Abstract
Previous research indicates that abnormal attention-emotion interactions are related to symptom presentation in individuals with schizophrenia. However, the individual components of attention responsible for this dysfunction are unclear. In the current study we examined the possibility that schizophrenia patients with higher levels of negative symptoms (HI-NEG: n = 14) have greater difficulty disengaging attention from unpleasant stimuli than patients with low negative symptoms (LOW-NEG: n = 18) or controls (CN: n = 27). Participants completed an exogenous emotional cueing task that required them to focus on an initial emotional or neutral cue and subsequently shift attention to a separate location outside of foveal vision to detect a target stimulus (letter). Results indicated that HI-NEG patients had greater difficulty disengaging attention from unpleasant stimuli than CN or LOW-NEG patients; however, behavioral performance did not differ among the groups for pleasant stimuli. Higher self-reported trait negative affect was also associated with greater difficulty disengaging attention from unpleasant stimuli. Abnormalities in disengaging attention from unpleasant stimuli may thus play a critical role in the formation and maintenance of both negative symptoms and trait negative affect in individuals with schizophrenia.
Keywords: Schizophrenia, Emotion, Attention, Negative Symptoms
1. Introduction
In recent years, there has been increased interest in studying affective disturbance in individuals with schizophrenia. This is due in part to what appears to be a consistent set of discrepancies among various methods of emotional self-report that have brought into question what anhedonia actually reflects in these patients. Specifically, previous findings indicate that patients report diminished levels of pleasure relative to controls when queried on clinical rating scales or self-report questionnaires, yet report experiencing similar levels of pleasure to controls when exposed to pleasant stimuli in laboratory paradigms (see Kring & Moran, 2008; Horan, Kring, Blanchard 2006; Cohen & Minor, 2010). Although patients do not appear to have reduced hedonic capacity when providing “online” (i.e., in the moment) self-report in response to stimuli, they do report experiencing greater negative affect than controls when exposed to unpleasant, neutral, and pleasant stimuli (Cohen & Minor, 2010). When coupled with the observation that patients have increases in trait negative mood (Horan et al., 2008), this consistent pattern of findings has led some researchers to suggest that abnormalities in emotional experience, and perhaps anhedonia itself, may reflect chronic elevations in negative mood that occur due to a problem in emotion regulation (Cohen & Minor, 2010; Cohen et al., 2011; Horan et al., 2006; Strauss & Herbener, 2011).
Studies examining the potential causes of anhedonia and these elevations in negative affect have been limited. Here, we offer the novel possibility that difficulty disengaging attention from salient unpleasant features of the environment makes it difficult for individuals with schizophrenia to attenuate negative emotional states, resulting in chronic elevations in negative mood and anhedonia. We found some evidence for this possibility in our previous study using the Emotional Stroop task, which indicated that patients with deficit schizophrenia (i.e., primary and enduring negative symptoms) had longer RTs for neutral words than they did for unpleasant words immediately preceding them, ostensibly signifying that negative symptom patients have difficulty disengaging attention once it had been engaged by a salient unpleasant stimulus (Strauss et al., 2008)1. However, the precise attentional mechanisms operating in that variant of the Emotional Stroop are unclear since the task did not require a shift in visual attention to a different spatial location.
In the current study, we used an exogenous emotional cueing task developed by Fox et al. (2001) (experiment 5) to further examine this emotion-attention interaction and more directly test the possibility that high negative symptom patients display difficulty disengaging visual-spatial attention from unpleasant stimuli. In this task, an emotional cue (word) presented in the center of the screen is immediately followed by a target letter (S or K) that is presented in one of 4 locations (above, below, left, or right). On each trial, participants are asked to attend to a cue (word) and indicate which target (letter) was presented. Unlike the Emotional Stroop, this task directly assesses disengagement by requiring a shift in spatial attention to multiple locations within the visual field. If participants display difficulty disengaging attention from a given emotional stimulus, they should display longer RTs while identifying targets (letters) immediately following an emotional cue than targets immediately following a neutral cue. In line with our previous study (Strauss et al., 2008), we hypothesized that patients with more severe negative symptoms would display greater difficulty disengaging attention from unpleasant stimuli than patients with low negative symptoms or controls. No differences were expected among the groups in relation to pleasant stimuli. We also predicted that increased trait negative mood on the Positive and Negative Affect Scale (PANAS-X: Watson & Clark, 1992) and clinical Anhedonia rated on the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) (but not alogia or restricted affect), would be associated with difficulty disengaging attention from unpleasant stimuli, in line with our theory that these dysfunctional emotion-attention interactions predict chronic elevations in negative emotion and anhedonia.
2. Methods and Materials
2.1. Participants
The current study included 32 participants meeting DSM-IV-TR criteria for schizophrenia or schizoaffective disorder, as determined by the Structured Clinical Interview for DSM-IV (SCID; First et al., 2001), and 27 healthy control participants (CN). Patients were recruited from the outpatient clinics at the Maryland Psychiatric Research Center (MPRC) and were studied during a period of clinical stability. Schizophrenia patients were divided into high (HI-NEG: n = 14) and low (LOW-NEG: n = 18) negative symptom groups based upon a median split on the Scale for Negative Assessment of Negative Symptoms (SANS; Andreasen, 1983; Buchanan et al., 2007) total score of all patients included in our outpatient clinics at the Maryland Psychiatric Research Center (MPRC) (Median score used = 34; n = 1374 ratings). The 22-item version of the SANS used in the current study was developed in the CONSIST clinical trial (Cognitive and Negative Symptoms in Schizophrenia Trial) (Buchanan et al., 2007). For the HI-NEG group the mean SANS item score was in the mild to moderate range (M = 2.12; range = 1.6 to 3.3), and scores in the LOW-NEG are in the normal to absent range (M = 0.88; range = 0.1 to 1.5).
Control subjects were recruited from random digit dialing and word of mouth among individuals recruited through random digit dialing. CN were administered a screening interview and denied a lifetime or family history of psychosis and any current Axis I or II disorders according to the SCID and SIDP-IV (Pfohl et al., 1997), respectively. The three participant groups did not significantly differ in age, gender, or ethnicity. Patients had fewer years of total education than controls (Table 1). The HI-NEG and LOW-NEG patients significantly differed on the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962) positive symptom factor and the disorganization symptom factor score. The two patient groups did not significantly differ on the BPRS total score (p = 0.07).
Table 1.
Characterizing data for individuals with schizophrenia and controls.
| LOW-NEG (n = 18) | HI-NEG (n = 14) | CN (n = 27) | Test Statistic | p | |
|---|---|---|---|---|---|
| Age | 44.28 (9.04) | 43.86 (8.68) | 42.26 (9.56) | F = 0.30 | p = 0.74 |
| Education | 13.11 (2.27) | 12.50 (2.47) | 15.11 (2.01) | F = 8.05 | p = 0.001 |
| % Male | 61.1% | 66.7% | 64.3% | χ2 = 1.54 | p = 0.82 |
| Race | |||||
| Caucasian | 55.6% | 60.0% | 64.3% | ||
| African- American | 33.3% | 26.7% | 32.1% | ||
| Asian | 5.6% | 0.0% | 0.0% | ||
| American Indian or Alaskan Native | 0.0% | 6.7% | 3.6% | ||
| Other | 5.6% | 0.0% | 0.0% | ||
| Antipsychotic Medications | χ2 = 2.18 | p = 0.34 | |||
| % Conventional | 23.5% | 6.7% | |||
| % Atypical | 64.7% | 86.7% | |||
| SANS Total | 21.61 (9.36) | 46.60 (11.04) | F = 49.59 | p < 0.001 | |
| BPRS Symptoms | |||||
| Positive | 1.97 (0.66) | 2.68 (0.96) | F = 6.11 | p = 0.02 | |
| Disorganized | 1.22 (0.24) | 1.67 (0.38) | F = 16.79 | p < 0.001 | |
| Total | 31.94 (8.11) | 37.79 (9.20) | F = 3.43 | P = 0.07 | |
| PANAS | |||||
| Positive affect | 31.0 (5.2) | 28.0 (7.4) | 33.0 (5.9) | F = 2.16 | P = 0.12 |
| Negative affect | 18.5 (7.7) | 22.3 (8.1) | 15.1 (6.3) | F = 3.98 | P = 0.02 |
Note. HI-NEG and LOW-NEG patients were prescribed a similar regimen of antipsychotic medications. The most frequently used medication was clozapine, used alone (n = 9), in conjunction with risperidone (n = 3), or in conjunction with quetiapine (n = 1). Risperidone used alone (n = 3) or in conjunction with clozapine (n = 3) or olanzapine (n = 2) was the second most frequently used antipsychotic. Patients were also prescribed olanzapine (n = 5), fluphenazine (n = 2), loxapine (n = 1), thiothixine (n =1), quetiapine (n = 1), haloperidol (n = 2), and ziprasidone (n = 2) alone. One patient was prescribed a combination of haloperidol and ziprazidone.
2.2. Measures
2.3.1. Symptom Ratings and Trait Emotional Self-Report
The Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962) and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) were administered to assess global psychiatric and negative symptoms, respectively. The Positive and Negative Affect Schedule- Version X (PANAS-X: Watson & Clark, 1992) was used to assess trait positive and negative emotional experience.
2.3.2. Exogenous Emotional Cueing Task
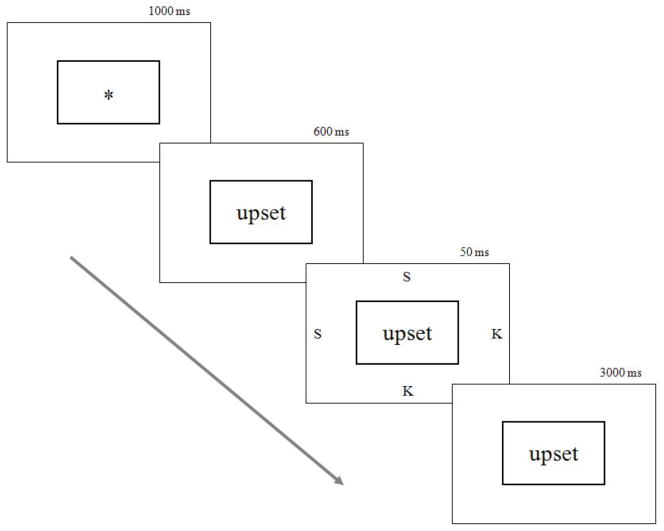
Participants completed a modified exogenous emotional cueing task based on the paradigm developed by Fox et al. (2001) (Experiment 5). To orient participants to the nature of the task, the experimenter walked participants through a set of instructions and examples that described the trial sequence. Participants were told that they would be presented with a small box on the computer screen containing an asterisk, and that the asterisk would be replaced by a word that would remain on the screen for a short time. While this word was on the screen, a letter (S or K) would briefly flash on the computer screen either above, below, to the left or to the right of the grey box. Participants were instructed to respond by pressing the correct letter button on the computer keyboard as accurately and quickly as possible, and asked to remember the words for later because they would be tested for whether or not they remembered them.
Throughout the experiment, a dark gray box of 1 mm line thickness, 1.5 cm height, and 2.0 cm length was displayed continuously in the center of the screen. An asterisk was presented at the start of each trial in the middle of the box for 1,000 ms. Subsequently, one of the word stimuli was displayed in the box, and after 600 ms the target stimulus (S or K) was presented for 50 ms at one of the four locations described above (3.5 cm away from the centrally fixated word). The word stimuli remained at fixation for 3,000 ms or until the participant responded. There was a 2,000 ms intertrial interval. Accuracy and reaction time were recorded in relation to participant responses (button press).
Participants completed a block of 18 practice words, which were all neutral in valence. This was followed by completion of one block of 120 experimental trials. Trials were equally divided into those appearing above, below, to the left, or the right of the central box (30 trials for each location). For each target location, the centrally fixated word was chosen from one of 20 neutral, 20 unpleasant, and 20 pleasant words. Each individual word was presented twice within the experimental block. See Figure 1 for an example trial sequence.
Figure 1.
Example trial sequence of the Exogenous Emotional Cueing Task.
Following the experimental block, participants completed a recognition testing session, which consisted of 120 trials, where 60 trials consisted of the 20 pleasant, 20 unpleasant, and 20 neutral words included in the experimental block. There were also 60 foil trials, consisting of 20 pleasant, 20 unpleasant, and 20 neutral words not included in the experimental session. The recognition testing phase was included to ensure that subjects adequately attended to word stimuli before disengaging attention and shifting to detect the targets. Recognition results are presented in Supplemental Materials.
Emotional words were selected to broadly represent unpleasant emotional content rather than any one discrete emotional category (Strauss & Allen, 2008). Tasks including such stimuli have produced reliable emotion-attention effects (Strauss et al., 2005; Strauss & Allen, 2006, 2009). Details of word stimuli can be found in Supplemental Materials online.
2.4. Data Analysis
Analyses were completed in several steps. First, we computed means for demographic and clinical variables to determine the appropriateness of controlling for these variables in primary analyses. The three groups significantly differed on participant education, and the two patient groups significantly differed on education and severity of psychotic and disorganized symptoms (see Table 1 and Participants section). Second, we examined the distributions of RT and Accuracy variables to ensure that they met assumptions for parametric analyses. It was found that accuracy data exhibited moderately negative skewness; however, RT difference scores calculated for positive and negative conditions (emotional RT – neutral RT) were approximately normally distributed. Third, MANOVA was used to examine group differences in RT difference scores for pleasant and unpleasant stimuli. MANCOVAs were then conducted to determine whether the inclusion of education, psychosis, and disorganization as covariates significantly altered results. Significant differences among the 3 groups for individual emotion conditions were followed-up by post hoc LSD contrasts. Fourth, the Kruskal-Wallis H-Test was selected to examine differences in accuracy for pleasant, unpleasant, and neutral conditions given that data violated assumptions for parametric analyses. Finally, Spearman correlations were calculated between behavioral test performance variables and symptom severity scores to further examine relationships with clinical presentation. Effect size is reported in terms of partial eta squared (0.01=low; 0.06=medium; and 0.14=large).
3.0. Results
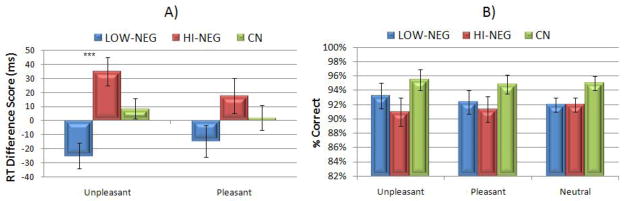
MANOVA was conducted to examine the effect of diagnosis on attentional disengagement. An emotional word RT difference score served as the dependent variable (calculated as emotional condition RT – neutral condition RT). MANOVA indicated a significant overall effect for group, F (4, 116) = 4.40, p= 0.002 (η2=.13), signifying differences in the extent to which emotional information created difficulty with attentional disengagement across CN, LOW-NEG, and HI-NEG groups. However, only unpleasant stimuli produced a significant difference as an individual variable, F (2, 61) = 10.22, p < 0.001 (η2= 0.26). Post hoc LSD contrasts indicated that HI-NEG had significantly greater difficulty disengaging attention from unpleasant stimuli than CN (p < 0.04) and LOW-NEG patients (p < 0.001), and that CN had slower disengagement than LOW-NEG from unpleasant stimuli (p < 0.01)(see Figure 2)2.
Figure 2.
Mean (SE) Accuracy and RT Difference Scores for Word Conditions in HI-NEG, LOW-NEG, and CN Participants.
Note. Panel A presents mean RT difference scores for pleasant and unpleasant conditions across the 3 groups. RT difference scores were calculated as Emotional Word RT – Neutral Word RT. Higher scores reflect greater difficulty disengaging attention from emotional relative to neutral stimuli. Panel B presents mean accuracy for pleasant and unpleasant words across the 3 groups. Higher scores reflect greater accuracy for target letters presented in conjunction with word stimuli. *** = p < 0.001.
The Kruskal-Wallis H-Test was conducted to examine differences in accuracy among the three groups. There were no significant differences in median level of accuracy for pleasant, unpleasant, or neutral conditions (see Figure 2).
Spearman correlations indicated a significant association between RT for unpleasant words and SANS total negative symptoms (r = 0.45, p < 0.01) and the BPRS disorganization factor (r = 0.48, p <0.01) in individuals with schizophrenia3. Correlations with SANS global items for the 4 individual subscales indicated a significant relationship between unpleasant word RT and Global Anhedonia (r = 0.35, p < 0.05) and Global Alogia (r = 0.37, p < 0.05), but not Global Affective Blunting or Avolition. Correlations between unpleasant word RT and the BPRS total score and BPRS psychosis factor were nonsignificant, as were all correlations with RT for pleasant words and accuracy for all word conditions. Correlations between RT and accuracy variables for pleasant and unpleasant conditions were also not significantly correlated with BPRS Anxiety (r = 0.26) or Depression (r = 0.13) items.
For individuals with schizophrenia, there was a significant correlation between unpleasant word RT and PANAS NA (r = 0.37, p < 0.05); however, there were no significant correlations between PANAS PA and RT for either condition. Accuracy was also not significantly correlated with PANAS self-report in patients. In controls, there were no significant correlations between behavioral performance and PANAS scores.
4.0. Discussion
Results were consistent with our hypothesis that HI-NEG patients would display greater difficulty disengaging attention from unpleasant stimuli than CN or LOW-NEG patients. Specifically, HI-NEG patients took significantly longer to disengage visual-spatial attention from unpleasant relative to neutral cues in order to successfully identify target letters presented outside of foveal vision. There were no group differences in RT difference scores for pleasant stimuli.
These findings are consistent with our prior study (Strauss et al., 2008), which found that patients meeting criteria for deficit schizophrenia displayed a significantly greater “lingering effect” for unpleasant words than non-deficit schizophrenia patients and controls on an Emotional Stroop task. We interpreted these previous results as reflecting an abnormality in attentional disengagement; however, the attentional mechanisms involved with our prior task were unclear since the task did not require a shift in visual attention to a different spatial location. The current results therefore provide some clarification of our previous study, indicating that HI-NEG patients do in fact display difficulty disengaging attention from unpleasant stimuli using a task that directly manipulated disengagement.
It is also interesting to note that the low negative symptom patients were faster at disengaging attention from emotional stimuli than controls. This may suggest that low negative symptom patients restrict their elaborative processing of emotional relative to neutral stimuli, allowing them to subsequently disengage their attention from these stimuli more rapidly. Alternatively, low negative symptom patients may be more likely to employ active attentional avoidance strategies when confronted with emotional stimuli, particularly those that they perceive as aversive.
There are several deleterious consequences of failing to effectively disengage attention from unpleasant stimuli. Problems with disengaging attention from unpleasant stimuli have been proposed to be a major contributor to elevated negative emotional experience in individuals with other psychiatric disorders (e.g., anxiety) (Fox et al., 2001), and our results suggest that this may be true of individuals with schizophrenia as well. We found significant associations with trait negative affect on the PANAS, but not BPRS anxiety or depression ratings, suggesting that attentional disengagement abnormalities may be less related to these specific clinical states and more related to chronic elevations in negative mood. Dwelling on unpleasant environmental stimuli may have the negative consequence of drawing significant cognitive resources and limiting the ability to process functionally important positive cues from the environment. In this sense, abnormalities in disengaging attention from aversive cues may be an important neurobehavioral predictor of both trait negative affect and anhedonia, preventing patients from orienting to potentially rewarding cues while perpetuating their focus on unpleasant information. This pattern of behavior is likely maladaptive, and it may be important to train individuals with schizophrenia to disengage attention from cues that they perceive as unpleasant to reduce their negative mood states and symptoms. There is support for this notion in the literature on anxiety disorders, which shows that intervention techniques that teach individuals to disengage attention from negative cues can lead to reductions in anxiety (Schmidt, Richey, Buckner, & Timpano, 2009).
In summary, we found that difficulty disengaging attention from unpleasant stimuli is associated with elevated trait negative affect and negative symptoms of schizophrenia, including anhedonia. These findings are highly relevant to the literature on emotional experience in schizophrenia. Given that patients generally report experiencing levels of positive emotion that are similar to controls when exposed to emotional stimuli, yet report elevated levels of negative emotion when exposed to emotional stimuli, some researchers have recently suggested that the emotional abnormalities seen in schizophrenia may primarily relate to negative emotion (for meta-analysis see Cohen & Minor, 2010; for review Kring & Moran, 2008). Elevations in negative emotion may reflect an emotion regulation problem, where patients have difficulty down-regulating negative mood (Cohen & Minor, 2010; Cohen et al., 2011; Horan et al., 2006). Here, we offer the novel suggestion that impairments in disengaging attention from unpleasant information may make it difficult for individuals with schizophrenia to attenuate negative emotional states, resulting in chronic elevations in negative mood.
Limitations of the study include being unable to specifically address the effects of antipsychotic medications, sample size, and not assessing the role of persistent or primary/secondary negative symptoms. Disorganization may also play a role in difficulty disengaging attention from unpleasant stimuli, and this should be addressed in future studies; however, we expect that the correlation with disorganization observed here may not be meaningful given the restricted range of scores and because ANCOVA indicated that there was no change in the pattern of results or the estimated marginal means when disorganization was added as a covariate. It is also important to note that there was not a clear demonstration of a differential deficit in disengaging attention from unpleasant relative to pleasant stimuli, and future studies will want to include stimuli of both valences when extending these findings. Future studies should also study the interaction between emotional stimuli and the orient, shift, and disengage components of attention in a single paradigm to determine whether these components interact with pleasant/unpleasant stimuli and negative symptoms differentially.
Supplementary Material
Acknowledgments
We would like to thank the subjects who participated in the study and staff at the Maryland Psychiatric Research Center who made the completion of the study possible. We are especially thankful to members of Dr. Gold’s laboratory, Jackie Kiwanuka, Sharon August, Zuzana Kasanova, Leeka Hubzin, and Tatyana Matveeva who conducted subject recruitment and testing.
Footnotes
In this Emotional Stroop task, 3 blocks of pleasant, unpleasant, and neutral words were presented and subjects were asked to identify the color of ink in which the word was presented while ignoring the meaning of the written word. Each block included a total of 25 words, which were further organized into 5 series of 5 words each. Within each series of 5 words, a target word, either a pleasant, unpleasant, or neutral word, was presented in the first position, and was subsequently followed by 4 neutral words (positions 2, 3, 4, 5) that were matched to the target word for frequency and length. To index the effect of emotional stimuli on neutral stimuli following them, a difference score was calculated as neutral word position 2 RT emotional word position 1 RT. Using this calculation, positive difference scores indicate that when an emotional word initially captures attention, its effect on the attentional system remains past its initial presentation, causing the string of neutral words immediately following it to have a longer RT than the initial emotional word itself.
MANCOVA indicated that the inclusion of education and BPRS psychosis did not appreciably alter these findings. However, disorganization showed a trend toward altering the significance of findings, as the overall effect of group was diminished when the BPRS disorganization factor was added as a covariate, as indicated by a reduced significance for the overall group effect (p = 0.04) and unpleasant RT condition (p = 0.04). However, we suspect that this trend is not clinically meaningful given that the mean severity of disorganization in our sample was very low, falling below a mild level of severity on average with a maximum disorganization factor score of 2.4 for the most severely disorganized patient (mild severity). There was therefore not enough range in the disorganization scores to reliably estimate their impact on the dependent variable. Furthermore, there was no change in the pattern of the estimated marginal means from any of the covariates suggesting that they had minimal effect on attentional disengagement.
There was no significant difference in the magnitude of correlation between the SANS total and pleasant and unpleasant stimulus RT (Z = 1.18, p = 0.24). Similarly, ANOVA also indicated that the Group X Condition interaction was nonsignificant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms. University of Iowa Press; Iowa City: 1983. [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36(1):143–50. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: Potential affective, cognitive and social-based mechanisms. Clin Psychol Rev. 2011;31:440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York: 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders- Patient Edition (SCID-I/P 2/2001 Revision) [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J Experimental Psych: General. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective Traits in Schizophrenia and Schizotypy. Schizophr Bull. 2008;34(5):856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol. 2006;115:496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–34. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pfohl BM, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality. 1. American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. J Abnorm Psycho. 2009;118(1):5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN. The experience of positive emotion is associated with the automatic processing of positive emotional words. The Journal of Positive Psychology. 2006;1:150–159. [Google Scholar]
- Strauss GP, Allen DN. Emotional intensity and categorisation ratings for emotional and nonemotional words. Cognition and Emotion. 2008;22(1):114–133. [Google Scholar]
- Strauss GP, Allen DN. Positive and negative emotions uniquely capture attention. Appl Neuropsycho. 2009;16:144–149. doi: 10.1080/09084280802636413. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Jorgensen ML, Cramer SL. Test-Retest Reliability of Standard and Emotional Stroop Tasks: An Investigation of Color-Word and Picture-Word Versions. Assessment. 2005;12:330–337. doi: 10.1177/1073191105276375. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Herbener ES. Patterns of Emotional Experience in Schizophrenia: Differences in Emotional Response to Visual Stimuli are Associated with Clinical Presentation and Functional Outcome. Schizophr Res. 2011;128:117–123. doi: 10.1016/j.schres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. Affects separable and inseparable: On the hierarchical arrangement of the negative affects. J Pers Soc Psycho. 1992;62(3):489–505. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.