Abstract
Mirror visual feedback has previously been found to reduce disproportionate interlimb variability and neuromuscular activity in the arm muscles in children with Spastic Hemiparetic Cerebral Palsy (SHCP). The aim of the current study was to determine whether these positive effects are generated by the mirror per se (i.e. the illusory perception of two symmetrically moving limbs, irrespective of which arm generates the mirror visual feedback) or by the visual illusion that the impaired arm has been substituted and appears to move with less jerk and in synchrony with the less-impaired arm (i.e. by mirror visual feedback of the less-impaired arm only). Therefore, we compared the effect of mirror visual feedback from the impaired and the less-impaired upper limb on the bimanual coupling and neuromuscular activity during a bimanual coordination task. Children with SHCP were asked to perform a bimanual symmetrical circular movement in three different visual feedback conditions (i.e. viewing the two arms, viewing only one arm, and viewing one arm and its mirror image), combined with two head orientation conditions (i.e. looking from the impaired and looking from the less-impaired body side). It was found that mirror visual feedback resulted in a reduction in the eccentric activity of the Biceps Brachii Brevis in the impaired limb compared to the condition with actual visual feedback from the two arms. More specifically, this effect was exclusive to mirror visual feedback from the less-impaired arm and absent when mirror visual feedback from the impaired arm was provided. Across conditions, the less-impaired arm was the leading limb, and the nature of this coupling was independent from visual condition or head orientation. Also, mirror visual feedback did not affect the intensity of the mean neuromuscular activity or the muscle activity of the Triceps Brachii Longus. It was concluded that the positive effects of mirror visual feedback in children with SHCP are not just the result of the perception of two symmetrically moving limbs. Instead, in order to induce a decrease in eccentric neuromuscular activity in the impaired limb, mirror visual feedback from the ‘unaffected’ less-impaired limb is required.
Keywords: Cerebral palsy, Hemiparesis, Mirror visual feedback, Neuromuscular activity, Electromyography, Bimanual coordination
Introduction
Children with Spastic Hemiparetic Cerebral Palsy (SHCP), who have unilateral motor impairments in both their arm and leg due to brain and/or pyramidal tract damage (Miller 2007),1 perform tasks requiring only the less-impaired hand reasonably well (e.g. Steenbergen et al. 1996; Utley and Sugden 1998). In contrast, tasks requiring bimanual coordination pose a huge challenge because of the inevitable involvement of the impaired arm and hand. In recent years, bimanual reaching and grasping has been thoroughly investigated in individuals with SHCP (e.g. Utley and Sugden 1998; Volman et al. 2002; Sugden and Utley 1995; Steenbergen et al. 1996). Interestingly, these studies suggest that, despite the unilateral impairment, bimanual actions of children with SHCP seem to be facilitated by bilateral connections at multiple levels of the central nervous system similar to what has been found in typical populations (e.g. corticospinal, cerebellar, brain stem, and propriospinal; Wiesendanger et al. 1994). For example, Volman et al. (2002) showed that when drawing circles in an in-phase (symmetrical) coordination mode, the spatiotemporal interlimb variability decreased. Furthermore, movement smoothness of the impaired limb increased compared with single-handed performance. Steenbergen et al. (2008) observed close temporal synchrony of the hands when grasping an object bimanually, which contrasted with the timing differences between both hands when they performed separately. It should be noted that some of these findings indicate adaptations of the less-impaired side to the behaviour of the affected side (e.g. Steenbergen et al. 1996), but combined these studies suggest that bilateral interactions exist in children with SHCP and that they can lead to favourable effects in the impaired arm.
A paradigm that has been used to further our understanding of how visual and spatial processes influence coordination and perception of the two hands is the ‘mirror box illusion’ (e.g. Franz and Packman 2004; Holmes and Spence 2005). This illusion is manifested when a mirror is placed in between the two upper limbs along the midsagittal plane. The reflection of the arm viewed in the mirror seems superimposed on the visual image of the arm behind the mirror. When the arm facing the reflective side is moved, this creates the illusory perception of a zero lag symmetrical movement of the two limbs. The effects of mirror visual feedback were first investigated by Ramachandran and Rogers-Ramachandran (1996) in amputees with phantom pain. After a short period of ‘mirror box’ therapy, which involved (bilateral) mirror-symmetric movements, amputees reported a decrease in phantom pain. These encouraging findings led to the adoption of mirror visual feedback in treating other acquired unilateral motor or pain disorders where the illusion appeared to result in positive effects on motor performance and pain perception (for a review see Ramachandran and Altschuler 2009). For instance, it was found that chronic stroke patients could benefit from therapy using mirror visual feedback, showing increases in the range of motion, speed and accuracy of arm movements (Altschuler et al. 1999; Stevens and Stoykov 2003), an improved functional use and a recovery of grip strength (Sathian et al. 2000). Likewise, in patients with Chronic Regional Pain Syndrome 1 (CRPS1) mirror visual feedback of the unaffected limb reduced the perception of pain and stiffness (McCabe et al. 2003).
Interestingly, Feltham et al. (2010a, c) demonstrated that the positive effects of mirror visual feedback may potentially be extended to individuals with congenital disorders such as SHCP, a finding that was recently supported by Gygax et al. (in press) who showed that mirror therapy in children with hemiplegia may improve strength and dynamic function of the impaired arm. Feltham et al. (2010a, c) used a task where participants performed continuous symmetrical circular movements with both upper limbs in three visual conditions (glass: seeing the two arms; screen: seeing only the less-impaired arm; mirror: seeing the less-impaired arm and its mirror reflection). An effect of mirror visual feedback was found on the nature of the bimanual coordination (Feltham et al. 2010a) and on the neuromuscular activation in children with SHCP (Feltham et al. 2010c). More specifically, in the first study, it was demonstrated that movement variability of the interlimb coupling was lower in the mirror condition in comparison with the screen condition. In addition, mirror visual feedback resulted in a reduction in the neuromuscular intensity in the shoulder muscles of the less-impaired limb and a shortening of the duration of eccentric and concentric activity in the elbow muscles of the impaired limb. In accordance with Perry et al. (2001), a phase where a flexor muscle (e.g. Biceps Brachii Brevis, BBB) was actively contributing to a flexion movement was defined as concentric, whereas flexor activity was eccentric when it contributed to an extension movement. For extensor muscles (e.g. Triceps Brachii Longus, TBL), the opposite classification was used. Note that an earlier study showed that children with SCHP performed this bimanual coordination task with higher levels of neuromuscular intensity in elbow and wrist muscles and longer periods of concentric and eccentric activity in elbow and shoulder muscles compared with typically developing children (Feltham et al. 2010b). More eccentric activity of the BBB might suggest more counteraction to the extension movement and hence indicates that the neuromuscular control is less efficient in children with SHCP. The finding of a decrease in interlimb variability and a reduction in eccentric and concentric muscle activity in a condition with mirror visual feedback thus shows that the mirror has the capacity to induce a general improvement of the kinematics and the neuromuscular efficiency during bimanual movements in children with SHCP.
A pertinent question is, however, whether the mirror effects observed in these children are caused by the illusory perception of seeing two arms moving in perfect symmetry, irrespective of which arm is seen in the mirror, or by the illusion that the impaired limb has been substituted with a less-impaired limb, which is not spastic. The studies by Feltham et al. (2010a, c) described above have only investigated the effect of mirror visual feedback from the unaffected arm and therefore were not able to discriminate between these two explanations. When Franz and Packman (2004) found that mirror visual feedback was powerful enough to enhance spatial coupling of the two hands in healthy adults performing a circle drawing task in a similar manner as actual vision of both hands, this effect was independent of the laterality of the mirror visual feedback. In a condition where only one hand was visible, the circles drawn by the hand in vision were found to be significantly larger than for the hand hidden behind the screen. Mirror visual feedback, regardless of which hand was viewed, had the capacity to wipe out this between-hand difference in circle size. Franz and Packman (2004) hypothesised that the illusion of the perfect symmetry between the two hands created by the mirror promoted the sensorimotor coupling at the central level.
In children with SHCP, however, the movement produced by the impaired and less-impaired arm is qualitatively different, and hence, the mirror visual feedback created by either arm is considerably different as well. Whilst there is an illusion of perfect symmetric movement in both situations, the mirror visual feedback of the impaired arm shows a less smooth movement hampered by the motor deficits. This discrepancy between the two sides and the mirror visual feedback they elicit enables us to investigate the mirror box illusion in this group of children in more detail. More specifically, the aim of the present study was to determine whether the mirror effects as found previously by Feltham et al. (2010a, c) are the result of the perception of visual symmetry per se, irrespective of which arm is viewed, or by the illusion that the impaired arm has been substituted and appears to move smoother and in synchrony with the less-impaired arm. For this purpose, we compared the effect of mirror visual feedback generated by the less-impaired and the impaired arm on the bimanual coupling and the neuromuscular activity in children with SHCP during a bimanual coordination task similar to the one used in Feltham et al. (2010a, c). Based on the studies of Feltham et al. (2010a, c) we anticipate that mirror visual feedback from the less-impaired arm will result in smaller interlimb variability and reduced eccentric activity in the arm muscles of the impaired limb compared to the visual feedback of both arms (glass condition). If the illusion of visual symmetry is the main trigger for the changes induced by the mirror, mirror visual feedback of the less-impaired arm is expected to induce similar effects on the kinematics and the neuromuscular activity as compared to mirror visual feedback of the impaired arm. Alternatively, if the mirror effect in children with SHCP is caused by a mechanism involving substitution of the visual information of the impaired arm by visual feedback from the less-impaired arm, we expect to find less favourable changes to the control of the movement when viewing the impaired upper limb and its mirror reflection than when viewing mirror visual feedback of the less-impaired limb.
Methods
Participants
Ten children (eight males and two females) with SHCP participated in the study (mean age 12.7 ± 3.2 years). Further participant characteristics can be found in Table 1. A subset of the data from seven children who took part in a previous study (Feltham et al. 2010c) was identified to be included in the present analysis. The participants did not have impaired vision or any neuromuscular disorders other than SHCP. Written informed consent was obtained from all participating children and their parents. The experiment was conducted in accordance with the Declaration of Helsinki, and all experimental procedures were approved by the institutional research ethics committee.
Table 1.
Participant characteristics
| Participant | Age | Sex | Hand dominance | MAS | GMFCS | WeeFIM | Aethiology |
|---|---|---|---|---|---|---|---|
| 1 | 12.8 | M | Left | 1 | I | 90 | Unknown |
| 2 | 9.3 | F | Left | 1+ | I | 89 | Cerebral haemorrhage |
| 3 | 13.2 | M | Left | 1 | I | 91 | Unknown |
| 4 | 14.3 | M | Left | 1+ | I | 91 | Cerebral haemorrhage during birth and meningitis just after birth |
| 5 | 11.0 | M | Left | 1 | II | 55 | Meningitis just after birth |
| 6 | 6.8 | M | Left | 1 | I | 83 | O2 shortage during birth |
| 7 | 17.1 | M | Left | 2 | I | 91 | Cerebral haemorrhage |
| 8 | 11.1 | M | Right | 1 | I | 91 | Unknown |
| 9 | 14.7 | M | Right | 2 | II | 62 | Schizencephaly |
| 10 | 16.3 | F | Right | 1 | I | 79 | O2 shortage during birth |
Severity of the impairment was assessed by a single experimenter with the Modified Ashworth Scale (MAS; spasticity levels increase from 1 to 4), Gross Motor Function Classification System (GMFCS; function deteriorates from I to V) and the functional independence measure for children (WeeFIM; motor items only, with a possible score range of 13–91. A higher score denotes a better functional independence of the child)
Test procedures
Each participant was seated on a height adjustable chair at a table with both feet flat on the floor and the knees 90° flexed. The elbows were flexed over 90°, and in each hand, the participant grasped a handle attached to a wooden disc (radius 0.10 m) which spun freely 360° around a vertical axis. The axes were fixed to a wooden plateau and were located 0.31 m apart.
Participants were asked to perform a continuous inward symmetrical circular bimanual movement (the right arm rotated anti-clockwise and the left arm rotated clockwise). Starting at the inner most part of each circle (9 o’clock for the right arm and 3 o’clock for the left arm), children were asked to rotate the discs continuously at a self-selected speed until they were instructed to stop. Additionally, they were instructed to keep the movement time per cycle (i.e. movement frequency) constant across the experimental trials and the different conditions.
The type of visual feedback was varied so that the participant (1) viewed both arms, (2) viewed only one arm and (3) viewed one arm and its mirror reflection, by placing a glass, opaque screen or mirror divide, respectively (all: width 0.06 m, depth 0.75 m, height 0.39 m), between the arms along the midsagittal plane (Fig. 1). The glass and the screen conditions were added as control conditions. In addition, in order to examine the difference between mirror visual feedback of the less-impaired arm (referred to as ‘uncompromised’ mirror visual feedback) and mirror visual feedback of the impaired arm (referred to as ‘compromised’ mirror visual feedback) on the nature of the bimanual coupling and the neuromuscular activity in the BBB and TBL muscle, the orientation of the head (i.e. viewing side) was varied; the participants orientated their head either towards the impaired side of the body (ViewImp) or to the less-impaired side of the body (ViewLessImp).
Fig. 1.
Experimental setup showing one of the experimenters demonstrating the task during the glass (left panel), screen (middle panel) and mirror (right panel) condition. The participant viewed the bimanual task either from the impaired or from the less-impaired side of the body. Note that the participants were considerably smaller than the experimenter and that their posture was more erect than shown in this picture
The six conditions (3 visual feedback × 2 viewing side conditions) were presented in a random order and per condition, three trials, each lasting approximately 15 s, were recorded. Prior to data collection, practice trials were conducted to familiarise the participants with the test setup. Short breaks were given between the trials in order to recover from any fatigue or decrease in concentration that might have occurred during the performance of the experiment. In order to keep the participants motivated, they were told that rotating the discs more symmetrically resulted in more points. At the end of the experiment, the children could trade their points for a small gift.
Recording and analysis procedures
The 3D position of the wrist, elbow and shoulder was determined by two serially connected units containing three infrared cameras at 200 Hz (3020 Optotrak, Northern Digital Inc., Waterloo, Canada). Light emitting diodes were bilaterally attached to the skin with double-sided tape over the dorsal tuberculum of the radius (wrist), lateral epicondyle of the humerus (elbow), greater tubercle of the humerus (shoulder) and the trochantor of the femur (hip). The phase of each limb was calculated according to the following formulas:
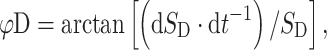 |
and
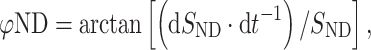 |
where φD and φND are the phase of the dominant (less-impaired) and the non-dominant (impaired) hand, respectively, S D and S ND are the position time series, and dS D·dt −1 and dS ND·dt −1 represent the instantaneous velocity. Before the calculation of φND, the sign of the position time series of the non-dominant arm was inversed to an anti-clockwise trajectory. The continuous relative phase (CRP) indicating the degree of coupling (i.e. synchronicity) between the arms is then:
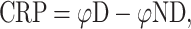 |
where a positive value for CRP implied the less-impaired arm lead and a negative value the impaired arm lead.
Superficial EMG (electromyography) was bilaterally recorded from the main muscles around the elbow: the Biceps Brachii Brevis (BBB) and the Triceps Brachii Longus (TBL), according to the SENIAM guidelines for surface EMG measurement (Hermens et al. 2000). The ground electrode was placed over the acromion on the side of the less-impaired hand. Disposable Ag/AgCl surface EMG electrodes with a gel-skin contact, active detection area of 15 mm2 for each electrode and a 20 mm centre to centre inter-electrode distance, were placed in parallel with the muscle fibre direction over the muscle bellies after cleaning and gentle abrasion of the skin. The EMG signals were amplified 20 times, high-pass pre-filtered at 10 Hz and AD-converted at 1,000 Hz with a 22-bit resolution and stored on a computer. The EMG signals were band-pass filtered with a zero lag 2nd order Butterworth filter between 10 and 400 Hz and then full-wave rectified. Finally, the EMG signals were smoothed with a zero lag 2nd order low-pass Butterworth filter at 6 Hz.
Bilateral EMG recordings were analyzed from the first two cycles of each trial.2 Typically, EMG amplitudes are scaled to the activation levels recorded either during an isometric maximal voluntary contraction or a specified steady-state sub-maximal contraction. However, this procedure is likely to be unreliable in people with neurological conditions since they are often unable or unwilling to perform maximum contractions (van Dieën et al. 2003; Smith et al. 2008). Therefore, to determine the intensity of the mean neuromuscular activity of each muscle during the bimanual movement, the mean amplitude was calculated from the smoothed raw EMG signals. In addition, the amount of concentric and eccentric muscle activity was determined. To this end, the EMG profile of each muscle was broken down into active and inactive phases, after the threshold for muscle contraction was determined. Consistent with Perry et al. (2001), it was assumed that a purposeful activation of a muscle causes an increase in the EMG signal within the frequency range of 0–160 Hz. The active/inactive threshold value was then calculated as follows: T = 15 + 1.5R, where T is the threshold value, R is the mean value of the EMG signal above 160 Hz and the constants are derived from Perry et al. (2001). A muscle was classified as active if the smoothed raw EMG signal was above the threshold level. Subsequently, the active phases were classified as eccentric, concentric or isometric depending on the observed elbow movement and the primary mechanical function of the muscle (i.e. flexion or extension). For example, BBB muscle activity above threshold was classified as concentric when the elbow was being flexed and as eccentric when the elbow was being extended. Above threshold, TBL muscle activity was classified as concentric for elbow extension and as eccentric activity for elbow flexion. If the muscle was active but no change in elbow angle was observed, it was classified as isometric activity. However, this isometric activity was not included in further analysis of this study since the task involved a dynamical movement with accordingly very short relative durations of isometric activity (1.25% of the total muscle activity). The duration of all eccentric and concentric phases was summed and expressed as a percentage of the total movement time (i.e. the movement time of the first two cycles), giving the relative duration of eccentric activity and the relative duration of concentric activity for each muscle.
Statistical analysis
The effect of viewing side and visual feedback condition on the bimanual coupling, EMG intensity and the phases of muscle activity in each arm, was tested using a repeated measurement ANOVA with three within factors: Limb (impaired, less-impaired), Viewing side (view impaired [ViewImp], view less-impaired [ViewLessImp]) and Visual condition (mirror, screen, glass). These analyses were conducted using mean data calculated from the three trials per combination of independent variables. In the event that the sphericity assumption was violated, Greenhouse-Geisser adjustments were applied. Fisher’s LSD tests were used for the post hoc analysis, and the level of significance was set at 0.05.
Results
Bimanual coupling
The CRP did not differ in the three visual conditions (mirror = 6.6° ± 6.3°; screen = 13.2° ± 7.2°; glass = 10.8° ± 7.4°) and the viewing side did not have an effect on the interlimb coupling either (ViewImp = 11.1° ± 6.4° and ViewLessImp = 9.3° ± 7.0°; see Table 2 for values per individual condition). The overall mean was 10.2° ± 6.6°, indicating that the less-impaired arm was the leading limb.
Table 2.
Mean and SE values of the continuous relative phase (CRP) in degrees for each visual condition and viewing condition
| ViewImp | ViewLessImp | |
|---|---|---|
| Mirror | 8.1 ± 7.7 | 5.0 ± 6.6 |
| Screen | 17.2 ± 7.1 | 9.3 ± 8.6 |
| Glass | 8.0 ± 6.6 | 13.6 ± 8.6 |
Intensity of the mean neuromuscular activity in BBB and TBL
There were no significant main or interaction effects on the mean neuromuscular activity in BBB and TBL of either Viewing side or Visual condition (see Table 3). This means that the EMG intensity in BBB and TBL did not change as a function of viewing side or the nature of visual feedback. Viewing the impaired arm and its mirror reflection did not result in higher levels of EMG intensity (BBB: 24.1 ± 3.1; TBL: 9.9 ± 1.2) than viewing the less-impaired arm and its mirror reflection (BBB: 21.7 ± 3.6; TBL: 11.2 ± 2.0). Inspection of Table 3 seems to indicate a trend (F 2,18 = 2.76, P = 0.09) towards lower intensities of neuromuscular activity in the mirror condition compared with the glass and the screen conditions (especially in the BBB of the less-impaired limb in the ViewLessImp condition). In addition, the mean neuromuscular activity tended to be higher in the impaired than in the less-impaired arm for both the BBB and TBL muscles (BBB: 29.0 ± 4.9 vs. 19.5 ± 3.9; TBL: 14.7 ± 3.3 vs. 8.5 ± 1.1); however, the ANOVA indicated that this effect of Limb was not statistically significant (BBB: F 1,9 = 2.29, P = 0.17; TBL: F 1,9 = 3.40, P = 0.10).
Table 3.
Mean and SE values of the intensity of mean neuromuscular activity (μV) for the BBB and the TBL muscle of the impaired and the less-impaired limb presented for each viewing condition (ViewImp, ViewLessImp)
| BBB | ||
|---|---|---|
| ViewImp | ViewLessImp | |
| Impaired limb | ||
| Mirror | 29.9 ± 4.2 | 27.4 ± 5.7 |
| Screen | 27.9 ± 4.2 | 27.3 ± 5.6 |
| Glass | 31.0 ± 6.3 | 30.6 ± 5.2 |
| Less-impaired limb | ||
| Mirror | 18.2 ± 3.8 | 16.2 ± 3.2 |
| Screen | 17.6 ± 3.4 | 21.3 ± 4.4 |
| Glass | 17.5 ± 4.5 | 26.2 ± 7.2 |
| TBL | ||
|---|---|---|
| ViewImp | ViewLessImp | |
| Impaired limb | ||
| Mirror | 12.4 ± 2.2 | 13.9 ± 3.5 |
| Screen | 12.4 ± 2.0 | 17.3 ± 5.4 |
| Glass | 15.4 ± 4.3 | 16.8 ± 3.9 |
| Less-impaired limb | ||
| Mirror | 7.3 ± 1.1 | 8.4 ± 1.4 |
| Screen | 8.8 ± 1.3 | 8.8 ± 1.4 |
| Glass | 6.8 ± 1.1 | 10.6 ± 1.9 |
Relative duration of concentric and eccentric activity in the BBB muscle
No significant main or interaction effects were found for the concentric activity of the BBB muscle (see Table 4). Mirror visual feedback, irrespective of which arm was viewed, did not have an effect on the relative contribution of concentric BBB activity to the execution of the movement in the impaired or less-impaired arm (F 2,18 = 0.36; P = 0.70). Additionally, there tended to be more concentric activation in the impaired limb than in the less-impaired limb (25.8 ± 3.9 vs. 17.2 ± 4.4), but this difference was insignificant (F 1,9 = 2.74, P = 0.13).
Table 4.
Mean and SE values of the eccentric and concentric muscle activity, expressed as a percentage of the total movement, of the Biceps Brachii Brevis (BBB) and the Triceps Brachii Longus (TBL) in the impaired and less-impaired limb for theViewImp (viewing the movement from the impaired side of the body) and ViewLessImp (viewing the movement from the less-impaired side of the body) conditions
| BBB (%muscle activity) | ||||
|---|---|---|---|---|
| Eccentric | Concentric | |||
| ViewImp | ViewLessImp | ViewImp | ViewLessImp | |
| Impaired limb | ||||
| Mirror | 34.2 ± 4.9 | 23.9 ± 6.5 | 26.6 ± 3.7 | 26.1 ± 4.2 |
| Screen | 30.2 ± 5.5 | 28.5 ± 7.2 | 25.7 ± 4.7 | 22.5 ± 3.6 |
| Glass | 28.0 ± 6.1 | 36.7 ± 6.3 | 25.1 ± 5.4 | 28.6 ± 4.1 |
| Less-impaired limb | ||||
| Mirror | 12.5 ± 4.1 | 13.2 ± 4.5 | 16.4 ± 5.1 | 16.2 ± 4.5 |
| Screen | 12.2 ± 4.1 | 16.3 ± 4.3 | 17.4 ± 5.0 | 18.8 ± 4.6 |
| Glass | 15.1 ± 5.6 | 14.5 ± 3.7 | 16.2 ± 5.3 | 18.3 ± 5.2 |
| TBL (%muscle activity) | ||||
|---|---|---|---|---|
| Eccentric | Concentric | |||
| ViewImp | ViewLessImp | ViewImp | ViewLessImp | |
| Impaired limb | ||||
| Mirror | 7.3 ± 2.8 | 11.6 ± 4.2 | 10.5 ± 3.7 | 9.9 ± 4.9 |
| Screen | 9.1 ± 3.4 | 11.7 ± 4.0 | 11.8 ± 3.4 | 13.5 ± 5.2 |
| Glass | 10.8 ± 4.6 | 13.0 ± 4.8 | 12.7 ± 4.5 | 13.0 ± 4.7 |
| Less-impaired limb | ||||
| Mirror | 3.4 ± 1.6 | 4.9 ± 2.3 | 1.7 ± 0.7 | 3.8 ± 1.4 |
| Screen | 5.2 ± 1.8 | 3.2 ± 1.2 | 4.3 ± 1.5 | 5.7 ± 2.0 |
| Glass | 2.2 ± 1.5 | 8.3 ± 2.6 | 1.8 ± 1.2 | 8.8 ± 3.0 |
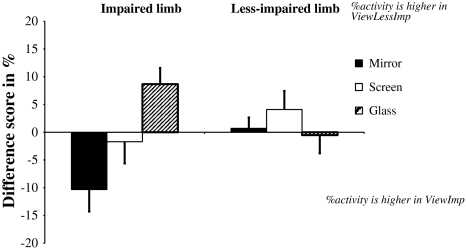
For the eccentric activity of the BBB muscle, a significant main effect of the Limb was found (F 1,9 = 7.53, P = 0.02) with the impaired limb having 16.3% more eccentric activity than the less-impaired limb. This effect was accompanied by a three-way interaction between Limb, Viewing side and Visual condition (F 2,18 = 4.67, P = 0.02). Figure 2 illustrates this interaction using the difference in eccentric activity between the two viewing sides (i.e. ViewImp and ViewLessImp) for the impaired and less-impaired limb and for each visual condition. This difference score was determined by subtracting the eccentric activity in the ViewImp condition from the eccentric activity in the ViewLessImp condition. A negative difference score then indicates lower eccentric activity in the ViewLessImp condition, whereas a positive difference score represents higher eccentric activity in the ViewLessImp condition. Inspection of Fig. 2 and post hoc examination of the three-way interaction indicated that there were no effects of Visual condition or Viewing side on the eccentric activity of the less-impaired arm. For the impaired arm, however, mirror visual feedback from the impaired arm resulted in 10.3% more eccentric activity than mirror visual feedback from the less-impaired arm (P = 0.007). Furthermore, a significant effect of Viewing side was also present in the glass condition, where looking from the less-impaired side resulted in more eccentric activity than looking from the impaired side (mean difference score = 8.7%, P = 0.02). Viewing side did not have an effect on the eccentric activity of the BBB in the screen condition. Finally, focusing on the differences in eccentric activity between the visual conditions (see Table 4), it was found that mirror visual feedback of the less-impaired arm resulted in less eccentric activity in the impaired arm than the glass condition when viewing from the same side (mean difference = 12.8%, P = 0.001). In addition, the glass condition was performed with more eccentric activity in the impaired arm than the screen condition (mean difference = 8.2%, P = 0.02).
Fig. 2.
Difference scores of the relative duration of eccentric activity (in percentage) in the BBB muscle of the impaired (left side of the figure) and the less-impaired limb (right side of the figure) for the mirror (black bars), screen (white bars) and glass (dashed bars) condition. A positive difference score means that the eccentric activity is higher in the ViewLessImp compared with the ViewImp condition, and a negative difference score means that the eccentric activity is lower in the ViewLessImp condition compared with the ViewImp condition
Relative duration of concentric and eccentric activity in the TBL muscle
For the concentric activity of the TBL muscle, a significant interaction effect between Limb and Viewing side was found (F 1,9 = 10.47, P = 0.01; see Table 4). The concentric activity in the impaired limb was larger than in the less-impaired limb for both the ViewImp and the ViewLessImp condition (mean difference = 8.56 and 4.56%, respectively). Furthermore, viewing from the less-impaired side resulted in longer durations of concentric activity in the less-impaired limb than viewing from the impaired side, irrespective of the visual condition (mean difference = 3.49%).
For the eccentric activity of the TBL, no effect of Limb, Visual condition or Viewing side was found.
Discussion
This study investigated the effect of mirror visual feedback from the impaired arm (‘compromised’) compared with the mirror visual feedback from the less-impaired arm (‘uncompromised’) on the interlimb coupling and the neuromuscular control during a bimanual coordination task in children with SHCP. In doing so, we wanted to determine whether previously found effects of the mirror box illusion in these children (Feltham et al. 2010a, c) were the result of the mirror and the related perception of visual symmetry per se or of the illusion that the impaired arm appears to move with less jerk and in synchrony with the less-impaired arm. While the former would mean that ‘compromised’ as well as ‘uncompromised’ mirror visual feedback can trigger an improvement of the bimanual coupling and/or the neuromuscular activation, the latter can only be elicited by ‘uncompromised’ mirror visual feedback.
The CRP, which gives an indication of the nature of the bimanual coupling during this task, i.e., the synchronicity of the two limbs, indicates that the less-impaired arm was ‘leading’ the impaired arm across all conditions. This is in congruence with earlier studies on bimanual coordination in typically developing children (Pellegrini et al. 2004) and adults (e.g. Amazeen et al. 1997; Stucchi and Viviani 1993; Treffner and Turvey 1995). The asynchrony of approximately 10° falls within the higher range of previously reported values in children with SHCP (Feltham et al. 2010a: −0.3°; Volman et al. 2002: −5° to 9°), but is still acceptable given the unilateral impairment of the children. Note that the phase lag between the two hands may indicate that the movement of the lagging impaired hand may be guided by visual feedback from the less-impaired hand. However, the CRP did not change as a function of visual condition or viewing side, which suggests that the bimanual coupling is clearly not solely governed by a visual feedback mechanism and that processes relying on central representations of action do contribute to the coupling as well (addressed below).
It thus seems that mirror visual feedback did not influence the interlimb coupling, and there was no difference between ‘compromised’ and ‘uncompromised’ mirror visual feedback. Interestingly, however, the mirror did have an effect on the neuromuscular activity required to perform the task. This suggests that, although the movement performance itself remained the same, the muscular effort responsible for this movement did change in response to the available visual information. Our results demonstrate that mirror visual feedback led to a reduction in eccentric BBB activity in the impaired arm compared with the glass condition, and importantly, this effect was exclusive to ‘uncompromised’ mirror visual feedback, i.e., viewing the less-impaired arm and its mirror reflection (ViewLessImp). In the impaired arm, mirror visual feedback of the less-impaired arm appears to have the capacity to improve the neuromuscular efficiency by reducing the disproportionally high eccentric activity. The finding that ‘compromised’ mirror visual feedback did not elicit a similar effect shows that the mirror effect in children with SHCP is not just a response to the visual symmetry, but is also dependent on the type of visual information generated by the mirror. The latter nuances the findings of Franz and Packman (2004) who found that mirror visual feedback enhanced the bimanual coupling (i.e. similarity in range of motion of the two hands) in typical adults, irrespective of viewing mirror feedback from the left or the right hand. However, unlike in typical adults, in children with SHCP, the nature of mirror visual feedback from the left and right hand is qualitatively different, which might explain the apparent discrepancy between the two studies.
The finding from the present study that mirror visual feedback of the impaired arm has the opposite effect of ‘uncompromised’ apparent symmetrical motion in children with SCHP qualifies the findings of Feltham et al. (2010c) who only looked at the effect of mirror feedback from the less-impaired arm. We demonstrated that the favourable results (i.e. the reduction in eccentric BBB activity in the impaired arm) are not just due to the visual perception of apparent bimanual symmetry per se. Instead, children with SHCP appear to benefit specifically of mirror visual feedback from the less-impaired arm, which seems to be in line with the notion of Ramachandran (2005). Ramachandran hypothesised that mirror visual feedback may assist the central control of movement in people with unilateral motor problems by restoring the congruence between disrupted sensory information and the central motor command signals. According to this view, the information provided by the mirror could assist in the neuromuscular control of the movement by replacing conflicting visual feedback of the impaired limb with feedback that is in accordance with the intended movement (i.e. ‘uncompromised’ visual feedback of the less-impaired limb). By showing that the mirror effect on motor performance in children with SHCP is specifically related to mirror visual feedback of the less-impaired arm, the current study provides a valuable contribution to the discussion about the underlying mechanisms of this effect. Nevertheless, the actual neural underpinnings will only be revealed using advanced neuro-imaging techniques. In addition, it may be surprising that a short exposure to the mirror already induces these effects on the neuromuscular activity and future studies should examine the impact of longer exercise or interventions with mirror feedback. Related to this issue is the fact that no (major) effect of the mirror was observed on the bimanual coupling or neuromuscular measures such as the intensity of mean neuromuscular activity, the eccentric activity in the TBL muscle and concentric activity in the BBB muscle. Furthermore, we cannot exclude the limited number of trials (three per condition) and the large age range of the participants to affect the precision and generalisation of the results. The precision of the measurement might be enhanced with larger number of trials, but in the current study, it was high enough to reveal significant differences between the conditions. One can expect that a larger number of trials will enhance the actual results but one must also consider that the limited attention span and fatigability of the participants with cerebral palsy might interfere. Considering that the present study used a repeated measures design each participant was his own control and the variability that the large age range may have introduced was nevertheless small enough to show a significant effect of the experimental conditions. While we did not anticipate an age effect, we cannot exclude it and suggest that this should be further investigated.
In conclusion, this study provided more insight into the effects of mirror visual feedback in children with SHCP. We showed that the effects found by Feltham et al. (2010a, c) on neuromuscular activity and bimanual coordination are likely not caused by the perception of two symmetrically moving limbs per se. Instead, for an increase in neuromuscular efficiency of bimanual movement (i.e. a decrease in excessive eccentric activity in the arm flexors), children with SHCP require mirror visual feedback of the (‘unaffected’) less-impaired limb.
Acknowledgments
We would like to thank the children and their parents for their participation in the study. In addition, we would like to thank Marjolein Smit and Anniek Geerlings for their help with the additional data collection.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Cerebral Palsy (CP) is a group of permanent disorders of movement and posture due to a non-progressive lesion in the foetal or infant brain (Miller 2007). CP is the most common cause of childhood disability and has an incidence of 2–2.5 per 1,000 living births (Lin 2003). A common form of CP is Spastic Hemiparetic Cerebral Palsy (SHCP). Children with SHCP have a brain lesion in one hemisphere and as a result have spasticity on the other side of the body.
Only the first two cycles of each trial could be analyzed since some children with SHCP could only fulfil 2 cycles before they adopted a different coordination mode than the one they were instructed to produce. Moreover, for some children the movement time allowed them to complete only 2 cycles within the allocated time of each trial or the hand slipped off the handle at which point the trial had to be terminated.
References
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, Ramachandran VS. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353(9169):2035–2036. doi: 10.1016/S0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- Amazeen EL, Amazeen PG, Treffner PJ, Turvey MT. Attention and handedness in bimanual coordination dynamics. J Exp Psychol. 1997;23:1552–1560. [Google Scholar]
- Feltham MG, Ledebt A, Bennett SJ, Deconinck FJ, Verheul MH, Savelsbergh GJ. The “mirror box” illusion: effect of visual information on bimanual coordination in children with spastic hemiparetic cerebral palsy. Mot Control. 2010;14(1):68–82. doi: 10.1123/mcj.14.1.68. [DOI] [PubMed] [Google Scholar]
- Feltham MG, Ledebt A, Deconinck FJ, Savelsbergh GJ. Assessment of neuromuscular activation of the upper limbs in children with spastic hemiparetic cerebral palsy during a dynamical task. J Electromyogr Kinesiol. 2010;20(3):448–456. doi: 10.1016/j.jelekin.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Feltham MG, Ledebt A, Deconinck FJ, Savelsbergh GJ. Mirror visual feedback induces lower neuromuscular activity in children with spastic hemiparetic cerebral palsy. Res Dev Disabil. 2010;31(6):1525–1535. doi: 10.1016/j.ridd.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Franz EA, Packman T. Fooling the brain into thinking it sees both hands moving enhances bimanual spatial coupling. Exp Brain Res. 2004;157(2):174–180. doi: 10.1007/s00221-004-1831-3. [DOI] [PubMed] [Google Scholar]
- Gygax MJ, Schneider P, Newman CJ (in press) Mirror therapy in children with hemiplegia: a pilot study. Dev Med Child Neurol 53(5):473–476. doi:10.1111/j.1469-8749.2011.03924.x [DOI] [PubMed]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Holmes NP, Spence C. Visual bias of unseen hand position with a mirror: spatial and temporal factors. Exp Brain Res. 2005;166(3–4):489–497. doi: 10.1007/s00221-005-2389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JP. The cerebral palsies: a physiological approach. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 1):i23–i29. doi: 10.1136/jnnp.74.suppl_1.i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, Blake DR. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1) Rheumatology (Oxford) 2003;42(1):97–101. doi: 10.1093/rheumatology/keg041. [DOI] [PubMed] [Google Scholar]
- Miller F. Physical therapy of cerebral palsy. New York: Springer; 2007. [Google Scholar]
- Pellegrini AM, Andrade EC, Teixeira LA. Attending to the non-preferred hand improves bimanual coordination in children. Hum Mov Sci. 2004;23(3–4):447–460. doi: 10.1016/j.humov.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Perry JE, Davis BL, Luciano MG. Quantifying muscle activity in non-ambulatory children with spastic cerebral palsy before and after selective dorsal rhizotomy. J Electromyogr Kinesiol. 2001;11(1):31–37. doi: 10.1016/S1050-6411(00)00035-3. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Plasticity and functional recovery in neurology. Clin Med. 2005;5(4):368–373. doi: 10.7861/clinmedicine.5-4-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132(Pt 7):1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263(1369):377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Sathian K, Greenspan AI, Wolf SL. Doing it with mirrors: a case study of a novel approach to neurorehabilitation. Neurorehabil Neural Repair. 2000;14(1):73–76. doi: 10.1177/154596830001400109. [DOI] [PubMed] [Google Scholar]
- Smith MD, Coppieters MW, Hodges PW. Is balance different in women with and without stress urinary incontinence? Neurourol Urodyn. 2008;27(1):71–78. doi: 10.1002/nau.20476. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Hulstijn W, de Vries A, Berger M. Bimanual movement coordination in spastic hemiparesis. Exp Brain Res. 1996;110(1):91–98. doi: 10.1007/BF00241378. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Charles J, Gordon AM. Fingertip force control during bimanual object lifting in hemiplegic cerebral palsy. Exp Brain Res. 2008;186(2):191–201. doi: 10.1007/s00221-007-1223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil. 2003;84(7):1090–1092. doi: 10.1016/S0003-9993(03)00042-X. [DOI] [PubMed] [Google Scholar]
- Stucchi N, Viviani P. Cerebral dominance and asynchrony between bimanual two-dimensional movements. J Exp Psychol Hum Percept Perform. 1993;19(6):1200–1220. doi: 10.1037/0096-1523.19.6.1200. [DOI] [PubMed] [Google Scholar]
- Sugden D, Utley A. Interlimb coupling in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1995;37(4):293–309. doi: 10.1111/j.1469-8749.1995.tb12008.x. [DOI] [PubMed] [Google Scholar]
- Treffner PJ, Turvey MT. Handedness and the asymmetric dynamics of bimanual rhythmic coordination. J Exp Psychol. 1995;21(2):318–333. [Google Scholar]
- Utley A, Sugden D. Interlimb coupling in children with hemiplegic cerebral palsy during reaching and grasping at speed. Dev Med Child Neurol. 1998;40(6):396–404. [PubMed] [Google Scholar]
- van Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13(4):333–351. doi: 10.1016/S1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Volman MJ, Wijnroks A, Vermeer A. Bimanual circle drawing in children with spastic hemiparesis: effect of coupling modes on the performance of the impaired and unimpaired arms. Acta Psychol (Amst) 2002;110(2–3):339–356. doi: 10.1016/S0001-6918(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Kaluzny P, Kazennikov O, Palmeri A, Perrig S. Temporal coordination in bimanual actions. Can J Physiol Pharmacol. 1994;72(5):591–594. doi: 10.1139/y94-084. [DOI] [PubMed] [Google Scholar]