Abstract
Recent studies of spontaneously vocalizing primates, cetaceans, bats and rodents suggests these animals possess a limited but meaningful capacity to manipulate the timing and acoustic structure of their vocalizations, yet the neural substrate for even the simplest forms of vocal modulation in mammals remains unknown. Echolocating bats rapidly and routinely manipulate the acoustic structure of their outgoing vocalizations to improve echolocation efficiency, reflecting cognitive rather than limbic control of the vocal motor pathways. In this study, we used immunohistochemical localization of immediate early gene (c-fos) expression to map neural activity in the brains of spontaneously echolocating stationary Mexican free-tailed bats. Our results support the current model of vocal control obtained largely through microstimulation studies, but also provide evidence for the contributions of two novel regions, the dorsolateral caudate nucleus and mediodorsal thalamic nucleus, which together suggest a striatothalamic feedback loop may be involved in the control of echolocation pulse production. Additionally, we found evidence of a motivation pathway, including the lateral habenula, substantia nigra pars compacta, and raphe nuclei. These data provide novel insights into where and how mammalian vocalizations may be regulated by sensory, contextual and motivational cues.
Keywords: echolocation, vocalization, basal ganglia, c-fos, bats
1. Introduction
Innate mammalian vocalizations are produced by a limbic triggering of brainstem vocal pattern generators. The current visceromotor model of vocal production [1] accounts for the basic acoustic properties of most non-human mammalian vocalizations, but important questions about the mechanisms by which sensory feedback and behavioral context influence vocalizations remain. Varying degrees of vocal flexibility have been described for a few species, most notably cetaceans, bats, and primates, which indicates that at least some mammals possess circuitry for expanding their vocal repertoire beyond the constraints of innate fixed action patterns. Identifying the brain structures and circuits that participate in this process is an important step towards understanding the evolutionary origins and biological basis of human speech motor control.
Whales, bats, and primates can modify the temporal and acoustic features of their calls in response to acoustic stimuli [2–11]. Echolocating bats should be of particular interest in this regard because they constantly fine-tune the temporal and acoustic properties of their vocalizations to match the sensory needs of their sonar system [12–14]. Some elements of audio-vocal integration may be accounted for by direct midbrain connections between ascending sensory pathways and the descending vocal motor pathways [12, 15], but currently even the simplest audio-vocal behaviors such as the Lombard response [16] remain unaccounted for in current models of vocal production. In both songbirds and humans, basal ganglia circuits provide an important substrate for vocal plasticity, but evidence of their involvement in mammalian vocalizing is sparse [17–20]. This highlights the need for more basic information about the neural circuits governing vocal production in mammals.
Previous investigations of the mammalian vocal motor pathway have relied heavily on chemical or electrical stimulations to drive neuronal activity leading to vocalizing [21], but these studies were not designed to identify brain regions associated with vocal plasticity. In songbirds, immediate early gene expression was used as a marker of neuronal activity to map vocalization-related activity patterns in the brains of spontaneously singing birds, and this technique successfully revealed novel aspects of vocal production in hummingbirds and parrots [22–24]. Increased expression of the immediate early gene c-fos was also used to map vocalization-related neuronal activity in tamarins that were electrically-stimulated to vocalize [25], and two recent papers have used c-fos expression to localize vocalization-related activity in the frontal cortex of primates [26, 27] but to our knowledge no studies have used immediate early gene expression to localize subcortical activity in naturally vocalizing mammals.
Here, we use c-fos immunohistochemistry to map brain activity in naturally echolocating bats (Tadarida brasiliensis). Echolocation is by nature a flexible vocal behavior guided by cognitive more so than limbic processes, which led us to predict that this combination of technique and species could reveal vocalization-related activity in novel brain regions in addition to those revealed by previous microstimulation studies. We first examined whether vocal control centers identified by microstimulation also showed increased neuronal activity in naturally vocalizing bats, and secondly we identified novel brain regions found to be more active during vocalizing than during listening or in silence. Evidence that key sites in the basal ganglia and thalamus are active during vocalizing provides the basis of an updated hypothesis of the vocal control networks in bats.
2. Methods
2.1. Animals
33 male and 2 female Mexican free-tailed bats (Tadarida brasiliensis) obtained from within buildings on the College Station campus of Texas A&M University and temporarily housed in the Biology Department vivarium were used in these experiments. Females were not excluded but rather rarely captured. All experimental procedures complied with NIH guidelines for experiments involving vertebrate animals and were approved by the Texas A&M University Institutional Animal Care and Use Committee.
2.2. Acoustic recording and playback
All experiments took place in a sound isolation chamber lined with echo-absorbing acoustic foam. During the experiments bats were allowed to move freely within a small wire cage (25cm×12cm×12cm). Vocalizations were monitored by a Brüel & Kjær free-field ¼ inch microphone (Type 4939) positioned directly beneath the cage. Output of the microphone amplifier was digitized (NI PCI-6251 DAQmx; 200 kHz, 16-bit sample rate) and viewed with Avisoft Recorder v3.0 (Avisoft Bioacoustics, Berlin). All auditory stimulations were played through a Sony amplifier (model # STR-DE598) driving a four-speaker array consisting of two Pioneer Ribbon Tweeters (model #ART-55D/301080) and two Pioneer Rifle Tweeters (model #ART-59F/301081), arranged 1 meter apart to project towards the cage located in the center. Each speaker provided a flat 85±3 dB output across the frequency range of the stimulus (20 kHz to 50 kHz). Artificial echolocation calls were synthesized using TDT OpenEX software and generated using the TDT System III hardware (TDT Systems, Alachua, FL). The acoustic stimuli used for these experiments were downward frequency-modulated pulses (FM sweeps) beginning at approximately 50 kHz and descending to 20 kHz over 5 milliseconds, repeated every 200 milliseconds for 30 minutes. The acoustic parameters of the stimulus were set to match the mean acoustic parameters of each bat’s echolocation calls.
2.3. Behavioral protocols
Three different experimental groups were compared for this experiment: 1) echolocating (vocalizing and hearing itself), 2) listening only (not vocalizing, hearing playback of echolocation calls), and 3) silent (not vocalizing, not hearing). All bats received the same handling procedures leading up to each experimental trial. All trials were initiated the night before by first placing a bat in the test cage and isolating it in the recording chamber for 12 hours prior to the experiment. All vocal activity during the 12 hour isolation period was recorded and reviewed prior to beginning experiments, and only animals that remained quiet during most of the night were used for subsequent experiments. Keeping animals in a quiet, isolated environment prior to stimulation has been shown to maximize gene expression in similar experiments [28]. Because c-fos protein becomes detectable by immunohistochemistry approximately 30 minutes after the onset of stimulation [29], each experiment consisted of a 30 minute test trial followed by an additional 30 minutes of quiet isolation before the animal was sacrificed.
For the “silent” trials, after 12 hours the environment was no longer novel and consequently most bats remained silent for the succeeding 60 minute experiment. Animals were visually inspected at the beginning and end of each trial to confirm they were awake and passively monitoring their surroundings. If an animal began to vocalize during a “silent” trial the experiment was discontinued. For the “echolocating” trials, the procedure was modified slightly by surrounding the test cage with a large box lined with acoustic foam the night before and then removing the box at experimental onset the next morning, thereby exposing the bat to a novel acoustic environment without handling it. Removing the box reliably evoked prolonged bouts of echolocation after the preceding long period of quiet. For the “listening” trials, the protocol was identical to the silent treatment except that the experimental trial consisted of 30 minutes of artificially-generated echolocation calls played back to the bat from the loudspeaker array. Playback rarely evoked vocalizing, and when it did those experiments were discontinued and not included in the study. For all three experimental conditions, a real-time analysis of each animal’s vocal behavior determined whether or not to sacrifice the bat for immunohistochemistry. For the echolocating group, only bats uttering more than 1000 calls during the thirty minute trial were sacrificed for neuroanatomical study; mean call rates ranged from 0.7 calls/second to 4.5 calls/second for this group. All of the bats included in the “silent” and “listening” trials were completely silent during the 30 minute experimental trials as well as the 30 minute post-trial isolation period. Visual inspection of the acoustic recordings accompanying each trial confirmed that echolocating bats exclusively uttered echolocation calls and not communication calls.
2.4. Locomotion measurements
Since spontaneous locomotor activity could contribute to changes in immediate early gene expression, we quantified total locomotor activity during all experimental trials. Movements were tracked with a motion detector (passive infrared sensor, Parallax Inc., Rocklin CA) attached to the cage, and monitored on a computer with the DATAPAC 2K2 hardware and software package (Run Technologies, Mission Viejo, CA). We quantified movement during the experimental trial as the percentage of time the animal spent moving, including locomotor activity, head, mouth, and ear movements, and grooming activity.
2.5. Brain Preparation and Immunohistochemistry
All animals were sacrificed by anesthetic overdose (sodium pentobarbital). This method of sacrifice is rapid enough (<5 minutes) to not have an effect on gene expression. Their brains were quickly removed, flash frozen in isopentane to minimize cell damage, and stored at −80°C [30]. Serial coronal sections (20 μm) of the entire brain were cryosectioned, thaw mounted on Histobond microscope slides (Statlab Medical Products Inc., Lewisville TX), and stored at −80°C until use. Serial adjacent brain sections were separated into four sets of slides. One slide set from each animal was used for the c-fos immunohistochemistry protocol (see below), and additional slide sets served as replacements for lab errors or use in other stains to help determine brain anatomy.
The primary antibody for c-fos, corresponding to N terminal amino acids 1–14 of human c-Fos, was obtained from Abcam (ab7963). The secondary antibody was part of a kit from Vector Labs (Vectastain Elite ABC Kit). The specificity of the c-fos antibody in Tadarida brasiliensis was confirmed using a western blot with a blocking peptide (ab7997). For the immunohistochemistry protocol, brain sections were fixed in ice cold methanol, incubated in a 0.3% H2O2 solution, and then rinsed. The slices were then blocked in normal goat serum and incubated with the primary antibody (1:5000) overnight at 4 C. The next day, the slides were incubated with the biotinylated secondary antibody solution (goat anti-rabbit, 1:2000). The slices were then incubated in Vectastain Elite ABC reagent and then in the chromogen solution, diaminobenzidine with nickel. They were then rinsed, dehydrated, cleared, and cover-slipped. In addition to c-fos, we also used this same immunohistochemistry protocol with a primary antibody to tyrosine hydroxylase (ab112, Abcam), the first enzyme in the dopamine synthesis pathway, to aid in determining the locations of regions in the striatum and substantia nigra.
2.6. Data Analysis
We focused our measurements of fos-like immunoreactivity (FLI) on 12 brain structures subdivided into 20 discrete regions of interest (ROIs). These ROIs were based on current published models of non-human vocal motor control [1, 20, 31] (table 1). The ROIs included one auditory region, the dorsal nucleus of the lateral lemniscus (dnLL) to serve as a positive control. The anterior cingulate cortex (ACg), periaqueductal gray (PAG), parabrachial nucleus (PB), basolateral amygdala (BLA), and the hypothalamic nuclei (lateral (LH) and periventricular (PA)) were of central interest because microstimulation of these regions elicits vocalizations [32, 33]. In addition to these regions, the lateral habenula (LHb) and raphe nuclei (dorsal (DR) and medial (MnR)) were previously shown to exhibit increased FLI following electrical stimulation of vocal initiation centers in monkeys [25]. The nucleus accumbens (Acb) and substantia nigra pars compacta (SNc) have been implicated in vocal control because they are reciprocally interconnected with vocal regions of the PAG [34]. The striatum, mediodorsal thalamic nucleus (MD), and PB all receive efferent projections from the larynx area of the motor cortex [35, 36]. For purposes of this analysis the striatum was divided into four parts following the nomenclature of Paxinos and Watson (1998), 1) the anterior-most region or caudate head (CPu), 2) the dorsolateral caudate nucleus (dlCdN), 3) the ventromedial caudate nucleus (vmCdN), and 4) the caudal putamen (Pu) (Fig. 1). The midbrain PAG is a large structure suspected of involvement in a wide variety of behaviors. To determine whether vocalization-related neuronal activity in PAG was limited to a specific subregion the PAG was analyzed as 16 separate parts: it was first divided dorsoventrally into dorsal (dPAG), ventral (vPAG), left lateral (lPAG), and right lateral (rPAG) regions, and then each of these areas was divided rostrocaudally into four bins, with bin 1 being most rostral and bin 4 most caudal.
Table 1.
| Echolocating | Listening | Silent | P | F (d.f.) | |
|---|---|---|---|---|---|
| Acg | 4.354 ± 0.887 (8) A |
0.840 ± 0.456 (5) B |
0.300 ± 0.151 (8) B |
<0.001 | 102.636 (2,18) |
|
| |||||
| BLA | 0.250 ± 0.177 (8) A |
0.160 ± 0.055 (5) A |
0.138 ± 0.074 (8) A |
0.164 | 1.827 (2,18) |
|
| |||||
| Acb | 0.338 ± 0.292 (8) A |
0.387 ± 0.189 (5) A |
0.186 ± 0.090 (7) A |
0.250 | 1.504 (2,17) |
|
| |||||
| CPu | 0.487 ± 0.285 (8) A |
0.380 ± 0.164 (5) A |
0.214 ± 0.157 (7) A |
0.083 | 2.886 (2,17) |
|
| |||||
| dlCdN | 8.677 ± 3.246 (8) A |
1.183 ± 0.346 (5) B |
1.240 ± 0.283 (8) B |
<0.001 | 33.160 (2,18) |
|
| |||||
| vmCdN | 0.912 ± 1.147 (8) A |
0.020 ± 0.045 (5) A |
0.488 ± 0.304 (8) A |
0.133 | 2.266 (2,18) |
|
| |||||
| Pu | 0.138 ± 0.130 (8) A |
0.124 ± 0.089 (5) A |
0.139 ± 0.118 (8) A |
0.974 | 0.0265 (2,18) |
|
| |||||
| PA | 7.417 ± 3.607 (8) A |
2.567 ± 1.610 (5) B |
3.083 ± 3.150 (8) B |
0.015 | 5.401 (2,18) |
|
| |||||
| LH | 3.979 ± 0.998 (8) A |
0.967 ± 0.380 (5) B |
1.000 ± 0.408 (8) B |
<0.001 | 45.800 (2,18) |
|
| |||||
| LHb | 7.438 ± 2.527 (8) A |
1.540 ± 1.004 (5) B |
2.275 ± 1.094 (8) B |
<0.001 | 23.404 (2,18) |
|
| |||||
| MD | 8.188 ± 2.302 (8) A |
6.280 ± 1.431 (5) A |
3.612 ± 1.336 (8) B |
<0.001 | 13.125 (2,18) |
|
| |||||
| SNc | 7.571 ± 2.290 (7) A |
1.740 ± 0.573 (5) B |
1.163 ± 0.550 (8) B |
<0.001 | 42.661 (2,17) |
|
| |||||
| dPAG | 10.332 ± 2.593 (8) A |
3.026 ± 1.352 (5) B |
2.245 ± 0.921 (8) B |
<0.001 | 45.081 (2,18) |
|
| |||||
| lPAG | 6.451 ± 2.113 (8) A |
1.904 ± 0.528 (5) B |
1.158 ± 0.354 (8) B |
<0.001 | 34.059 (2,18) |
|
| |||||
| rPAG | 6.924 ± 1.943 (8) A |
2.122 ± 1.010 (5) B |
1.477 ± 0.896 (8) B |
<0.001 | 33.681 (2,18) |
|
| |||||
| vPAG | 6.207 ± 2.150 (8) A |
1.844 ± 0.771 (5) B |
0.891± 0.528 (8) B |
<0.001 | 30.442 (2,18) |
|
| |||||
| DR | 8.091 ± 2.569 (8) A |
2.363 ± 0.553 (5) B |
1.511 ± 0.937 (8) B |
<0.001 | 32.887 (2,18) |
|
| |||||
| MnR | 7.250 ± 1.900 (8) A |
1.280 ± 0.179 (5) B |
1.543 ± 1.176 (7) B |
<0.001 | 41.031 (2,17) |
|
| |||||
| PB | 7.119 ± 1.904 (7) A |
1.833 ± 1.381 (4) B |
0.500 ± 0.385 (7) B |
<0.001 | 43.747 (2,15) |
| m | 5.690 ± 3.056(7) A |
20.538 (2,15) | |||
| l | 3.286 ± 2.277 (7) B |
9.921 (2,15) | |||
|
| |||||
| dnLL | 6.333 ± 2.594 (8) A |
5.867 ± 1.609 (5) A |
1.542 ± 0.434 (8) B |
<0.001 | 16.233 (2, 18) |
Figure 1.
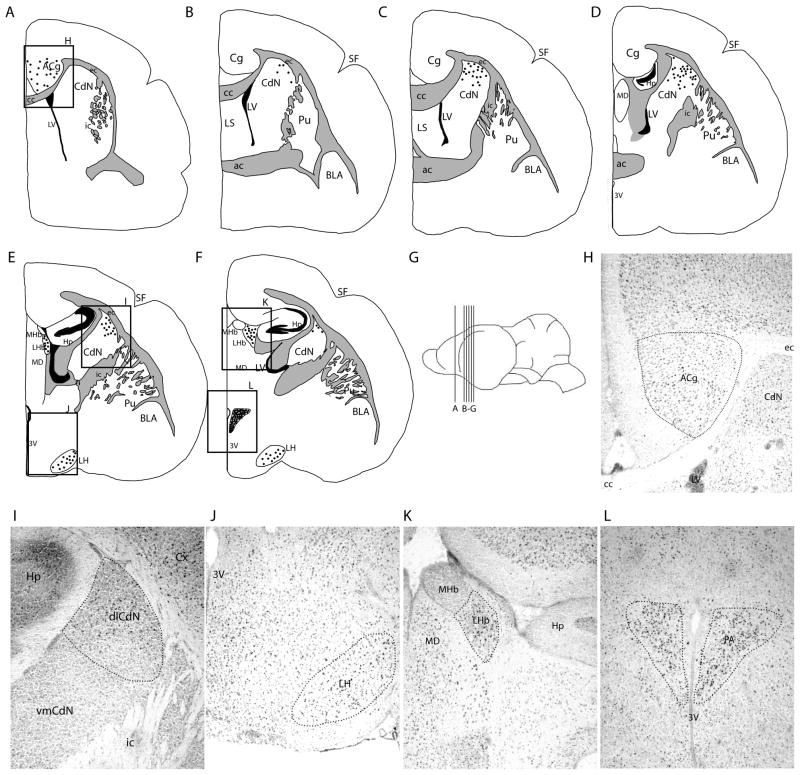
Fos-like immunoreactivity in the forebrain of spontaneously echolocating bats. A–E are atlas drawings progressing through the Tadarida brasiliensis forebrain. Each dot represents two cells. G provides a map of the location of each coronal brain atlas drawing. The boxed areas in A, E–F are an illustration of the positions of H–L, showing FLI in the anterior cingulate cortex (H), dorsolateral caudate nucleus (I), lateral hypothalamus (J), lateral habenula (K), and paraventricular nucleus (L).
Coordinates of all brain regions were determined from our own histochemical analysis of the free-tailed bat brain and other descriptions of bat neuroanatomy [37–39]. All ROI coordinates were similar to their counterparts in rodents [40, 41], because the free-tailed bat brain is very similar in size, structure and organization to the mouse brain. To better delineate the locations and borders of the basal ganglia structures and PB, we used the sections immunostained with the tyrosine hydroxylase antibody as a guide. The cytoarchitectonic delineations of cortical vocal control centers in bats are unknown, however it is known that the vocal initiation region of the ACg was dorsal and rostral to the anterior commissure [33], which is similar to its position in primates above the genu of the corpus callosum [42]. Because we lack further details about the functional organization and cytoarchitecture of the bat cortex as it pertains to vocalizing, we did not investigate c-fos expression in cortical regions other than cingulate cortex.
Pictures of each brain area were taken using an Infinity 2 microscope camera connected to a computer running Infinity Capture application software (version 3.7.5, Lumenera Corporation). Consecutive sections from both the right and left sides for each ROI were analyzed for each animal. Cells exhibiting FLI (5–40 pixels in size) were counted using NIH Image J [43]. Cells were counted in 0.012 × 0.012 mm square areas randomly positioned within each ROI [44–46]. Data from the right and left sides for bilateral structures were not significantly different, so they were combined and the mean for each ROI for each animal was calculated. The animals were then divided into their respective treatment groups and cell counts were compared between groups of bats using an analysis of variance (ANOVA) with treatment as the factor (echolocating, listening, or silent). The comparisons of interest were echolocating vs. listening, echolocating vs. silent, and listening vs. silent. Multiple pairwise comparisons were analyzed using the Holm-Sidak method. In order to assess the relative contributions of mean call rate and locomotor activity on the FLI in echolocating bats we performed a multiple linear regression analysis on the FLI for each brain region versus call rate and percent of time spent moving.
3. Results
3.1. Overview
Table 1 presents the complete results of our analyses comparing the amount of FLI in 20 ROIs distributed throughout the forebrain, midbrain, and pons of echolocating, listening, and silent free-tailed bats. In two instances (one echolocating bat and one silent bat) tissue damage during cryosectioning resulted in the loss of some brain sections. In echolocating bats, we found significantly elevated numbers of cells expressing FLI relative to both listening and silent bats in each of the major components of the known visceromotor vocal pathway, including ACg (Fig. 1), PAG (Fig. 2) and PB (Fig. 3) (see table 1 for statistics). We also found significant increases in FLI in the hypothalamic nuclei previously linked with vocal production in rodents and primates, the PA and LH (Fig. 1). Other motivational centers that showed significant vocalization-related elevations in cell counts were the lateral habenula (LHb; Fig. 1) and the raphe nuclei (MRn and DR; Fig. 3). Cell counts were elevated in the auditory pathway (dnLL) for both echolocating and listening bats relative to the silent group. The MD was the only ROI that exhibited significantly higher FLI in both the echolocating and listening bats relative to silent bats. Within the basal ganglia we found significantly higher FLI in vocalizing bats compared to listening and silent bats in the dlCdN and SNc (Fig. 1 and 3), but not in other parts of the striatum, including Acb. No FLI was found in the BLA of any groups. An atlas representation of the cell locations for each ROI is provided in figures 2, 3, and 4, showing in particular that the distribution of cells in the dlCdN and SNc appeared limited within these structures.
Figure 2.
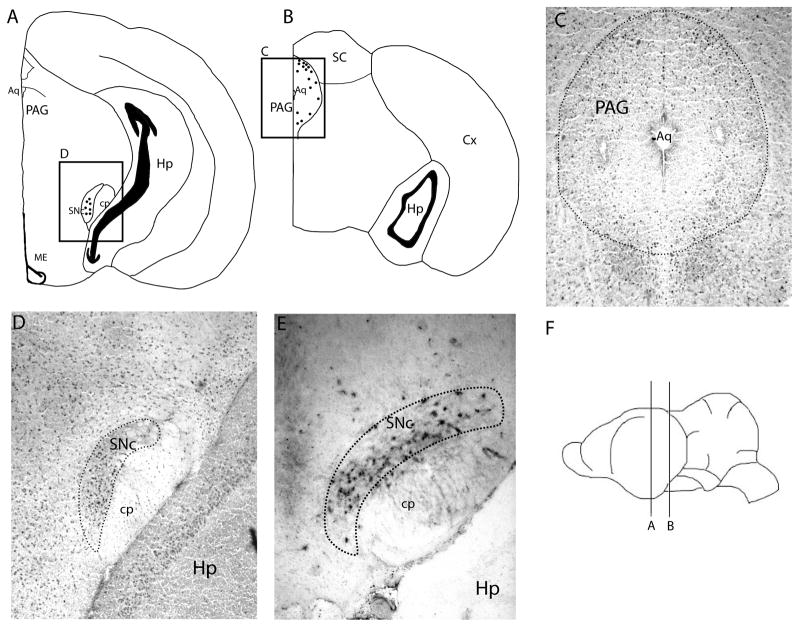
Fos-like immunoreactivity in the midbrain of spontaneously echolocating bats. A–B are atlas drawings of the Tadarida brasiliensis midbrain. Each dot represents two cells. The boxed areas provide the placement of C–D, which show FLI in the periaqueductal gray (C) and substantia nigra pars compacta (D). E shows tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta. F gives the placement of the brain slices in A–B.
Figure 3.
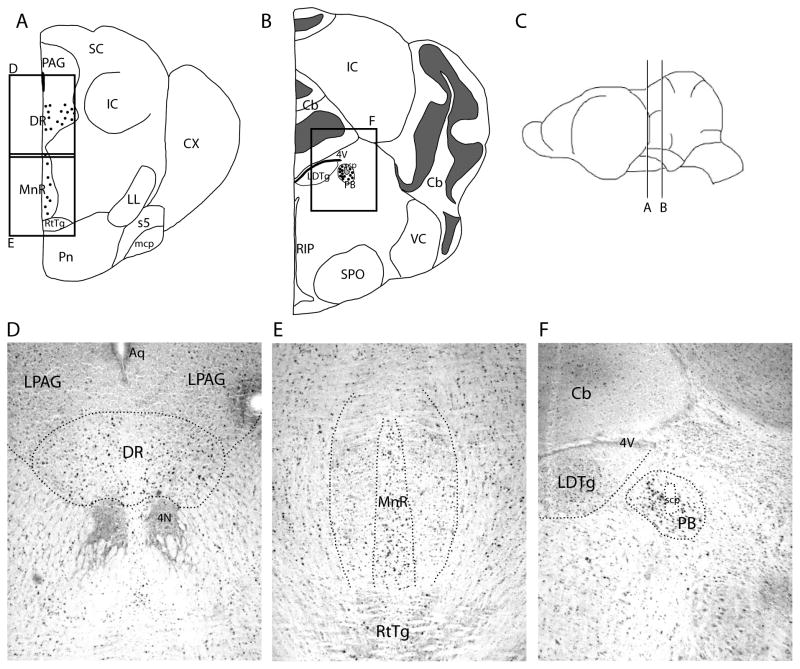
Fos-like immunoreactivity in the hindbrain of spontaneously echolocating bats. A–B are atlas drawings of the free-tailed bat hindbrain. C shows the location in the brain of A–B. Each dot represents two cells. The boxed areas represent the positions of D–F, which show FLI in the dorsal raphe nucleus (D), median raphe nucleus (E), and parabrachial nucleus (F).
Figure 4.
The relationship between movement, call rate, and FLI. A. This figure provides data from a large pool of bats (n=21) and a first order regression shows that movement and call rate are weakly correlated (R2=0.3788). B. This figure shows that there was no correlation between call rate and FLI in the dorsal PAG (n=7). C. This figure shows that there was no correlation between percent of time spent moving and FLI in the dorsal PAG. B–C are representative of all the ROIs, none of which showed any correlation between FLI and call rate or movement.
3.2. Midbrain regions of interest
In the PAG we mapped the relative distribution of FLI throughout this relatively large structure. All sections of the PAG in the echolocating group had significantly higher FLI than both the listening and silent bats (table 1, Fig. 2). We found no significant differences in FLI across PAG regions within treatment groups, however some trends were apparent (table 2). In descending order, the areas of the PAG that had the highest FLI cell count were the caudal-most bins 4, 3, and 2 of the dorsal PAG and the rostral-most bin 1 of lateral PAG. The dPAG consistently had the highest density of FLI of all ROIs. Surrounding the PAG is the reticular formation, which has also been implicated in vocal motor control, including echolocation pulse production. In general we observed modestly elevated background levels of FLI broadly distributed throughout the midbrain reticular formation. We looked for but did not find any distinct regions of the reticular formation that had localized elevations in FLI which might otherwise have warranted the inclusion of an additional ROI.
Table 2.
Anatomical distribution of FLI within the periaqueductal grey.
| Echolocating | Listening | Silent | P | F (d.f.) | |
|---|---|---|---|---|---|
|
| |||||
| dPAG | |||||
| 1 | 7.425 ± 3.986 A |
2.160 ± 1.941 B |
1.875 ± 1.666 B |
<0.001 | 9.067 (2,18) |
| 2 | 10.479 ± 3.572 A |
3.667 ± 1.312 B |
2.271 ± 1.188 B |
<0.001 | 25.236 (2,18) |
| 3 | 10.771 ± 2.577 A |
2.633 ± 1.325 B |
2.250 ± 1.330 B |
<0.001 | 47.478 (2,18) |
| 4 | 12.167 ± 3.234 A |
3.500 ± 1.532 B |
2.521 ± 1.364 B |
<0.001 | 40.319 (2,18) |
|
| |||||
| lPAG | |||||
| 1 | 8.275 ± 3.061 A |
2.040 ± 1.187 B |
1.900 ± 0.793 B |
<0.001 | 23.556 (2,18) |
| 2 | 5.813 ± 2.498 A |
1.700 ± 0.681 B |
0.646 ± 0.663 B |
<0.001 | 21.414 (2,18) |
| 3 | 6.583 ± 2.136 A |
1.733 ± 0.279 B |
0.833 ± 0.684 B |
<0.001 | 37.260 (2,18) |
| 4 | 5.438 ± 2.121 A |
2.167 ± 0.645 B |
1.375 ± 0.635 B |
<0.001 | 17.977 (2,18) |
|
| |||||
| rPAG | |||||
| 1 | 9.325 ± 2.496 A |
2.360 ± 1.322 B |
2.275 ± 1.126 B |
<0.001 | 36.915 (2,18) |
| 2 | 5.771 ± 2.870 A |
1.867 ± 0.877 B |
0.616 ± 0.322 B |
<0.001 | 16.549 (2,18) |
| 3 | 7.229 ± 3.094 A |
2.000 ± 1.505 B |
0.875 ± 0.659 B |
<0.001 | 20.201 (2,18) |
| 4 | 5.771 ± 2.415 A |
2.300 ± 1.181 B |
1.458 ± 0.689 B |
<0.001 | 14.657 (2,18) |
|
| |||||
| vPAG | |||||
| 1 | 2.225 ± 1.202 A |
0.800 ± 0.548 B |
0.125 ± 0.149 B |
<0.001 | 14.262 (2,18) |
| 2 | 5.979 ± 2.837 A |
1.733 ± 1.593 B |
0.396 ± 0.408 B |
<0.001 | 17.658 (2,18) |
FLI was also found to be significantly higher in the PB of echolocating bats compared to the silent and listening bats (table 1, Fig. 3). Since the medial and lateral components of the PB are suspected of subserving different functions in vocal control [47], we analyzed the medial and lateral PB separately to see if either region had significantly more FLI. The medial PB had significantly higher FLI than the lateral PB. The FLI counts of the lateral PB alone were not significantly different from those of the listening bats.
3.3. Hypothalamic nuclei
In the hypothalamus, PA and LH both exhibited significantly higher FLI in the echolocating bats relative to the silent and listening bats (table 1, Fig. 1). No other regions of the hypothalamus displayed elevated FLI in any bats. We encountered substantial inter-individual variability in FLI counts within the PA; although the echolocating group ended up having significantly higher FLI than the other two groups, there were animals in the listening and silent groups that had cell counts in the range of the echolocating group. However this was not the case for the LH. This region had the lowest FLI count for all echolocation-positive ROIs analyzed, but FLI measurements were very consistent within treatment groups.
3.4. Basal ganglia
Two ROIs in the basal ganglia resulted in higher FLI in echolocating bats relative to the other two treatment groups. The FLI in the forebrain dlCdN was focused within a very discrete area of the caudate nucleus (Fig. 1). In general, the caudate nucleus area is encompassed by the external capsule on the dorsal side, internal capsule laterally, and corpus callosum medially. The dlCdN is the upper half of this structure. Where the FLI begins, the cells are scattered throughout the dlCdN, but their distribution appeared to become more concentrated medially, where this structure comes to a point between the external capsule and the corpus callosum. Progressing caudally, the dlCdN gradually diminishes in size and the FLI became more concentrated. FLI in the dlCdN was observed bilaterally. Within the same serial sections FLI was generally absent in all three treatment groups in the vmCdN. The second basal ganglia region with significantly higher FLI in echolocating bats was the midbrain SNc (Fig. 2d). Within SN we confirmed that the observed FLI overlapped and was consistent with the distribution of TH-positive (dopamine producing) cells (Fig. 2e), which was the basis for designating this as the pars compacta region of the SN. However, we did not attempt to establish whether the cells expressing FLI were the same cells expressing TH. In general it appeared that FLI in this ROI was not broadly distributed throughout the structure but rather appeared limited to a discrete area within the SNc, concentrated centrally and in the ventromedial compartment of SNc.
3.5. The relationship between call rate, locomotion, and cell counts
The above results showed that echolocating bats exhibited increased FLI relative to silent bats in several brain regions. Since there was some variability in call rate among bats, we investigated whether mean call rate influenced the amount of FLI in these brain regions. Within the echolocating group we looked for a significant correlation between call rate (calls/sec) and FLI or alternatively the percent time spent moving during the experimental trial and FLI in any of the brain regions expressing echolocation-related FLI. To first quantify the relationship between locomotion and call rate, we pooled data from previous behavioral studies in the lab with data collected from these bats (n=21 adult male free-tailed bats) and found that movement and call rate were weakly correlated (R2=0.3788) in stationary bats. This indicates that bats were slightly more likely to call while moving (Fig. 4), but also illustrates that locomotion within the cage was not the only motivation for echolocation pulse emissions. We found no positive correlations between cell counts and either call rate or time spent moving (or in combination) in any of the brain regions showing increased FLI in echolocating bats. Fig. 4 illustrates our analyses of the relationship between these parameters and FLI within the dorsal PAG, the ROI showing the highest levels of FLI.
4. Discussion
This study describes the neuroanatomical distribution of c-fos immunoreactivity in the brains of spontaneously vocalizing Mexican free-tailed bats, Tadarida brasiliensis. We took advantage of the solitary bat’s natural tendency to emit large numbers of vocalizations as part of its echolocation behavior, but we were also able to exploit normal variations among bats to identify brain areas that were preferentially active during vocal production rather than during auditory stimulation or in silence. A similar behavioral strategy coupled with c-fos IHC was used in marmosets to identify frontal cortical regions involved in primate communication [27]. Miller et al. [26] also used c-fos expression in spontaneously vocalizing primates to characterize cortical brain activity patterns associated with audio-vocal communication. Cytoarchitectonic details of frontal cortical regions analogous to those identified in vocalizing primates are as yet undefined in bats, which caused us to limit our analyses of cortical c-fos expression to just one area previously implicated in vocalizing, the anterior cingulate cortex. Overall we found vocalization-related increases in c-fos expression in the cingulate cortex, dorsolateral caudate nucleus, paraventricular and lateral hypothalamic nuclei, lateral habenula, substantia nigra pars compacta, PAG, median and dorsal raphe nuclei, and parabrachial nucleus of echolocating animals but not listening or silent animals (Fig.s 1–3, table 1). Most of these areas had been previously linked with vocal production through the use of various microstimulation techniques but not in spontaneously vocalizing animals. The basal ganglia structures represent novel observations that may be more important for vocalizing in the context of spontaneous echolocation than for the production of evoked communication calls. Importantly however, the overarching conclusion of this study is that the free-tailed bat uses most of the same brain structures for producing echolocation pulses that other mammals, including primates, use for producing communication sounds. Although echolocation pulse production undoubtedly represents a specialized form of mammalian vocalization and the underlying circuitry may include some features unique to bats [48], the composition of the descending motor pathways appears largely consistent with the standard architecture responsible for other stereotyped mammalian vocalizations.
4.1. Vocalization-induced c-fos expression in the vocal motor pathway
It was hypothesized that c-fos expression should be elevated in those brain regions that had been previously identified by other techniques as central components of the mammalian vocal motor pathway. The functional significance of the ACg, PAG and PB has been implied through a combination of microstimulation and lesion studies in a variety of mammals, and now their contributions to spontaneous vocal behaviors is supported by this investigation Previous microstimulation studies in primates [32] and bats [33] identified a region of the ACg rostral to the anterior commissure and above the genu of the corpus callosum as a vocal initiation center. Our results confirmed that this region of cortex showed increased neuronal activity in naturally vocalizing free-tailed bats compared to awake but silent bats. In addition to its known role in the vocal initiation pathway, there is a body of evidence that suggests the ACg is important for audio-vocal integration [42]. This ROI receives extensive input from auditory regions in the superior temporal gyrus, including auditory association cortex, in non-human primates [49, 50]. Also, a subset of neurons in the superior temporal gyrus reacted to both auditory stimuli and electrical stimulation of vocal regions of the ACg [51]. In mustached bats the ACg is involved in the control of vocal pitch, and in experienced human singers fMRI revealed that the ACg is activated during vocal pitch-compensation when auditory feedback to the subject was artificially manipulated to indicate a shift in pitch [52, 53]. This suggests that the ACg is important for vocal modulation in addition to vocal initiation.
Microstimulation studies in the PAG have identified the dorsolateral PAG as a gating center of the vocal motor pathway in both the macaque and the bat [54, 55]. In the mustached bat, the best site for eliciting vocalizations appeared to be localized to the caudal dorsolateral PAG [55]. Our analyses revealed increased neuronal activity in all regions of the PAG in echolocating bats relative to listening and silent animals. It is possible that larger areas of the PAG are involved in vocalizing but from which microstimulation cannot evoke vocalizations. Alternatively the widespread increase in FLI within the PAG may reflect multiple mechanisms by which vocalizing is physiologically coupled to other systems, such as respiration and locomotion. Although we found no significant differences in FLI among PAG regions, our highest FLI count was found in the caudal dorsal PAG around the same midbrain area identified by Suga and colleagues using microstimulation [55].
The pontine PB was divided into two sections in our analyses, the medial and lateral PB (Fig. 3, table 1), because these sub-compartments are hypothesized to loosely correspond to separate laryngeal and respiratory proprioceptive feedback pathways respectively [47]. We found that FLI in the medial PB was significantly higher than in the lateral PB in echolocating bats, and also that FLI in the lateral PB was by itself not significantly different among the three treatment groups. If our assumptions about the functional compartmentalization of the PB are correct, then these results imply that laryngeal somatosensory pathways exhibit greater increases in immediate early gene expression than do pulmonary somatosensory relays within the PB during vocalizing.
4.2. Hypothalamic vocalization areas
The paraventricular nucleus of the hypothalamus is a central component of the mammalian stress response, coordinating both the endocrine system and autonomic nervous system [56, 57]. There is little evidence to support the involvement of this region in vocalization, aside from a stimulation study where electrical stimulation of this area resulted in aggressive vocalizations and displays, which could be categorized as a stress response [58]. We found significantly higher FLI in the PVN of echolocating bats in our experiment, but there were bats in each experimental group with similarly high levels of FLI. Due to the extensive evidence in the literature that this area is involved in the stress response, we attribute high FLI in the PVN of some bats to be in response to the stress associated with participation in the experiments, which may in turn have been the motivation for vocalizing.
Similarly, it is unclear what role the lateral hypothalamus plays in vocal behavior. The LH receives projections via the medial forebrain bundle, which collects inputs from the olfactory regions, striatum, prefrontal cortex, and limbic regions, among others [59]. The LH is also interconnected with several of our designated ROIs, including the ACg, LHb, PVN, raphe nuclei, PAG, and PB [60, 61]. These connections suggest that the LH could provide an integrative role, integrating sensory input arriving through the medial forebrain bundle to influence vocal behavior through the visceromotor pathway or the serotonin system.
4.3. Vocalization-induced c-fos expression in the basal ganglia
Two components of the bat basal ganglia expressed vocalization-related neuronal activity; the dorsolateral caudate nucleus and the substantia nigra pars compacta. It is proposed that in bats the basal ganglia circuitry contributes to the active regulation of the descending vocal motor pathways but is not, per se, an essential component of syllable production. For this reason their activity might not be revealed through microstimulation studies of the descending motor pathway but may appear during spontaneous vocal production involving more extended or variable sequences of vocalizations.
The dorsolateral striatum is considered the sensorimotor portion of the striatum because it receives inputs from motor, premotor, and sensory cortical areas [62] and it is hypothesized to perform functions in vocalizing similar to its presumed role in motor skill learning and production [63, 64]. Nonhuman primate vocalization are innate and exclusively emotional [65], thus the significance of the basal ganglia for primate vocalizations is ambiguous. In monkeys no areas of the basal ganglia exhibited increased FLI following electrically-stimulated vocalizations [25]. However, the nucleus accumbens and globus pallidus project afferents to the vocal PAG [34], and the caudate/putamen receives afferent projections from the larynx area of the primate motor cortex [20, 35], which leaves open the possibility that the basal ganglia might yet support some unknown functions in naturalistic primate vocalizations.
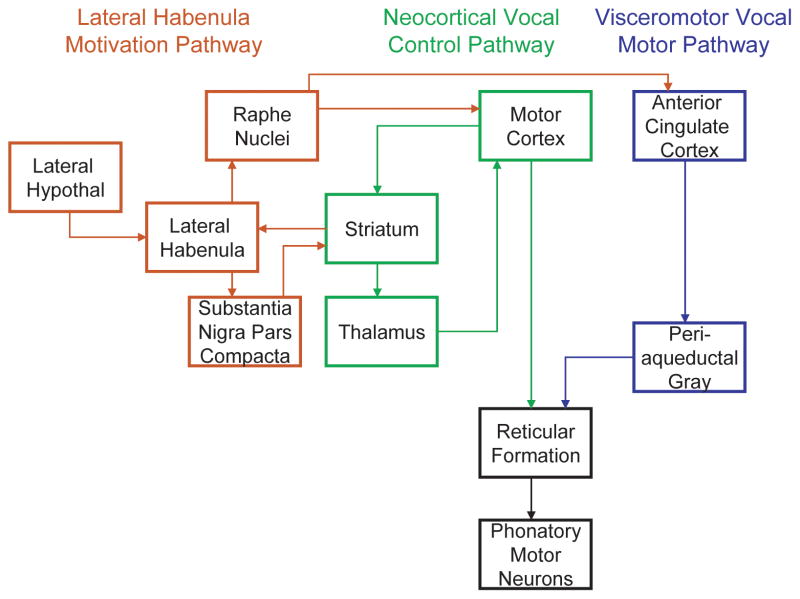
The basal ganglia are generally suspected of playing an important role in action timing and motor sequence ordering, as evidenced by their contributions to several human speech pathologies. Patients with obsessive-compulsive disorder and Tourette’s syndrome, both disorders involving uncontrolled repetitions, show abnormal activity patterns in the striatum when observed with positron emission tomography (PET) and fMRI [66]. Hypo- and hyperkinetic speech disorders arising due to Parkinson’s disease or Huntington’s disease respectively are linked to changes in basal ganglia physiology and generally reflect reduced control of the loudness, pitch and timing of syllables [66]. Elevated dopamine levels within the basal ganglia have been implicated in stuttering [67] and in hyperkinetic speech disorders [68]. Whether or not the basal ganglia perform similar functions in human speech and other mammalian vocalizations remains an open question. Incorporating the basal ganglia circuitry into the bat vocal control network as illustrated in Fig. 5 could provide a hypothetical basis for explaining many of the more sophisticated vocal behaviors exhibited by bats, such as vocal learning, singing, and the precise manipulation of syllable acoustics [2, 5, 69–71].
Figure 5.
The mammalian vocal motor pathway. The primitive visceromotor vocal pathway is shown in blue, with hypothesized contributions of a neocortical pathway shown in green and the motivation pathway highlighted in red.
4.4. Dopamine and serotonin in the vocal motor pathway
The neuroanatomical sites of synthesis and release of two major neurotransmitter systems, dopamine and serotonin, were found to have vocalization-related FLI in free-tailed bats. In the dopaminergic system, dopamine is produced and released by neurons in the substantia nigra pars compacta, and we observed increased FLI in this area indicating that this structure was more active during vocalizing than in silence. Dopaminergic neurons of the SNc project primarily to the striatum, which also exhibited increased FLI. Serotonin is produced and released by the raphe nuclei, and projection neurons send serotonin to many areas of the mammalian brain, including the cingulate cortex and the striatum [72]. The serotonin system is generally involved in anxiety and affect [73]. The observed increase in FLI within the raphe nucleus might be accounted for by anxiety if some bats were echolocating more due to the anxiogenic effects of being held in captivity or exposure to a novel environment.
The dopamine and serotonin systems closely interact with one another and have been implicated previously in vocal control systems. Monoamine oxidase inhibitors increased the duration and decreased the frequency of squirrel monkey isolation calls. A recent study in rats by Ciucci et al. [74, 75] showed that striatal dopamine depletion decreased the loudness and bandwidth of ultrasonic vocalizations [74]. In humans, selective serotonin reuptake inhibitors can cause dysphonia [76], and Parkinsonian dysarthria can be treated with Levodopa, the precursor to dopamine [77]. These results all imply that serotonin and dopamine play important general roles in human speech and other mammalian vocalizations.
4.5. Lateral habenula circuit: Possible vocal loop modulator
The lateral habenula is not known as a vocal region, but it may be involved in vocal behavior indirectly. It receives input from the basal ganglia by way of the globus pallidus internal segment [78]. It also receives input from the lateral hypothalamus. It projects to the substantia nigra pars compacta and ventral tegmental area (SNc/VTA) and also to the dorsal and medial raphe nuclei. The lateral habenula inhibits dopamine neurons of the SNc/VTA and also modulates serotonin release in the raphe nuclei [78, 79]. While it is unclear what role this structure plays in vocalization, it was previously reported that c-fos expression was increased in lateral habenula by vocalizing in cotton-topped tamarins, along with increased FLI in the raphe nuclei [25]. Since echolocation-induced c-fos activity was also found in the SNc, dorsolateral caudate nucleus, lateral hypothalamus, and raphe nuclei, all regions reciprocally innervating the lateral habenula, then this structure might be serving a central role in a motivation pathway that regulates the rate of vocalizations. This hypothesized lateral habenula motivation pathway is included in our proposed model of the bat vocal motor pathway (Fig. 5).
4.6. Relationship between moving and calling
Our results showed a weak correlation between moving and echolocating in stationary bats. A strong relationship is to be expected during flight, but bats must also echolocate in the roost to monitor their surroundings. How much bats echolocate while stationary is bound to be highly variable depending on many motivating factors, and we exploited this to identify brain areas more active in actively echolocating bats than in silent but attentive bats. Because vocalizing is at least partly subservient to locomotion the observed increase in c-fos immunoreactivity may not be exclusively due to vocalizing. However, the results also indicated that the c-fos immunoreactivity was not accounted for by the amount of time spent moving. Our primary results illustrated that bats calling at high rates exhibited much higher c-fos expression in specific brain regions than those not calling. Within-group comparisons failed to identify a correlation between call rate and c-fos expression. This may be overcome by analyzing c-fos expression patterns in a larger number of bats, but having identified target brain regions we felt that other techniques, especially electrophysiological techniques, might more efficiently address this question in the future. The main benefit of this study is that it provides a motivation and coordinates for investigating novel brain regions that may be involved in more complex aspects of mammalian vocal behaviors.
4.7. Conclusions
Audio-vocal integration is an important aspect of many of the complex vocal behaviors exhibited by echolocating bats. The current model of mammalian vocal production provides little basis for how these behaviors are managed by the mammalian brain. We hypothesized that additional brain areas might reveal their contributions to vocalizing if studied in naturally behaving animals. The basal ganglia appear to play an important role in both human speech and birdsong production, but these circuits are ignored in most models of non-human mammalian vocal production. This study provides indirect evidence that the basal ganglia are active during bat vocalizations, although their precise functions remain unclear. The extent to which this is transferable to other mammals may be limited by the unique nature of bat echolocation behavior, but related studies in rats [74, 75] suggest that the basal ganglia might indeed carry a more general significance in the control of syllable acoustics than previously reported. In addition to audio-vocal integration, the basal ganglia appear to be important for vocal learning in humans and songbirds [19]. Free-tailed bats sing elaborate courtship songs [69], and vocal learning has been demonstrated in bats [70, 71]. While it remains unknown whether Tadarida brasiliensis requires vocal learning for the development of its courtship song, audio-vocal integration clearly underlies many other vocal behaviors for this species as well as the 800 or more other species of echolocating bats. The proposed model of vocal production in free-tailed bats (Fig. 5) offers a framework for generating testable hypotheses about how and where in the bat brain these behaviors arise.
Highlights.
c-fos expression was used to identify brain areas active during echolocation.
Vocalization-related activity was distinguished from hearing-related activity.
Regions previously identified via evoked vocalizations were also active during spontaneous vocalizing.
Novel foci of vocalization-related activity were found in basal ganglia and thalamus.
Acknowledgments
We are grateful to the Texas A&M athletic department for granting us access to the bats living in their facilities. We thank Barb Earnest for her assistance in developing experimental protocols and maintaining the animals. We thank Drs. Kirsten Bohn, Jed Tressler, Thierry Lints, Heather Bortfeld and Vincent Cassone, for many helpful discussions, advice and comments. This study was funded by Texas A&M University and NIH NIDCD Grant No. DC007962 to MS.
List of Abbreviations
- 3V
third ventricle
- 4V
fourth ventricle
- ac
anterior commissure
- Acb
nucleus accumbens
- ACg
anterior cingulate cortex
- Aq
aqueduct
- BLA
basolateral amygdala
- Cb
cerebellum
- cc
corpus callosum
- CdN
caudate nucleus
- cp
cerebral peduncle
- CPu
caudate putamen
- Cx
cortex
- ec
external capsule
- dlCdN
dorsolateral caudate nucleus
- dnLL
dorsal nucleus lateral lemsiscus
- dPAG
dorsal periaqueductal gray
- DR
dorsal raphe nucleus
- FLI
fos-like immunoreactivity
- Hp
hippocampus
- ic
internal capsule
- IC
inferior colliculus
- LC
locus coeruleus
- LDTg
laterodorsal tegmental nucleus
- LH
lateral hypothalamus
- LHb
lateral habenula
- LL
lateral lemniscus
- lPAG
left lateral periaqueductal gray
- LS
lateral septal nucleus
- LV
lateral ventricle
- mcp
middle cerebellar peduncle
- MD
mediodorsal thalamic nucleus
- ME
median eminence
- MHb
medial habenula
- MnR
medial raphe nucleus
- PA
paraventricular hypothalamic nucleus
- PAG
periaqueductal gray
- PB
parabrachial nucleus
- Pn
pontine nuclei
- Pu
putamen
- RIP
raphe interpositus nucleus
- ROI
region of interest
- rPAG
right lateral periaqueductal gray
- RtTg
reticulotegmental nucleus of the pons
- s5
sensory root of trigeminal nerve
- SC
superior colliculus
- scp
superior cerebellar peduncle
- SF
sylvian fissure
- SNc
substantia nigra pars compacta
- SPO
superior paraolivary nucleus
- VC
ventral cochlear nucleus
- vmCdN
ventromedial caudate nucleus
- vPAG
ventral periaqueductal gray
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hage SR. Neuronal networks involved in the generation of vocalization. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. Elsevier; 2010. pp. 339–349. [Google Scholar]
- 2.Tressler J, Smotherman M. Context-dependent effects of noise on echolocation pulse characteristics in free-tailed bats. J Comp Physiol A. 2009;195:923–934. doi: 10.1007/s00359-009-0468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillam EH, Ulanovsky N, McCracken GF. Rapid jamming avoidance in biosonar. Proc Biol Sci. 2007;274(1610):651–60. doi: 10.1098/rspb.2006.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulanovsky N, et al. Dynamics of jamming avoidance in echolocating bats. Proc Biol Sci. 2004;271(1547):1467–75. doi: 10.1098/rspb.2004.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis J, et al. A mechanism for antiphonal echolocation by free-tailed bats. Anim Behav. 2010;79:787–796. doi: 10.1016/j.anbehav.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egnor SE, Hauser MD. Noise-induced vocal modulation in cotton-top tamarins (Saguinus oedipus) Am J Primatol. 2006;68:1183–1190. doi: 10.1002/ajp.20317. [DOI] [PubMed] [Google Scholar]
- 7.Egnor SE, Wickelgren JG, Hauser MD. Tracking silence: adjusting vocal production to avoid acoustic interference. J Comp Physiol A. 2007;193(4):477–83. doi: 10.1007/s00359-006-0205-7. [DOI] [PubMed] [Google Scholar]
- 8.Versace E, Endress AD, Hauser MD. Pattern recognition mediates flexible timing of vocalizations in nonhuman primates: experiments with cottontop tamarins. Anim Behav. 2008;76:1885–1892. [Google Scholar]
- 9.Foote AD, Osborne RW, Hoelzel AR. Environment: whale-call response to masking boat noise. Nature. 2004;428(6986):910. doi: 10.1038/428910a. [DOI] [PubMed] [Google Scholar]
- 10.Scheifele PM, et al. Indication of a Lombard vocal response in the St. Lawrence River Beluga. J Acoust Soc Am. 2005;117(3 Pt 1):1486–92. doi: 10.1121/1.1835508. [DOI] [PubMed] [Google Scholar]
- 11.Lane H, Tranel B. The Lombard sign and the role of hearing in speech. J Speech Hearing Sci. 1971;14:677–709. [Google Scholar]
- 12.Smotherman MS. Sensory feedback control of mammalian vocalizations. Behav Brain Res. 2007;182(2):315–26. doi: 10.1016/j.bbr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin DR. Listening in the dark: the acoustic orientation of bats and men. New Haven, CT: Yale University Press; 1958. p. 413. [Google Scholar]
- 14.Chiu C, Xian W, Moss CF. Adaptive echolocation behavior in bats for the analysis of auditory scenes. J Exp Biol. 2009;212:1392–1404. doi: 10.1242/jeb.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suga N, Yajima Y. Auditory-vocal integration in the midbrain of the mustached bat. In: Newman JD, editor. The physiological control of mammalian vocalization. Plenum Press; New York: 1986. pp. 87–108. [Google Scholar]
- 16.Lombard E. Le signe de l’élévation de la voix. Annales des Maladies de l’Oreille et du Larynx. 1911;37:101–119. [Google Scholar]
- 17.Doupe AJ, et al. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28(7):353–63. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis ED, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6(2):151–9. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 20.Jürgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26(2):235–58. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA. 1997;94(8):4097–102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis ED, Mello CV. Molecular mapping of brain areas involved in parrot vocal communication. J Comp Neurol. 2000;419(1):1–31. doi: 10.1002/(sici)1096-9861(20000327)419:1<1::aid-cne1>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis ED, et al. Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000;406(6796):628–32. doi: 10.1038/35020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jürgens U, Lu CL, Quondamatteo F. C-fos expression during vocal mobbing in the new world monkey, Saguinus fuscicollis. Eur J Neurosci. 1996;8(1):2–10. doi: 10.1111/j.1460-9568.1996.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller CT, et al. Vocalization Induced CFos Expression in Marmoset Cortex. Front Integr Neurosci. 2011;4:128. doi: 10.3389/fnint.2010.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simoes CS, et al. Activation of frontal neocortical areas by vocal production in marmosets. Front Integr Neurosci. 2011:4. doi: 10.3389/fnint.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8(13):iii–vii. [PubMed] [Google Scholar]
- 29.Chaudhuri A, et al. Molecular maps of neural activity and quiescence. Acta Neurobiol Exp. 2000;60(3):403–10. doi: 10.55782/ane-2000-1359. [DOI] [PubMed] [Google Scholar]
- 30.Sundquist SJ, Nisenbaum LK. Fast Fos: rapid protocols for single- and double-labeling c-fos immunohistochemistry in fresh frozen brain sections. J Neurosci Methods. 2005;141(1):9–20. doi: 10.1016/j.jneumeth.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Lima F. Responses of limbic, midbrain and brainstem structures to electrically-induced vocalizations. In: Brudzynski SM, editor. Handbook of Mammalian Vocalizations. Elsevier; 2010. pp. 293–301. [Google Scholar]
- 32.Jürgens U, Ploog D. Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res. 1970;10(5):532–54. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- 33.Gooler DM, O’Neill WE. Topographic representation of vocal frequency demonstrated by microstimulation of anterior cingulate cortex in the echolocating bat, Pteronotus parnelli parnelli. J Comp Physiol A. 1987;161(2):283–94. doi: 10.1007/BF00615248. [DOI] [PubMed] [Google Scholar]
- 34.Dujardin E, Jurgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034(1–2):114–31. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 35.Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974(1–2):43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- 36.Simonyan K, Jurgens U. Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res. 2002;949(1–2):23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- 37.Maseko BC, Manger PR. Distribution and morphology of cholinergic, catecholaminergic and serotonergic neurons in the brain of Schreiber’s long-fingered bat, Miniopterus schreibersii. J Chem Neuroanat. 2007;34(3–4):80–94. doi: 10.1016/j.jchemneu.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Prasada Rao PD, Kanwal JS. Oxytocin and vasopressin immunoreactivity within the forebrain and limbic-related areas in the mustached bat, Pteronotus parnellii. Brain Behav Evol. 2004;63(3):151–68. doi: 10.1159/000076241. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz C, et al. Distribution of 2-[125I]iodomelatonin binding in the brain of Mexican free-tailed bats (Tadarida brasiliensis) Brain Behav Evol. 2009;73:16–25. doi: 10.1159/000202987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. New York: Academic Press; 2008. [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 42.Newman JD. Evolution of the communication brain in control of mammalian vocalzation. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. Elevier; 2010. pp. 23–28. [Google Scholar]
- 43.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, MD: 1997. p. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 44.Beckett SRG, et al. Central c-fos expression following 20kHz/ultrasound induced defence behaviour in the rat. Brain Res Bull. 1997;42:421–426. doi: 10.1016/s0361-9230(96)00332-2. [DOI] [PubMed] [Google Scholar]
- 45.Neophytou SI, et al. Strain differences to the effects of aversive frequency ultrasound on behaviour and brain topography of c-fos expression in the rat. Brain Res. 2000;854:158–164. doi: 10.1016/s0006-8993(99)02334-3. [DOI] [PubMed] [Google Scholar]
- 46.Sadananda M, Wöhr M, Schwarting RKW. Playback of 22-kHz and 50 kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett. 2008;435:17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Smotherman M, Schwartz C, Metzner W. Vocal-respiratory interactions in the parabrachial nucleus. In: Brudzynski SM, editor. Handbook of mammalian vocalization. Elsevier; 2010. pp. 383–392. [Google Scholar]
- 48.Metzner W, Schuller G. Vocal control in echolocating bats. In: Brudzynski SM, editor. Handbook of mammalian vocalizations. Elsevier; 2007. pp. 403–415. [Google Scholar]
- 49.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 50.Barbas H, et al. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Müller-Preuss P, Newman JD, Jürgens U. Anatomical and physiological evidence for a relationship between the ‘cingular’ vocalization area and the auditory cortex in the squirrel monkey. Brain Res. 1980;202(2):307–15. doi: 10.1016/0006-8993(80)90143-2. [DOI] [PubMed] [Google Scholar]
- 52.Zarate JM, Zatorre RJ. Neural substrates governing audiovocal integration for vocal pitch regulation in singing. Ann NY Acad Sci. 2005;1060:404–408. doi: 10.1196/annals.1360.058. [DOI] [PubMed] [Google Scholar]
- 53.Zarate JM, Zatorre RJ. Experience-dependent neural substrates involved in vocal pitch regulation during singing. Neuroimage. 2008;40:1871–1887. doi: 10.1016/j.neuroimage.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Larson CR. The midbrain periaqueductal gray: a brainstem structure involved in vocalization. J Speech Hear Res. 1985;28(2):241–9. doi: 10.1044/jshr.2802.241. [DOI] [PubMed] [Google Scholar]
- 55.Suga N, et al. Orientation sounds evoked from echolocating bats by electrical stimulation of the brain. J Acoust Soc Am. 1973;54:793–7. doi: 10.1121/1.1913662. [DOI] [PubMed] [Google Scholar]
- 56.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 57.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrin. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Jürgens U, et al. Vocalization in the squirrel monkey (Saimiri sciureus) elicited by brain stimulation. Exp Brain Res. 1967;4:114–117. doi: 10.1007/BF00240356. [DOI] [PubMed] [Google Scholar]
- 59.Nieuwenhuys R, Geeraedts LMG, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- 60.Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 1982;8:511–526. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- 61.Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- 62.Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 64.Hikosaka O, et al. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 65.Simonyan K, Horwitz B. Laryngeal motor cortex and control of human speech. The Neuroscientist. 2011;17(2):197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graybiel AM. The basal ganglia. Curr Biol. 2000;10(14):R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 67.Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord. 2004;37(4):325–69. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Duffy JR. Motor speech disorders. Elsevier Mosby; St. Louis: 2005. [Google Scholar]
- 69.Bohn K, et al. Versatility and stereotypy of free-tailed bat songs. PLoS ONE. 2009;4:e6746. doi: 10.1371/journal.pone.0006746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boughman JW. Vocal learning by greater spear-nosed bats. Proc Biol Sci. 1998;265(1392):227–33. doi: 10.1098/rspb.1998.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esser KH. Audio-vocal learning in a non-human mammal: the lesser spear-nosed bat, Phyllostomus discolor. Neuroreport. 1994;5(14):1718–20. doi: 10.1097/00001756-199409080-00007. [DOI] [PubMed] [Google Scholar]
- 72.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat--Cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 73.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology. 2001;155(1):1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 74.Ciucci MR, et al. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res. 2007;182(2):284–9. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciucci M, et al. Reduction of dopamine synaptic activity: Degradation of 50-kHz ultrasonic vocalization in rats. Behav Neurosci. 2009;123(2):328–338. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petitpain N, et al. Dysphonia induced by selective serotonin reuptake inhibitors. South Med J. 2005;98:843. doi: 10.1097/01.smj.0000172786.09545.b5. [DOI] [PubMed] [Google Scholar]
- 77.Sanabria J, et al. The effect of levodopa on vocal function in Parkinson’s disease. Clin Neuropharmacol. 2001;24:99–102. doi: 10.1097/00002826-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Hikosaka O, et al. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang L, et al. Lateral habenula lesions improve the behavioral response in depressed rats via increasing serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]