjunctive antifungal therapy, the overall mortality of mucormycosis remains approximately ≥40%, and it approaches 100% in patients with disseminated disease, or persistent neutropenia.[2,4–6,11,12] Clearly new strategies to prevent and treat mucormycosis are urgently needed and such strategies can be facilitated by clear understanding of the pathogenesis of the disease.
Mucorales infect the host either through inhalation, ingestion and/or through direct inoculation of fungal spores through an abraded skin due to trauma.[13] Therefore, during early steps of the infection Mucorales interact directly with epithelial cells and basement membranes which separate the host cells from the underlying stroma. Further, clinical hallmarks of R. oryzae infection include its remarkable angiotropism and the susceptibility of patients with increased available serum iron and/or altered phagocytic function. The angioinvasion and subsequent hematogenous dissemination during mucormycosis indicate that the organism interacts in vivo with: 1) endothelial cells lining the vasculature; 2) the subendothelial membrane which is made accessible to the fungus upon damaging endothelial cells. The hypersusceptibility of patients with increased available serum iron to infection by Mucorales, but not other pathogenic fungi, highlights the central role of iron metabolism in the organisms’ virulence strategy. For example, patients in DKA are uniquely susceptible to mucormycosis. These patients are known to have elevated free iron generated by proton-mediated liberation from transferrin due to the acidic pH of their blood.[14, 15] Finally, the susceptibility of patients lacking functional phagocytes underscores the vital role of these cells in the host defense against Mucorales. Consequently, for Mucorales to be successful pathogens, they must adhere to and invade host tissues while evading host defense mechanisms. Therefore, understanding the mechanisms by which these processes occur may lead to new approaches to prevent and/or treat mucormycosis.
Currently no information is available on how the fungus invades lung, sinus or intestinal epithelial cells. Therefore, this review will focus on the interaction between R. oryzae, and phagocytes as well as its interactions with extracellular matrix proteins and endothelial cells. Further, because increased free serum iron represents a major risk for mucormycosis,[2,14,15] the effect of iron on these interactions is discussed.
A. Interactions between R. oryzae and extracellular matrix components
Basement membranes are extracellular protein matrices that separate epithelial and endothelial cells from underlying stroma.[16] They provide structural support for these cells and serve as barriers to the passage of macromolecules and invading pathogens. The majority of basement membrane proteins consist of laminin and collagen IV. Epithelial cell damage has been reported in patients who are susceptible to mucormycosis, such as diabetics or patients receiving chemotherapy. This damage in turn exposes the extracellular matrix proteins so that they can directly interact with the pathogen. In this respect, an early study showed that R. oryzae can adhere to laminin and type IV collagen, but not to fibronectin [17]. This attachment occurs with spores prior to germination and decreases dramatically when the spores germinate. Furthermore, adherence of R. oryzae to laminin and collagen is specific as determined by anti-laminin and anti-collagen antibodies blocking studies as well as receptor competition experiments.[17]
R. oryzae is known to harbor an expanded family of genes encoding for the proteolytic enzymes, including secreted aspartic proteinase (SAP) and subtilases gene families[18,19] and multiple studies demonstrate the production of these enzymes by R. oryzae.[20–23] Genes specifying these lytic enzymes, which have been shown to contribute to the virulence of other organisms,[24,25] are present in larger numbers in R. oryzae compared to other fungi (28 SAP genes and 23 subtilases genes).[18] These genes are expressed in patients with mucormycosis[26,27] and their products likely facilitate the penetration of the organism through extracellular matrix proteins and invasion of host cells.
B. Host Defense against mucormycosis
Phagocytes are the major line of defense against Mucorales.[28,29] Inhalation of Mucorales spores by immunocompetent animals does not result in the development of mucormycosis (unpublished data).[29] In contrast, neutropenic hosts are at increased risk for developing mucormycosis. Furthermore, corticosteroids and diabetes, both of which are known to suppress phagocyte functions, cause animals inhaling R. oryzae spores to die from progressive pulmonary and hematogenously disseminated infection.[29–31]
Both mononuclear and polymorphonuclear phagocytes of normal hosts kill Mucorales by the generation of oxidative metabolites and the cationic peptides, defensins.[29,32,33] However, during DKA where there is hyperglycemia and acidosis, phagocytes display dysfunctional chemotaxis and intracellular killing of R. oryzae by both oxidative and non-oxidative mechanisms.[34] The dysfunction in phagocyte anti-Rhizopus activities during DKA is likely due to the direct effects of hyperglycemia and acidosis. Also the elevated free iron found in DKA patients[14, 15] might be toxic to phagocytes.[35–37] Additionally, spleen cells of mice fed excess levels of iron secrete less IFN-γ, [38] a cytokine that upregulates killing of several Mucorales family members (including R. oryzae) by human polymorphonuclear leukocytes (PMNLs).[39] Therefore, it is likely that during DKA excess levels of iron lead to impaired phagocytic function. This hypothesis is supported by impaired chemotaxis of neutrophils in response to R. oryzae infection in mice given excessive amount of iron compared to normal mice.[40] This impaired chemotaxis is reversed upon treating mice with the Mucorales cidal iron chelator, deferasirox.[40]
It is also likely that members of the Mucorales order suppress the immune recognition during infection. In this respect, a study using whole genome expression profiling in Drosophila melanogaster after infection with R. oryzae identified host genes that was selectively down-regulated, act in pathogen recognition, innate immune defense mechanisms and tissue repair mechanisms.[41] These findings might further explain the success of Mucorales in evading host defenses and in causing extensive tissue necrosis.
C. Endothelial cell-R. oryzae interactions
Damage of and penetration through the endothelial cell lining of the blood vessels is likely a critical step in the pathogenetic strategy of Mucorales because angioinvasion is a hallmark of mucormycosis.[1,2,13,42] This angioinvasion often results in vessel thrombosis and subsequent tissue necrosis which can prevent delivery of leukocytes and antifungal agents to the foci of infection, thereby further exacerbating the disease.[2,43,44] A focus of our research is how Mucorales adhere to and invade the endothelium. These studies mainly utilize germinated R. oryzae (germlings) since this is the form that is likely to interact with endothelial cells during tissue invasion.
1. Adherence and invasion of human umbilical vein endothelial cells by R. oryzae.
R. oryzae germlings adhere avidly to endothelial cells but not to bare plastic.[45] Furthermore, R. oryzae germlings are able to cause endothelial cell injury in vitro independent of any serum factors.[45] This process requires direct contact between the organism and endothelial cells because membrane inserts separating R. oryzae from endothelial cells completely abrogates injury.[45] Endothelial cell injury requires internalization of R. oryzae (i.e. invasion of the endothelium) because the use of the endothelial cell microfilament inhibitor, cytochalasin D, blocks internalization of and R. oryzae-induced endothelial cell injury.[45] In addition, chelation of endothelial cell iron prevents R. oryzae from invading and damaging endothelial cells, which suggests that host iron can modulate the ability of Mucorales to cause disease.[46] Of note, mice treated with the deferiprone and deferasirox (two iron chelators that deprive R. oryzae from acquiring external iron) are protected from hematogenously disseminated mucormycosis.[40,47]
Unlike other fungi (e.g. C. albicans,[48] Cryptococcus neoformans,[49] etc.), we have determined that fungal-induced endothelial cell injury does not require fungal viability since dead germlings (heat-, ethanol-, or gluteraldehyde-killed) are able to cause a similar degree of injury to endothelial cells as do live organisms.[45] This ability of dead germlings to cause injury is also dependent on their internalization by endothelial cells. Injury mediated by dead germlings is induced by cell-associated rather than soluble factors since cell debris, but not the supernatant, from broken germlings as well as cell wall material from regenerating protoplasts of R. oryzae germlings cause equivalent injury to endothelial cells as live organisms do (unpublished data). Similar results are obtained when cell wall materials are collected from other members of the Mucorales order such as R. microsporus, Mucor, Cunninghamella, and Absidia (unpublished data). These results indicate that endothelial cell injury caused by Mucorales is dependent, at least in part, on a toxin like substance(s) that is associated with the cell wall.
2. GRP78 is a novel endothelial cell receptor for Mucorales
To identify the host receptor(s) that are utilized by Mucorales to invade endothelial cells, we used the affinity purification process developed by Isberg and Leong,[50] in which extracts of endothelial cell membrane proteins were incubated with intact R. oryzae germlings. A 78 kDa endothelial cell protein found to bind to R. oryzae but not S. cerevisiae (which does not adhere to or invade endothelial cells).[45] The major band at 78 kDa was identified as Glucose Regulated Protein 78 (GRP78). This protein is a novel host receptor which mediates invasion and subsequent injury of endothelial cells by Mucorales, but not C. albicans or A. fumigatus.[46] Additionally, GRP78 is a specific and universal receptor for germlings and not spores of several Mucorales members.[46] Although GRP78 is utilized by R. oryzae to invade endothelial cells, it does not play a role in initial fungal adherence to host cells.[46] These results provide support to a model by which the fungus invades endothelial cells through a two step approach that initially involves adherence of the fungus to a receptor followed by binding to GRP78, which triggers invasion.
GRP78 (also known as BiP/HSPA5) was discovered as a cellular protein induced by glucose starvation [51]. It is a member of the HSP70 protein family that is mainly present in the endoplasmic reticulum. It functions as a major chaperone that is involved in many cellular processes, including protein folding and assembly, marking misfolded proteins for proteosome degradation, [52] regulating Ca2+ homeostasis, and serving as a sensor for endoplasmic reticulum stress.[53] Despite its main function as a cellular chaperone protein, recent studies reported the translocation of a fraction of GRP78 to the cell surface in a variety of cells.[54]
Of interest, we found that elevated concentrations of glucose and iron, consistent with those seen during DKA, enhance GRP78 surface expression and resulting invasion and injury of endothelial cells in a receptor-dependent manner. These results are concordant with our finding that chelation of endothelial cell iron protects these cells from R. oryzae-induced injury in vitro.[46] We also found that mice in DKA, which have enhanced susceptibility to mucormycosis, have increased expression of GRP78 in their sinus, lungs, and brain versus normal mice and that anti-Grp78 immune serum protects these mice from mucormycosis.[46] Collectively, these data offer an explanation for the longstanding mystery as to why hosts in DKA are uniquely predisposed to mucormycosis infection and provide a foundation for novel therapeutic interventions against this deadly infection.
Conclusions
Mucormycosis has a remarkably high morbidity and mortality and its incidence is on the rise. This disease represents the third most common fungal infection in hematologic malignancy patients.[55–57] The availability of iron in the host environment likely plays a critical role in predisposing the host to mucormycosis. Therefore, strategies focused on depriving the fungus from host iron can be beneficial in preventing or treating the disease.
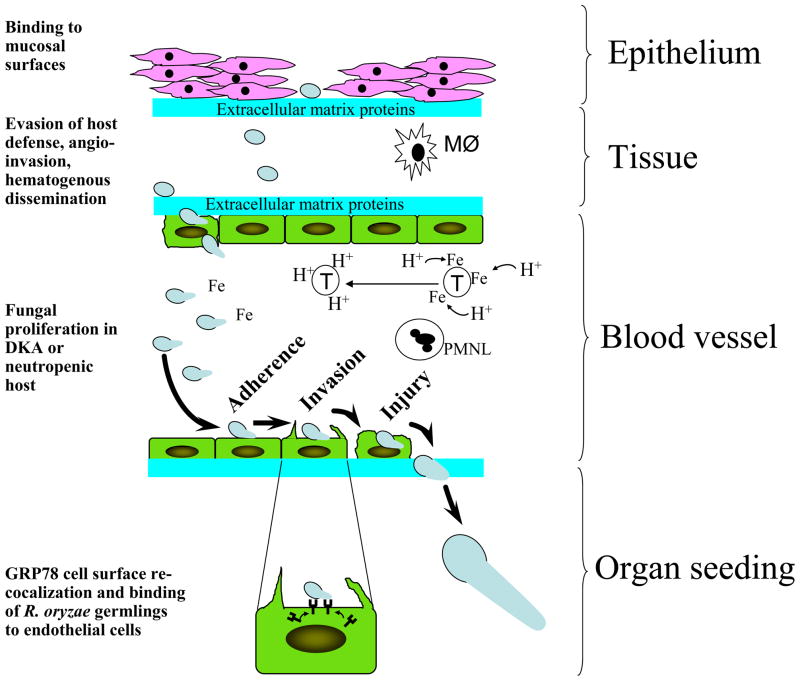
Mucorales enter the host through inhalation, ingestion, or through direct inoculation via abraded skin. The organism invades the host through attachment to extracellular matrix proteins and/or possibly via adherence to epithelial cells while evading killing by resident phagocytes. Further, blood vessel invasion and subsequent hematogenous dissemination happens through interacting with extracellular matrix proteins and endothelial cells via attachment to GRP78 which is upregulated in elevated iron and glucose concentrations seen in susceptible hosts such as DKA. Lack of vasculature phagocytes (e.g. neutropenia) or presence of dysfunctional PMNL (e.g. DKA) further exacerbates the infection. Figure 2 summaries our hypothesis of how R. oryzae causes disease in a susceptible host. However, many questions remain unanswered. For example, it is still unknown what is the fungal ligand(s) that mediates attachment/invasion of the organisms to host cells? Furthermore, since adherence and invasion are two independent processes and GRP78 is a receptor for Mucorales during invasion but not adherence, it is still to be determined what other host cell receptor(s) mediates the initial steps of Mucorales adherence to endothelial cells. Finally, studies focused on investigating the interactions of Mucorales with endothelial cells only address mucormycosis pathogenesis during hematogenous dissemination and organ seeding but not during the early stages of infection. Investigations focused on studying interactions between Mucorales and lung, sinus, or intestinal epithelial cells will better enhance our understanding of how the infection occurs and likely to provide promising targets for immuno-prophylactic or therapeutic strategies against mucormycosis.
Figure 2.
Proposed mechanisms of host invasion by Mucorales during mucormycosis. Mucormycosis is contracted through inhalation, ingestion, or direct inoculation into an abraded skin of Mucorales spores. These spores enter tissues by binding to exposed extracellular matrix proteins due to epithelial cell damage in a susceptible host. It is also possible that Mucorales first invade epithelial cells then penetrate the extracellular matrix protein via the action of secreted proteases. Once inside the tissues and due to immunosuppressive predisposing factors, Mucorales evade tissue macrophage (MØ)-mediated killing and invade blood vessels by binding to extracellular matrix proteins followed by invading endothelial cells. In a susceptible host, Mucorales thrive in an invaded blood vessel due to the abundance of free iron (e.g. release of iron from transferrin (T) via a proton-mediated mechanism in DKA patients) and the lack of or the presence of ineffective PMNL. Germlings adhere to endothelial cell through a receptor that is yet to be determined. Next, Mucorales invade endothelial cells by binding to GRP78 which is overexpressed and re-localized to the endothelial cell surface in high concentrations of glucose and iron often seen in DKA patients. Finally, fungal hyphae penetrate the blood vessel and seed target organs.
Highlights.
We review the how Mucorales invade host constituents. Elevated available serum iron compromises phagocyte killing of Mucorales. Mucorales binds to extracellular matrix proteins and invade endothelial cells. Iron enhances host cell invasion by increasing expression of the host receptor GRP78. Iron chelation therapy in animals is protective against mucormycosis.
Acknowledgments
This work was supported in part by Public Health Service grant R01 AI063503 and R21 AI082414 from the National Institutes of Allergy and Infectious Diseases.
Footnotes
Disclosures: Grant Support: Astellas, Enzon, Gilead, Merck, Pfizer, NovaDigm Therapeutics, Novartis; Consultant: NovaDigm Therapeutics, Novartis; Shareholder: NovaDigm Therapeutics
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18 :556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 4.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45 :1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30:851–856. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr Opin Infect Dis. 2004;17 :517–525. doi: 10.1097/00001432-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, Desenclos JC, Lortholary O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15:1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti A, Chatterjee SS, Shivaprakash MR. Overview of opportunistic fungal infections in India. Nippon Ishinkin Gakkai Zasshi. 2008;49:165–172. doi: 10.3314/jjmm.49.165. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 11.Kauffman CA. Zygomycosis: reemergence of an old pathogen. Clin Infect Dis. 2004;39:588–590. doi: 10.1086/422729. [DOI] [PubMed] [Google Scholar]
- 12.Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, Bartelt LA, Kilborn SB, Hoth PL, Diekema DJ, Pfaller MA. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39 :584–587. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- 13.Sugar AM. Agents of Mucormycosis and Related Species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6. Elsevier; 2005. p. 2979. [Google Scholar]
- 14.Ibrahim AS, Spellberg B, Edwards J., Jr Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–1114. doi: 10.2337/diacare.31.12.1109. This study introduced the first link between susceptibility of DKA patients and availability of serum iron. [DOI] [PubMed] [Google Scholar]
- 16.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- *17.Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70:76–83. This study demonstrated that spores and not germlings of R. oryzae can bind to the extracellular matrix proteins, laminin and type IV collagen. [PubMed] [Google Scholar]
- 18.Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, Elias M, Idnurm A, Lang BF, Sone T, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5 :e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farley PC, Sullivan PA. The Rhizopus oryzae secreted aspartic proteinase gene family: an analysis of gene expression. Microbiology. 1998;144 ( Pt 8):2355–2366. doi: 10.1099/00221287-144-8-2355. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Cho YC, Lai CC, Hsu WH. Purification and characterization of a new Rhizopuspepsin from Rhizopus oryzae NBRC 4749. J Agric Food Chem. 2009;57 :6742–6747. doi: 10.1021/jf8040337. [DOI] [PubMed] [Google Scholar]
- 21.M'Hir S, Rizzello CG, Di Cagno R, Cassone A, Hamdi M. Use of selected enterococci and Rhizopus oryzae proteases to hydrolyse wheat proteins responsible for celiac disease. J Appl Microbiol. 2009;106:421–431. doi: 10.1111/j.1365-2672.2008.04008.x. [DOI] [PubMed] [Google Scholar]
- 22.Egusa S, Otani H. Characterization of a cellular immunostimulating peptide from a soybean protein fraction digested with peptidase R. J Nutr Sci Vitaminol (Tokyo) 2009;55:428–433. doi: 10.3177/jnsv.55.428. [DOI] [PubMed] [Google Scholar]
- 23.Egusa S, Otani H. Soybean protein fraction digested with neutral protease preparation, "Peptidase R", produced by Rhizopus oryzae, stimulates innate cellular immune system in mouse. Int Immunopharmacol. 2009;9:931–936. doi: 10.1016/j.intimp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Sanglard D, Hube B, Monod M, Odds FC, Gow NA. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hube B, Sanglard D, Odds FC, Hess D, Monod M, Schafer W, Brown AJ, Gow NA. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoen C, Reichard U, Monod M, Kratzin HD, Ruchel R. Molecular cloning of an extracellular aspartic proteinase from Rhizopus microsporus and evidence for its expression during infection. Med Mycol. 2002;40:61–71. doi: 10.1080/mmy.40.1.61.71. [DOI] [PubMed] [Google Scholar]
- 27.Spreer A, Ruchel R, Reichard U. Characterization of an extracellular subtilisin protease of Rhizopus microsporus and evidence for its expression during invasive rhinoorbital mycosis. Med Mycol. 2006;44:723–731. doi: 10.1080/13693780600936399. [DOI] [PubMed] [Google Scholar]
- 28.Sugar AM. Agent of mucormycosis and related species. In: Mandell G, Bennett J, Dolin R, editors. Principles and practices of infectious diseases. 4. Churchill Livingstone; 1995. pp. 2311–2321. [Google Scholar]
- 29.Waldorf AR, Diamond RD. Cerebral mucormycosis in diabetic mice after intrasinus challenge. Infection and Immunity. 1984;44:194–195. doi: 10.1128/iai.44.1.194-195.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldorf AR, Ruderman N, Diamon;d RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. Journal of Clinical Investigation. 1984;74:150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldorf AR, Halde C, Vedros NA. Murine model of pulmonary mucormycosis in cortisone-treated mice. Sabouraudia. 1982;20:217–224. doi: 10.1080/00362178285380321. [DOI] [PubMed] [Google Scholar]
- 32.Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunology Series. 1989;47:243–271. [PubMed] [Google Scholar]
- 33.Diamond RD, Haudenschild CC, Erickson NF., 3rd Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982;38:292–297. doi: 10.1128/iai.38.1.292-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infection and Immunity. 1982;38:1123–1129. doi: 10.1128/iai.38.3.1123-1129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oren H, Sahin B, Irken G, Ates H, Duman M, Yilmaz S, Turker M, Atabay B, Yaprak I. Neutrophil apoptosis in patients with beta-thalassemia major. Pediatr Hematol Oncol. 2003;20:237–243. doi: 10.1080/713842280. [DOI] [PubMed] [Google Scholar]
- 36.Cantinieaux B, Janssens A, Boelaert JR, Lejeune M, Vermylen C, Kerrels V, Cornu G, Winand J, Fondu P. Ferritin-associated iron induces neutrophil dysfunction in hemosiderosis. J Lab Clin Med. 1999;133:353–361. doi: 10.1016/s0022-2143(99)90066-5. [DOI] [PubMed] [Google Scholar]
- 37.Guo D, Jaber BL, Lee S, Perianayagam MC, King AJ, Pereira BJ, Balakrishnan VS. Impact of iron dextran on polymorphonuclear cell function among hemodialysis patients. Clin Nephrol. 2002;58:134–142. doi: 10.5414/cnp58134. [DOI] [PubMed] [Google Scholar]
- 38.Omara FO, Blakley BR. The effects of iron deficiency and iron overload on cell-mediated immunity in the mouse. Br J Nutr. 1994;72:899–909. doi: 10.1079/bjn19940094. [DOI] [PubMed] [Google Scholar]
- 39.Gil-Lamaignere C, Simitsopoulou M, Roilides E, Maloukou A, Winn RM, Walsh TJ. Interferon- gamma and Granulocyte-Macrophage Colony-Stimulating Factor Augment the Activity of Polymorphonuclear Leukocytes against Medically Important Zygomycetes. J Infect Dis. 2005;191:1180–1187. doi: 10.1086/428503. [DOI] [PubMed] [Google Scholar]
- *40.Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. We show that iron acquisition is an important virulence factor for R. oryzae and chelation of iron protects mice from hematogenously disseminated mucormycosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, Halder G, Kontoyiannis DP. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A. 2008;105:9367–9372. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon-Chung KJ, Bennett JE. Medical Mycology. Lea & Febiger; 1992. Mucormycosis; pp. 524–559. [Google Scholar]
- 43.Ibrahim AS, Edwards JEJ, Filler SG. Zygomycosis. In: Dismukes WE, Pappas PG, Sobel JD, editors. Clinical mycology. Oxford University Press; 2003. pp. 241–251. [Google Scholar]
- 44.Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009;59:134–138. doi: 10.1016/j.jinf.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE., Jr Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778–783. doi: 10.1128/IAI.73.2.778-783.2005. In this study we demonstrate that R. oryzae adheres to, invades through and damages human umbilical vein endothelial cells in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **46.Liu M, Spellberg B, Phan QT, Fu Y, Lee AS, Edwards JE, Jr, Filler SG, Ibrahim AS. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 120:1914–1924. doi: 10.1172/JCI42164. In this paper we show that GRP78 is the receptor utilized by Mucorales to invade endothelial cells and we provide an explanation to why DKA patients are uniquely predisposed to mucormycosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim AS, Edwards JE, Jr, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother. 2006;58 :1070–1073. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- 48.Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim AS, Filler SG, Alcouloumre MS, Kozel TR, Edwards JE, Jr, Ghannoum MA. Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect Immun. 1995;63:4368–4374. doi: 10.1128/iai.63.11.4368-4374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60 :861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 51.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 52.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, Safdar A, Raad, Kontoyiannis DP. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91:986–989. [PubMed] [Google Scholar]
- 56.Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, Corvatta L, D'Antonio D, Girmenia C, Martino P, et al. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–214. [PubMed] [Google Scholar]
- 57.Eucker J, Sezer O, Graf B, Possinger K. Mucormycoses. Mycoses. 2001;44:253–260. [PubMed] [Google Scholar]