Abstract
We previously reported that extracellular histones are major mediators of death in sepsis. Infusion of extracellular histones leads to increased cytokine levels. Histones activate TLR 2 and 4 in a process that is enhanced by binding to DNA. Activation of TLR4 is responsible for the histone dependent increase in cytokine levels. To study the impact of histone release on pathology we used two models: a concanavalin A (ConA) triggered activation of T cells to mimic sterile inflammation and acetaminophen (APAP), to model drug induced tissue toxicity. Histones were released in both models and anti-histone antibodies were protective. TLR 2 or TLR 4 null mice were also protected. These studies imply that histone release contributes to death in inflammatory injury and in chemical induced cellular injury, both of which are mediated in part through the toll-like receptors.
Introduction
Systemic inflammatory responses syndrome (SIRS) occurs in a variety of medical conditions including infection, trauma, ischemia-reperfusion as well as autoimmunity. Sepsis is a typical example of SIRS caused by infection. Both gram-negative and positive bacteria can cause SIRS through TLR signaling pathways 1. The clinical sequelae of SIRS include organ injury, increases in coagulation that may result in macro and microvascular thrombosis and tissue injury mediated by cytokines and leukocytes 1,2. In sepsis and trauma, there are elevated levels of circulating nucleosomes that could be derived either from apoptotic cells 3 or from the degradation of neutrophil extracellular traps (NETs) 4,5. Apoptosis and tissue necrosis increases in severe diseases like sepsis and trauma and correlates with mortality 3,6,7. Nucleosomes result from chromatin degradation and consist of a core octamer of two copies of histones H2A,H2B, H3 and H4 wrapped by a 146 basepairs of DNA 3. Recently, NETs were shown to activate platelets leading to thrombosis 5 and the major contributor to this process was histone H4. We previously reported that extracellular histones released in response to bacterial challenge are mediators contributing to endothelial dysfunction, organ failure and death during sepsis. In that study, Histone H4 was also shown to kill endothelial cells 8. Most importantly, blocking histone mediated cytotoxicity was shown to protect mice from lipopolysaccharide mediated death. Infusion of histones alone into mice resulted in a pathological response similar to sepsis including migration of leukocytes into tissues, microvascular thrombosis and organ failure, ultimately culminating in death.
The molecular mechanisms of histone mediated tissue injury and organ failure remain unclear. In this study we demonstrate that TLR2 and TLR4 are major receptors for extracellular histone mediated sterile inflammation, tissue injury and death in mouse models. Extracellular histones and their signaling pathways through TLRs are potential novel therapeutic targets in a variety of inflammatory and chemical toxin mediated diseases.
Materials and Methods
Reagents
We purchased ConA (C2010), calf thymus histones (H9250), calf thymus DNA (D1501) and acetaminophen (A5000) from Sigma, and goat antibody to H3 (Santa Cruz). Dr. Marc Monestier provided mouse monoclonal antibodies to H3 (LG2-1), H4 (BWA-3) and DNA-H2A-H2B (PR1-3).
Animals
We used 6–12 week old male wild type (C57BL/6), TLR2 KO (Stock #004650, backcrossed at least 7 generations) and TLR4 KO (Stock #007227, backcrossed at least 6 generations) mice (Jackson Laboratory) according to an animal protocol approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Mouse plasma chemistry, cytokine assay and western blotting
We collected whole mouse blood in 11 mM sodium citrate by heart puncture and isolated plasma by centrifuging the blood immediately after collection. We added 20 mM benzamidine to the citrated blood to isolate the plasma for western blotting of H3 with goat antibody to H3. We measured mouse plasma ALT, AST, BUN, creatine kinase (CK), creatinine, total bilirubin (TBIL) with a Vitros 250 chemistry analyzer (Ortho-Clinical Diagnostics) and IFNγ, IL-1β, IL-6, IL-10, IL-12p70, KC and TNFα with a MSD 96-well Multi-Array for mouse cytokines (Meso Scale Diagnostics). The original histone TLR screening assay was performed by InvivoGen.
Cell culture and TLR signaling assay
We cultured human TLR2 (HEK-Blue-2) or human TLR4 (HEK-Blue-4) expressing HEK293 cells in DMEM supplemented with 10% FBS and selective antibiotics according to the manufacturer’s instructions (InvivoGen). The TLR4 expressing cells also express CD14. We measured secreted alkaline phosphatase as a reporter gene under the control of a promoter inducible by NF-kB to monitor the signaling by histones through TLR2 or TLR4 using HEK-Blue Detection medium according to the manufacturer’s instructions (InvivoGen). In experiments to determine the effect of DNA on histone signaling in vitro, the DNA was sonicated into 100 to 1000 bp fragments, mixed with the histones and immediately added to the cells.
Statistical analysis
We expressed the results as mean ± SD and used Student’s t-test to compare two groups. We analyzed survival studies with the log-rank test in the program Prism (GraphPad). We considered differences statistically significant at a P-value < 0.05.
Results
Extracellular histones trigger TLR2 and TLR4 signaling
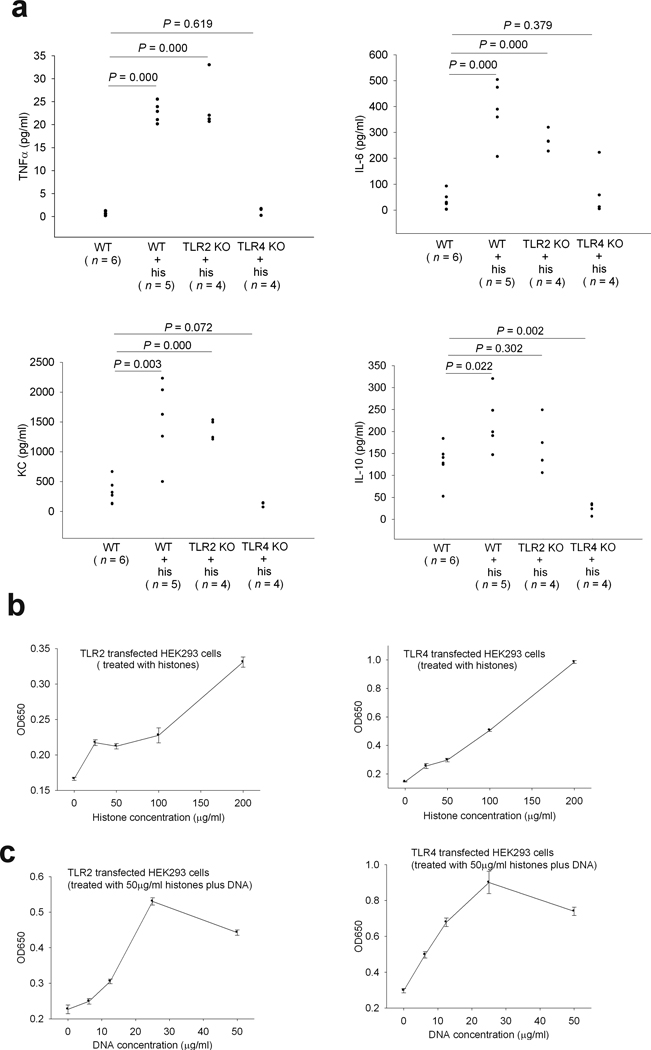
Extracellular histones released in response to inflammatory challenge contribute to endothelial dysfunction, organ failure and death during sepsis8. We wished to further explore the molecular mechanisms of histone mediated tissue injury and lethality. To avoid the direct histone toxicity towards cells, a sublethal dose (25 mg/kg) of histones was injected into wild type (C57BL/6) mice. We found high levels of TNFα, IL-6, KC and IL-10 in the circulation of wild type mice 2 h after histone injection (Fig. 1a). Since TLRs are obvious candidates for signaling inflammatory pathways, we screened seven TLRs as possible histone signaling receptors. TLR2 and TLR4, but not TLR3, TLR5, TLR7, TLR8, or TLR9 were stimulated by histones in TLR transfected cell lines. The presence of DNA further increased the histone induced TLR2 and TLR4 signaling (Fig. 1b and 1c and data not shown). Boiling the histones and DNA abolished TLR2 and TLR4 signaling, ruling out possible LPS effects in these experiments (data not shown). The massive proinflammatory cytokine production induced by histones was abolished in TLR4 KO mice but not in TLR2 KO mice (Fig. 1a), suggesting that TLR4 is the major receptor for a histone induced cytokine inflammatory response.
Fig.1. Extracellular histones trigger TLR2 and TLR4 signaling.
(a) TNFα, IL-6, KC and IL-10 levels in wild type, TLR2 KO or TLR4 KO mouse plasma collected 2 h after an intravenous injection of calf thymus histones (25 mg per kg body weight). Data from 5 experiments. (b) Reporter gene expression levels in TLR2 or TLR4 transfected HEK293 cells stimulated with calf thymus histones at the indicated concentrations. The results shown are the means ± SD performed in triplicate. (c) Reporter gene expression levels in TLR2 or TLR4 transfected HEK293 cells stimulated with calf thymus histones (50 µg/ml) and calf thymus DNA at the indicated concentrations. The results shown are the means ± SD performed in triplicate.
Extracellular histones are major mediators of death through TLR2 and TLR4 in ConA induced sterile inflammation model
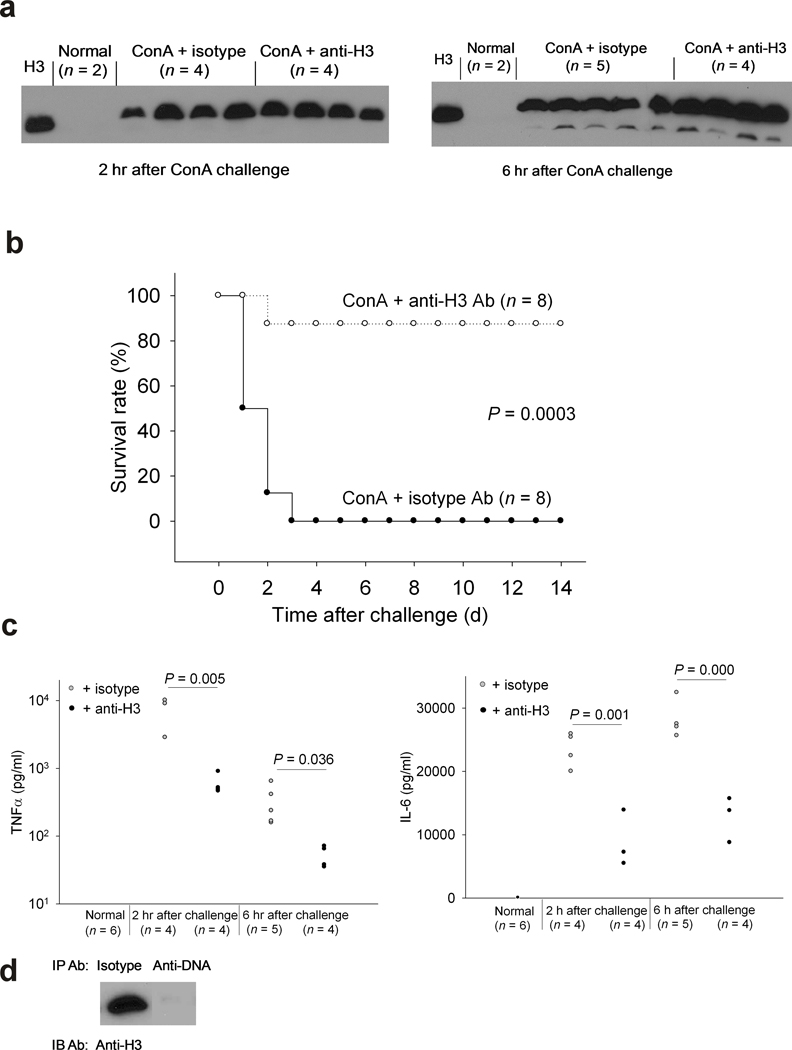
In our previous report, we demonstrated that extracellular histones released in response to bacterial inflammatory challenges contributed to endothelial dysfunction, organ failure and death during sepsis 8. To determine if the same is true for a sterile inflammatory challenge, a ConA challenge was used to induce a T cell activation dependent inflammatory reaction 9. A lethal dose of ConA (30 mg/kg) was injected intravenously and 40 min later we injected monoclonal antibody to histone H3 (H3) or mouse isotype control antibody. Among the effects of ConA infusion, liver injury is caused by the infiltration of activated T cells which results in massive necrotic tissue injury and inflammation 9. We hypothesized that tissue necrosis in this model might result in histone release, contributing to inflammation and death. Indeed, we detected intact H3 in the plasma after ConA challenge by western blotting (Fig 2a) which was sustained for at least 4 h. Injection of H3 antibody rescued 7/8 mice (Fig. 2b), indicating extracellular histones are major mediators of death in this sterile inflammation model, although the H3 antibody appeared to have no influence on the level of histone present in plasma after ConA challenge (Fig. 2a). H3 antibody had no significant protective effect on liver injury caused by ConA since H3 antibody injection did not decrease the high levels of ALT or AST in mouse plasma either 2 h or 6 h after ConA challenge (Supplemental Fig. 1). This result is consistent with a previous finding that liver damage itself was not a major contributor to death in the ConA model 10. The H3 antibody also did not decrease the high levels of creatine kinase or total bilirubin in the circulation after ConA challenge (Supplemental Fig. 2). However, we found that H3 antibody did significantly reduce TNFα and IL-6 levels in the circulation of the ConA challenged mice (Fig. 2c). The observed reduction in cytokine levels by antibody against H3 in this sterile inflammation model is consistent with extracellular histones being able to signal TLRs for cytokine production. To test whether the extracellular histones in the circulation of ConA challenged mice were still complexed with DNA, we immunoprecipitated DNA in a plasma sample with antibody against DNA-H2A-H2B complex and measured the H3 level remaining in the supernatant by western blotting. We found that the DNA-H2A-H2B antibody removed most of the H3 in the sample compared to the isotype control antibody (Fig. 2d), indicating that the bulk of the extracellular histones detected by western blotting were still complexed with DNA in the circulation in this mouse model.
Fig.2. Extracellular histones are major mediators of death in a ConA induced sterile inflammation model.
(a) Western blot analysis using a goat polyclonal anti-H3 in individual mouse plasmas collected 2 or 6 h after lethal ConA (30 mg per kg body weight) challenge in the presence of mouse monoclonal antibody to H3 or mouse isotype control antibody (10 mg per kg body weight). The very left lane is the H3 positive control. (b) Survival rates of C57BL/6 mice challenged with a lethal dose of ConA (30 mg per kg body weight, iv) and 40 min later treated with antibody to H3 or mouse isotype control antibody (10 mg per kg body weight, iv). Data from 3 experiments. (c) Plasma TNFα and IL-6 levels in mice without challenge or in ConA challenged mice as in (a) Data from 3 experiments. (d) Western blot analysis for H3 in a ConA challenged mouse plasma sample after immunoprecipitation by either isotype control or DNA antibody.
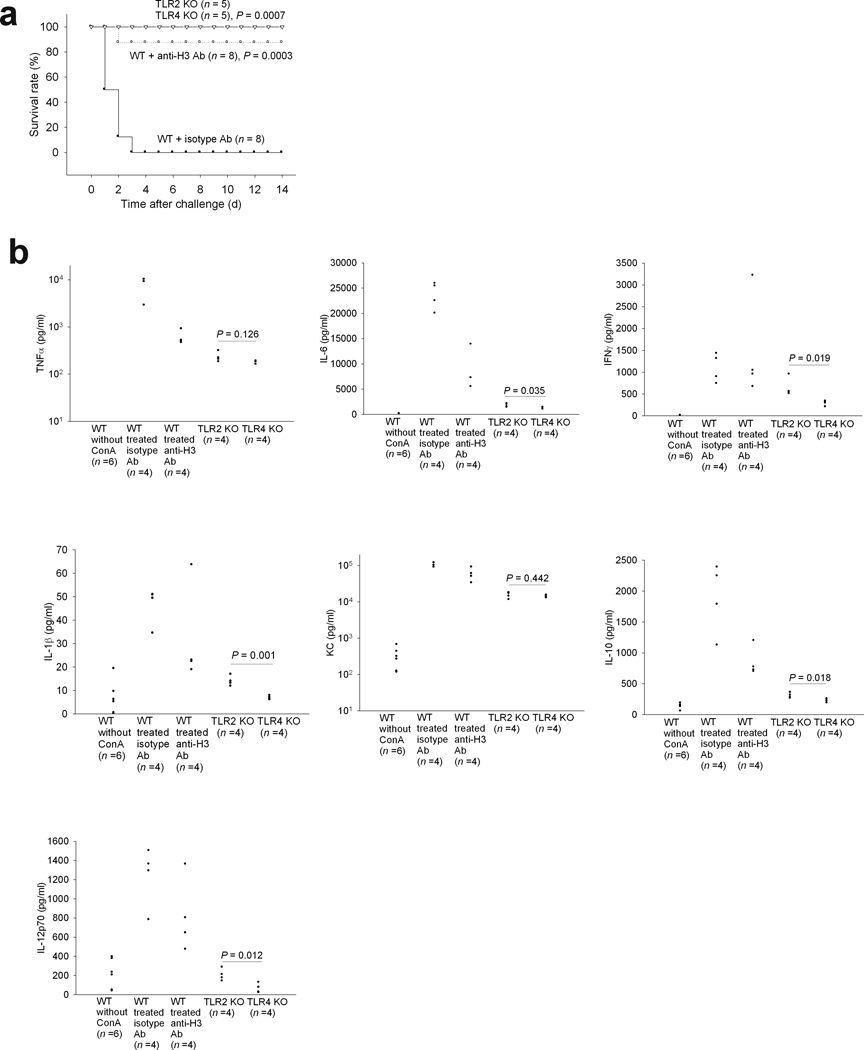
To test whether TLR2 and/or TLR4 contributed to the ConA induced lethality in this fatal inflammation model, we injected a lethal dose of ConA into TLR2 KO and TLR4 KO mice and found that both were protected from the challenge (Fig. 3a). Even though liver injury still occurred in the KO mice 2 h after ConA challenge (Supplemental Fig. 3), circulating levels of TNFα, IL-6, IFNγ, IL-1β, KC, IL-10 and IL-12p70 levels were lower than those observed in H3 antibody treated wild type mice (Fig. 3b). We also found that IL-6, IFNγ, IL-1β, IL-10 and IL-12p70 levels were significantly lower in the circulation of ConA challenged TLR4 KO mice than in the TLR2 KO mice (Fig. 3b), consistent with our previous finding that TLR4 might be a major receptor for histone induced cytokine production.
Fig. 3. TLR2 KO or TLR4 KO mice are resistant to a lethal challenge of ConA.
(a) Survival rates of TLR2 KO or TLR4 KO mice injected intravenously with a lethal dose of ConA (30 mg per kg body weight), combined with the data for WT mice shown in Fig. 2b. P values are as compared to WT mice treated with isotype control antibody. Data from 5 experiments. (b) TNFα, IL-6, IFNγ, IL-1β, KC, IL-10 and IL-12p70 levels in TLR2 KO, TLR4 KO or WT mouse plasma collected 2 h after the lethal challenge of ConA. Data for TNF and IL-6 from untreated WT mice are repeated from Fig. 2c. Data from 5 experiments.
Extracellular histones are major mediators of death through TLR2 and TLR4 in APAP induced tissue injury model
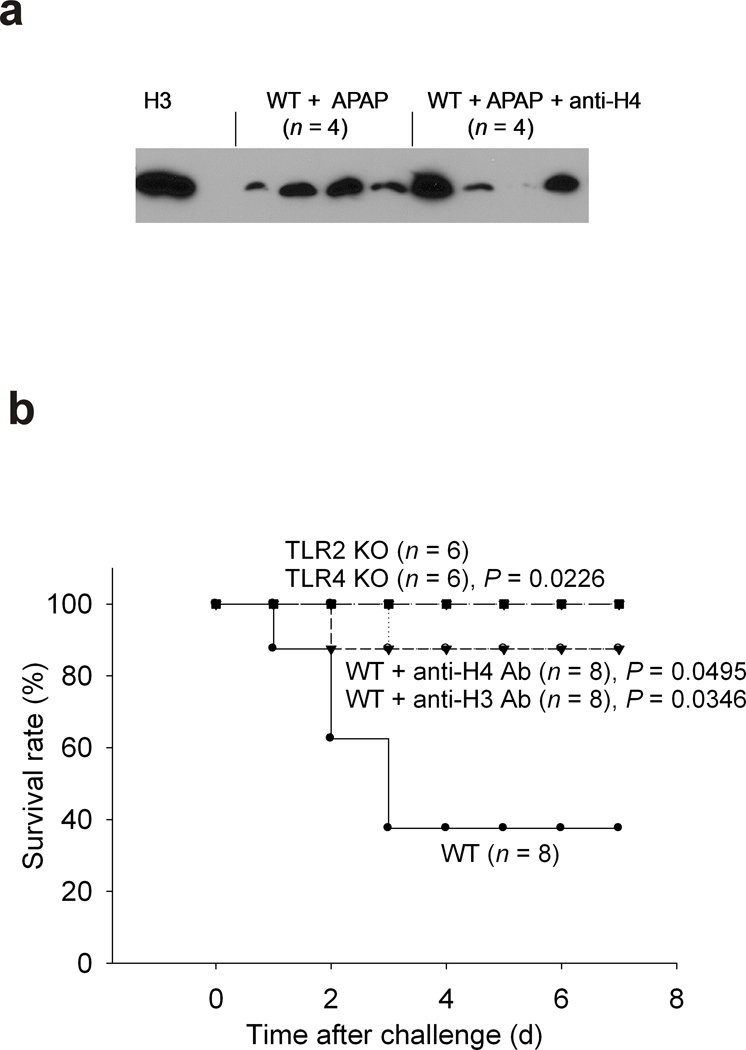
Having established an important role for histone mediated lethality which involves TLRs 2 and 4 in both pathogen and sterile inflammatory challenges, we wished to determine if cell injury in the absence of a massive inflammatory reaction also involved histones and TLR signaling. Acetaminophen (APAP) is known to induce liver injury and lethality in mice mimicking drug induced liver injury in patients with APAP overdose 11. HMGB1 and DNA released from dying hepatocytes as damage associated molecular pattern (DAMP) molecules were reported to be responsible for the activation of innate immunity and tissue damage following APAP hepatotoxicity in this mouse model 11,12. We hypothesized that extracellular histones as unrecognized endogenous DAMP molecules are also important mediators in APAP induced liver injury and lethality through TLR2 and/or TLR4 signaling. Although we found extracellular histone in the circulation after APAP challenge (Fig. 4a), we did not observe detectable TNFα and IL-1β in the circulation (data not shown). Antibodies to histone H3 or H4 protected the WT mice from APAP induced lethality and both TLR2 and TLR4 KO mice were also resistant to the APAP challenge (Fig. 4b). These results are consistent with the previous finding that TLR4 mutant mice were less susceptible to liver injury after a sublethal APAP challenge and the notions that endogenous DAMP molecules augment inflammation and/or tissue injury through TLRs and other signaling molecules 13,14.
Fig. 4. TLR2 KO or TLR4 KO mice are resistant to a lethal challenge of APAP.
(a) Western blot analysis for H3 in individual wild type mouse plasmas collected 6 h after a high dose of APAP (500 mg per kg body weight, ip) in the absence or presence of antibody to H4 (10 mg per kg body weight, iv). The very left lane is the H3 positive control. (b) Survival rates of wild type and TLR2 KO or TLR4 KO mice challenged with a high dose of APAP (500 mg per kg body weight, ip) and WT mice in the absence or presence of antibody to H3 or H4 (10 mg per kg body weight, iv). P value is compared to WT mice challenged with APAP. Data from 5 experiments.
Discussion
Superficially, there appears to be a conflict in the data presented in this report. We show that histones induce the production of circulating cytokines. We also show that APAP leads to the appearance of a significant concentration of histones in the circulation. However, we do not detect concomitant cytokines. This could be due to retention of the proteins in the liver without release into the bloodstream or intracellular destruction. Consistent with this observation, Imaeda, et al, 11 found that although the message for pro-IL1β, IL18, TNFα and IFNγ were increased by APAP 2 fold or more in a similar model, only a 5% increase in IL-1β over baseline was observed in the circulation.
The cytokine storm is a hallmark of the systemic host inflammatory response to infection as well as injury. LPS-induced TLR4 signaling is a well known pathway responsible for cytokine production in infectious diseases including sepsis 15. Endogenous TLR4 ligands such as heat shock proteins, fibrinogen, HMGB1 and tenascin-C have been reported but only HMGB1 and tenascin-C were able to induce proinflammatory cytokine synthesis in vitro and in vivo 15–17. We now show that extracellular histones activate TLR4 signaling in vitro and induce cytokine production in vivo primarily through TLR4 activation. Extracellular histones, DNA or both were found in the circulation of the ConA-induced sterile inflammatory mouse model in this study and in patients of stroke, trauma and autoimmune diseases 18. However, TLR2 KO mice were resistant to the lethal challenges of ConA, suggesting that histone mediated TLR2 signaling might be responsible for tissue dysfunction at late stages after the cytokine storm.
The ability of histones to activate TLR 2 and 4 is indicated by the reporter gene experiments in the transfected HEK 293 cells. In vivo, the TLR2 null mice were protected to the same extent from Tylenol toxicity as was achieved by blocking histone H4. The simplest interpretation of this result is that histones contribute to toxicity by activating TLR2 but we cannot exclude the possibility that other mediators might be released by the histones that activate TLR2 and contribute to death. Similar considerations apply to the interpretation of the TLR4 null mice and the impact of histone blockade in ConA mediated injury. Recent studies demonstrated TLR2 KO mice were more resistant to atherosclerosis, ischemic tissue injury, type I diabetes and mouse lupus 19–22, suggesting the presence of endogenous TLR2 agonists which might contribute to the pathogenesis of these diseases. Extracellular histones are good candidates for this endogenous ligand. The exact molecular mechanisms of histone mediated TLR2 signaling are under investigation.
A massive inflammatory response often follows septic tissue injury or trauma 23. Previously, the role of activated macrophages recruited to the site of injury has been considered a major culprit 24. Consistent with our observations on the effect of histones, especially when complexed with DNA, nucleosomes (our unpublished results) or DNA are readily detected in trauma patients and correlate with the severity of the disease 25,26. Our studies demonstrating that DNA-histone complexes, as seen in Fig. 2d, can activate TLR 2 and TLR4 leading to cytokine production and tissue injury also complement another recent study showing that mitochondrial formyl peptides and mitochondrial DNA can activate TLR9 and lead to activation of human polymorphonuclear neutrophils 27. Thus, cellular damage elicits at least two distinct mechanisms that trigger inflammation and can contribute to the association of inflammation with trauma.
Supplementary Material
Acknowledgements
We thank the OMRF Proteomic Research Facility for conducting mouse cytokine assay; P. Kincade for providing TLR KO mice and K. Deatherage for preparing the manuscript.
Footnotes
This work was supported by a Recovery Act challenge grant awarded by the National Institutes of Health (grant no. RC1 GM091739). C.T.E. is an investigator of the Howard Hughes Medical Institute.
Reference List
- 1.Opal SM, Esmon CT. Bench-to-bedside review: Functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Critical Care. 2003;7:23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgel D, Bornstain C, Reitsma PH, et al. A comparative study of the protein C pathway in septic and nonseptic patients with organ failure. American Journal of Respiratory and Critical Care Medicine. 2007;176:878–885. doi: 10.1164/rccm.200611-1692OC. [DOI] [PubMed] [Google Scholar]
- 3.Zeerleder S, Zwart B, Wuillemin WA, et al. Elevated nucleosome levels in systemic inflammation and sepsis. Critical Care Medicine. 2003;31:1947–1951. doi: 10.1097/01.CCM.0000074719.40109.95. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature Medicine. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 7.Zeerleder S, Zwart B, Velthuis HT, et al. A plasma nucleosome releasing factor (NRF) with serine protease activity is instrumental in removal of nucleosomes from secondary necrotic cells. Febs Letters. 2007;581:5382–5388. doi: 10.1016/j.febslet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Zhang XM, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nature Medicine. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiegs G, Hentschel J, Wendel A. A T-Cell-Dependent Experimental Liver-Injury in Mice Inducible by Concanavalin-A. Journal of Clinical Investigation. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Subudhi SK, Anders RA, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. Journal of Clinical Investigation. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. Journal of Clinical Investigation. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicology Letters. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yohe HC, O'Hara KA, Hunt JA, et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 14.Maher JJ. DAMPs ramp up drug toxicity. Journal of Clinical Investigation. 2009;119:246–249. doi: 10.1172/JCI38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujimoto H, Ono S, Efron PA, et al. Role of toll-like receptors in the development of sepsis. Shock. 2008;29:315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 16.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature Medicine. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 18.Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Critical Reviews in Clinical Laboratory Sciences. 2009;46:1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Han MS, Chung KW, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Lartigue A, Colliou N, Calbo S, et al. Critical Role of TLR2 and TLR4 in Autoantibody Production and Glomerulonephritis in lpr Mutation-Induced Mouse Lupus. Journal of Immunology. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 21.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. Journal of Clinical Investigation. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigeoka AA, Holscher TD, King AJ, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. Journal of Immunology. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 23.Hawksworth JS, Stojadinovic A, Gage FA, et al. Inflammatory Biomarkers in Combat Wound Healing. Annals of Surgery. 2009;250:1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 24.McCarter MD, Mack VE, Daly JM, Naama HA, Calvano SE. Trauma-induced alterations in macrophage function. Surgery. 1998;123:96–101. [PubMed] [Google Scholar]
- 25.Lam NYL, Rainer TH, Chan LYS, Joynt GM, Lo YMD. Time course of early and late changes in plasma DNA in trauma patients. Clinical Chemistry. 2003;49:1286–1291. doi: 10.1373/49.8.1286. [DOI] [PubMed] [Google Scholar]
- 26.Lo YMD, Rainer TH, Chan LYS, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clinical Chemistry. 2000;46:319–323. [PubMed] [Google Scholar]
- 27.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.