Abstract
Activation of CNS cannabinoid subtype-1 (CB1) receptors has been shown to mediate the antinociceptive and other effects of systemically administered CB receptor agonists. The endogenous peptide CB receptor ligand hemopressin (HE) has previously demonstrated an antinociceptive effect in rats with a hind paw inflammation, without exhibiting characteristic CB1 receptor-mediated side-effects. The current study evaluated the effect of intrathecal (i.t.) and intracerebroventricular (i.c.v.) injection of HE in a rat model of neuropathic spinal cord injury (SCI) pain. The non-subtype selective CB receptor agonist WIN 55,212-2 was also centrally administered in SCI rats as a comparator. Four weeks following an acute compression of the mid-thoracic spinal cord, rats displayed markedly decreased hind paw withdrawal thresholds, indicative of below-level neuropathic pain. Central administration of WIN 55,212-2 significantly increased withdrawal thresholds, whereas HE did not. Hemopressin has been reported to block CB1 receptors in vitro, similar to the CB1 receptor antagonist rimonabant. Pretreatment with rimonabant completely blocked the antinociceptive effect of centrally administered WIN 55,212-2, but pretreatment with HE did not. While the data confirm that activation of either supraspinal or spinal CB1 receptors leads to significant antinociception in SCI rats, the current data do not support an antinociceptive effect from an acute blockade of central CB1 receptors, HE’s putative antinociceptive mechanism, in neuropathic SCI rats. Although such a mechanism could be useful in other models of pain with a significant inflammatory component, the current data indicate that activation of CB1 receptors is needed to ameliorate neuropathic SCI pain.
Keywords: allodynia; hemopressin; neuropathic pain; SR 141716; WIN 55,212-2
1. Introduction
Neuropathic spinal cord injury (SCI) pain presents unique challenges to clinicians in that both spontaneous and evoked pain present at various levels relative to the injury, including below the level of the injury (Finnerup et al., 2007; Widerstrom-Noga et al., 2009). Drugs that are effective for other pain states do not appear to be as effective for neuropathic SCI pain (Cardenas et al., 2002). Furthermore, analgesic drugs that may be useful could be contraindicated for SCI patients. For example, opiates and tricyclic antidepressants may lead to visceral distention, which, in turn, could lead to an acute, life-threatening condition known as autonomic dysreflexia (Finnerup and Jensen, 2004; Rabchevsky, 2006).
Surveys of SCI patients suggest that Cannabis sativa confers significant pain relief (Cardenas and Jensen, 2006; Warms et al., 2002). Limited clinical findings support the medical use of Cannabis for other types of chronic pain, and other concurrent physiological effects, such as sedation, anti-emesis and appetite improvement, may enhance patient quality of life (Russo et al., 2007). A number of bioactive substances have been isolated from Cannabis sativa, including Δ9-tetrahydrocannabinol (THC), which potently bind to the CNS cannabinoid subtype-1 (CB1) receptor (Pertwee, 2008). In vitro autoradiography and in vivo positron emission tomography imaging studies with CB1 receptor radioligands have identified CB1 receptors in CNS regions (e.g. spinal cord, thalamus) which may be associated with the analgesic effects obtained with Cannabis (Burns et al., 2007; Herkenham et al., 1991; Howlett et al., 2004). However, CB1 receptors have also been identified in brain regions (e.g. limbic system, prefrontal cortex) associated with the mood-altering and cognitive-impairing effects of Cannabis which limit its widespread clinical use (Svendsen et al., 2004). Thus, there is a need for CB receptor ligands with not only robust analgesic efficacy but minimal adverse side-effects.
The nonapeptide hemopressin (HE), derived from the α-chain of hemoglobin, was identified in rat brain homogenates and initially characterized as having hypotensive effects following intravenous administration in rats (Rioli et al., 2003). Interestingly, HE potently binds to the rat brain CB1 receptor (Heimann et al., 2007). When combined with a CB receptor agonist in in vitro functional assays, HE inhibits CB1 receptor agonist-mediated effects, functioning as an antagonist. However, in the absence of an agonist in these assays, HE demonstrated the pharmacological property of an inverse agonist. The in vitro effects of HE are similar to those of rimonabant, a well-characterized CB1 receptor antagonist (Rinaldi-Carmona et al., 1996).
Surprisingly, given its in vitro profile, intrathecal (i.t.) HE has been shown to potently ameliorate inflammation-induced hind paw hypersensitivity to noxious stimulation, with the effect limited to the inflamed paw, and no effect reported following i.t. injection in uninjured rats (Heimann et al., 2007). Although the majority of studies have entirely focused on the antinociceptive effects of CB1 receptor agonists, a number of studies have demonstrated significant antinociceptive effects of CB1 receptor antagonists in chronic pain models (Costa et al., 2005; Croci and Zarini, 2007). The antinociceptive effect of rimonabant could be due to one of several mechanisms. It has been suggested that by blocking CB1 receptors, endocannabinoids synthesized following injury induce antinociception by non-CB1 receptor-mediated mechanisms, such as activation of CB2 receptors or desensitization of the transient receptor potential vanilloid type I (TRPV1) found on primary afferent nociceptors. (Costa, 2007). In this scenario, then, endocannabinoid binding to the CB1 receptor leads to nociception. Also, the production of pro-nociceptive cytokines, such as tumor necrosis factor-α (TNF-α) following tissue injury has been shown to be CB1 receptor-mediated, in that blockade leads to decreased tissue concentrations of cytokines (Costa et al., 2005; Croci et al., 2003). An additional, attractive feature of antinociception following CB1 receptor block, with either HE or rimonabant, is that adverse side-effects commonly observed with CB1 receptor agonists have not been observed. Thus, blocking of the CB1 receptor is a potentially novel antinociceptive strategy. It is unknown if i.t. HE is antinociceptive in the neuropathic state, specifically, following a SCI.
Because of a potential CB1 receptor-mediated antinociceptive effect and at the same time a possible in vivo antagonistic effect of HE, there were two main goals in the current study. The first goal was to determine whether injection of HE is antinociceptive in rats with below-level neuropathic SCI pain. As mentioned earlier, CB1 receptors are found in CNS nuclei, both spinal and supraspinal, that modulate nociception. A spinal site of action appears to be likely given that hind paw cutaneous hypersensitivity induced by inflammation was significantly ameliorated with HE treatment (Heimann et al., 2007). There is also increased cytokine expression in spinal cord caudal to a spinal injury, which could be CB1 receptor-mediated (Detloff et al., 2008; Peng et al., 2006). The effect of HE injected into the lateral ventricular space (intracerebroventricular; i.c.v.) on injury-induced cutaneous hypersensitivity has yet to be evaluated. Thus, HE was injected i.c.v. As a positive control, in a separate group of SCI rats, the non-subtype selective CB receptor agonist WIN 55,212-2 was tested, which has shown significant CB1 receptor-mediated antinociception following systemic administration in SCI rats (Hama and Sagen, 2007a).
The second goal was to determine if HE demonstrated properties of a CB1 receptor antagonist in vivo in the presence of a CB1 receptor agonist, as HE does in vitro. To this end, SCI rats were pretreated, either i.t. or i.c.v., with HE, followed by an i.t. or i.c.v. antinociceptive dose of WIN 55,212-2. As a positive control, SCI rats were treated with rimonabant prior to treatment with WIN 55,212-2.
2. Results
Prior to spinal compression surgery, hind paw withdrawal thresholds were 15 g. Four weeks after mid-thoracic spinal compression and prior to drug injections, the mean withdrawal threshold of all SCI rats decreased to 2.3 ± 0.1 g.
2.1 Effect of centrally administered CB receptor ligands in SCI rats
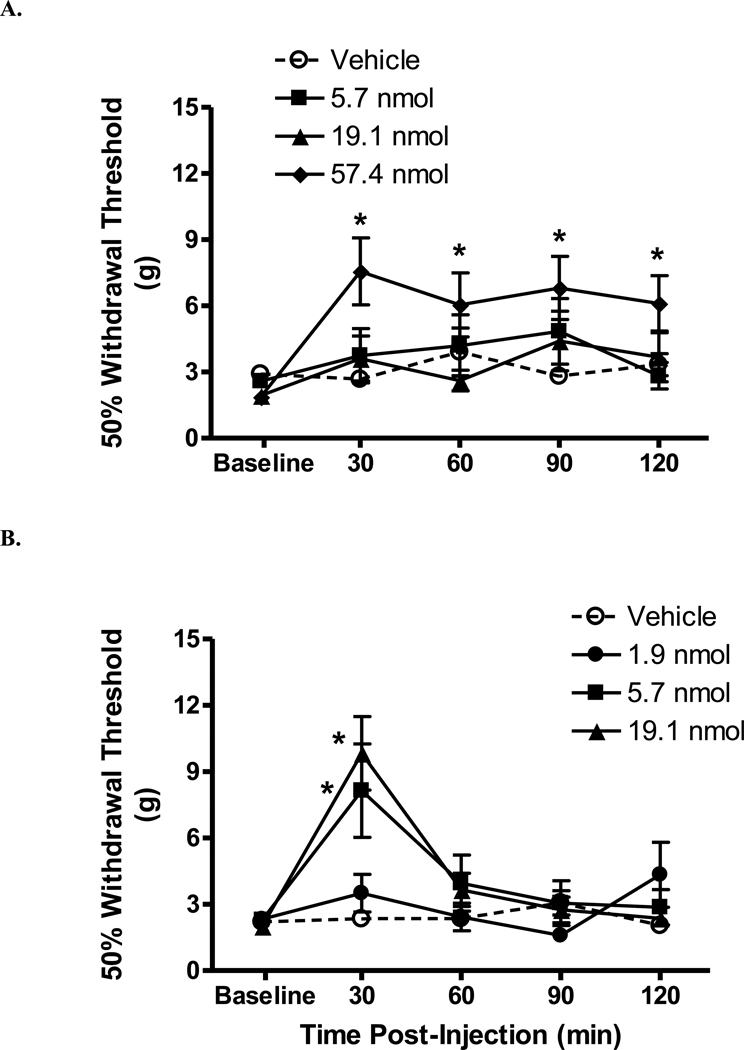
Central administration of the non-selective CB receptor agonist WIN 55,212-2 lead to robust antinociceptive effects. Following i.t. injection of 57.4 nmol WIN 55,212-2, withdrawal thresholds were significantly increased beginning 30 min post-i.t. injection, lasting at least 120 min post-injection (Fig. 1A; p < 0.05 vs. vehicle). At 30 min post-injection, the percent maximum possible effect (MPE) was 42.5 ± 12.0% (Table 1). No significant effects on withdrawal thresholds were observed with lower doses of WIN 55,212-2 or vehicle. Because the effect at the highest tested dose was under 50%, the 50% antinociceptive dose (A50) was not calculated. A dose-dependent antinociceptive effect was observed at 30 min following i.c.v. injection of WIN 55,212-2 (Fig 1B; p < 0.05 vs. vehicle). At 30 min, the A50 (95% confidence limits) was 5.0 (2.7–9.3) µg. No significant antinociceptive effect was observed at any other time post-injection. Vehicle injection did not significantly alter withdrawal thresholds.
Figure 1.
Antinociceptive effect of WIN 55,212-2 injected into rats with a spinal cord injury. The horizontal axis is time post-injection (min) and the vertical axis is withdrawal threshold (g). A. Intrathecal injection of the highest dose of WIN 55,212-2 lead to increased hind paw withdrawal thresholds. N = 6–8/group. B. Dose-dependent antinociceptive effect of i.c.v. injected WIN 55,212-2. Data are expressed as mean ± S.E.M. N = 7/group. *p < 0.05 vs. vehicle.
Table 1.
Effect of cannabinoid receptor ligands in rats with a SCI.
| WIN 55,212-2 | Rimonabant | Hemopressin | |
|---|---|---|---|
| Molecular Weight | 426.5 | 463.8 | 1088.3 |
| Intrathecal | |||
| Highest tested dose, nmol | 57.4 | 64.7 | 9.2 |
| Percent Maximum Possible Effect (S.E.M.) | 42.5 (12.0)* | 3.8 (2.0) | 7.1 (4.0) |
| Intracerebroventricular | |||
| Highest tested dose, nmol | 19.1 | NT | 2.8 |
| Percent Maximum Possible Effect (S.E.M.) | 60.6 (12.9)* | NT | 4(4) |
Molecular weight of the free base.
Rats tested 30 min injection.
NT, not tested
N = 7–8/treatment group.
P < 0.05 vs. vehicle
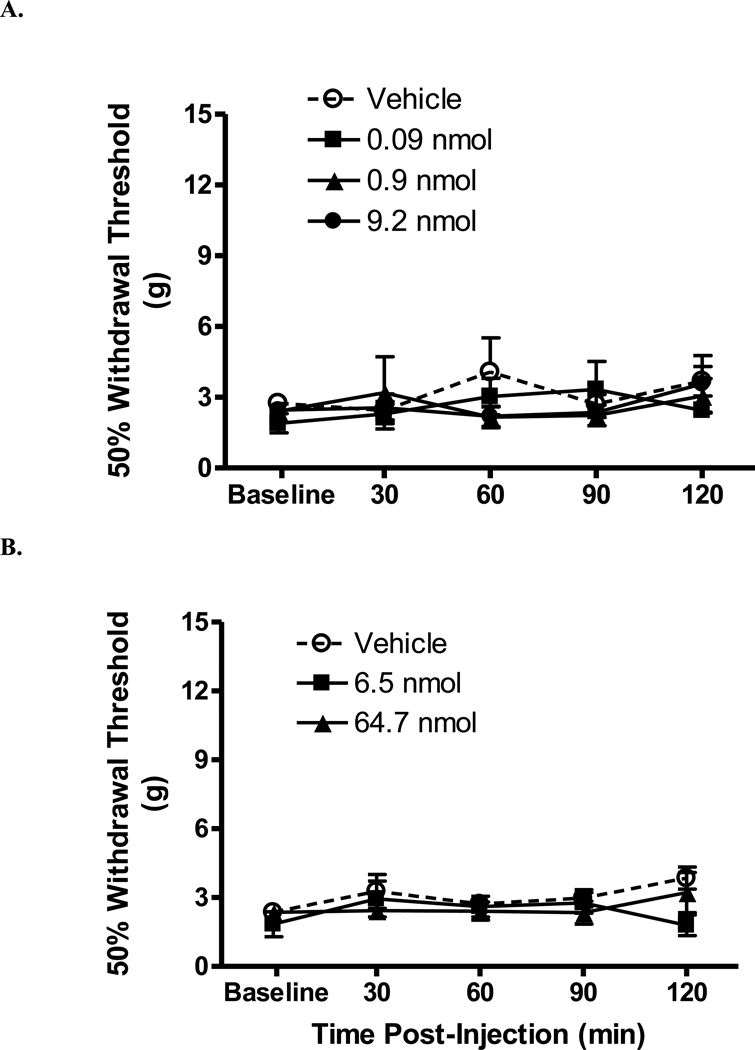
By contrast, i.t. HE (0.09, 0.9, 9.2 nmol) did not did not affect withdrawal thresholds in SCI rats (Fig. 2A, Table 1; p > 0.05 vs. vehicle). Likewise, i.c.v. injection of HE (0.09, 0.9, 2.8 nmol) did not affect withdrawal thresholds (data not shown). To determine if an acute spinal antagonism of CB1 receptors was antinociceptive in SCI rats, rats were injected with either vehicle, 6.47 nmol or 64.7 nmol rimonabant and tested beginning 30 min post-injection. No significant effect on threshold was observed (Fig. 2B, Table 1; p > 0.05 vs. vehicle). Neither i.t. nor i.c.v. vehicle injection altered withdrawal thresholds.
Figure 2.
Hemopressin and rimonabant intrathecally injected into rats with a spinal cord injury. The horizontal axis is time post-injection (min) and the vertical axis is withdrawal threshold (g). Intrathecally injected hemopressin (A), rimonabant (B) did not significantly alter hind paw withdrawal threshold. Vehicle did not alter withdrawal threshold. Data are expressed as mean ± S.E.M. N = 6/group.
2.2 Effect of pretreatments on the antinociceptive effect of WIN 55,212-2
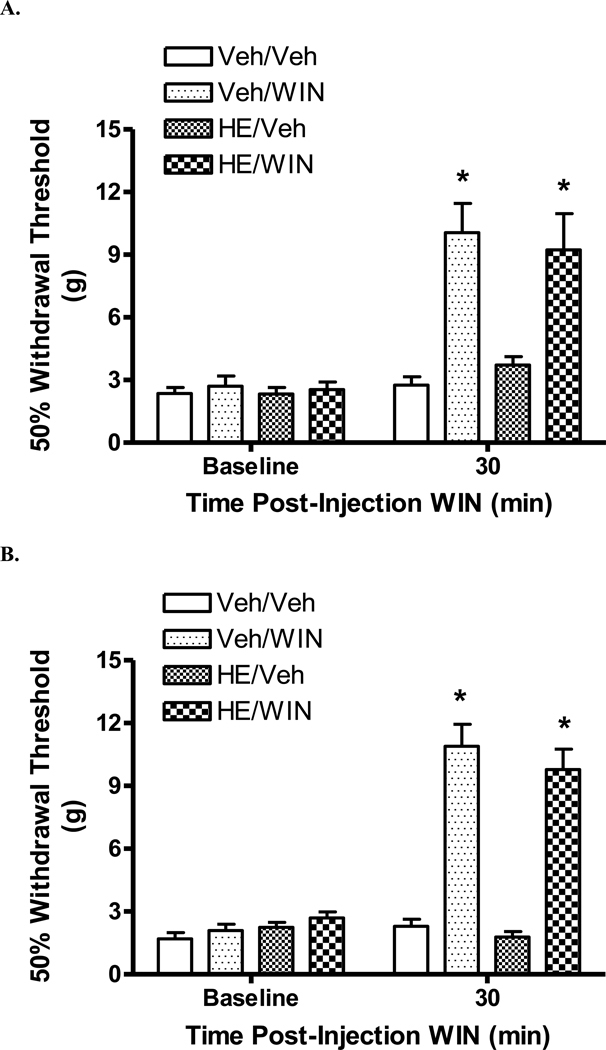
Hemopressin did not alter the antinociceptive effect of WIN 55,212-2, either as an i.t. pretreatment (Fig. 3A) or as an i.c.v. co-treatment (Fig. 3B), (p > 0.05, HE/WIN 55,212-2 vs. Vehicle/WIN 55,212-2).
Figure 3.
Effect of hemopressin pretreatment on the antinociceptive effect of WIN 55,212-2 in rats with a spinal cord injury. Baseline withdrawal thresholds were determined prior to i.t. (A) or i.c.v. (B) injection of either HE or vehicle (Veh). There are four groups (pretreatment/post-treatment): Veh/Veh; Veh/WIN; HE/Veh and HE/WIN. A. Thirty min following i.t. injection of 2.8 nmol HE or vehicle, rats were injected with either 57.4 nmol WIN 55,212-2 (WIN) or vehicle and tested 30 min thereafter. Intrathecal pretreatment with HE did not affect the antinociceptive effect of i.t. WIN 55,212-2. B. Rats were tested 30 min following i.c.v. co-injection of 2.8 nmol HE and 19.1 nmol WIN 55,212-2. Hemopressin co-treatment did not affect the antinociceptive effect of WIN 55,212-2. Data are expressed as mean ± S.E.M. N = 7/group. * p < 0.05 vs. Veh/Veh.
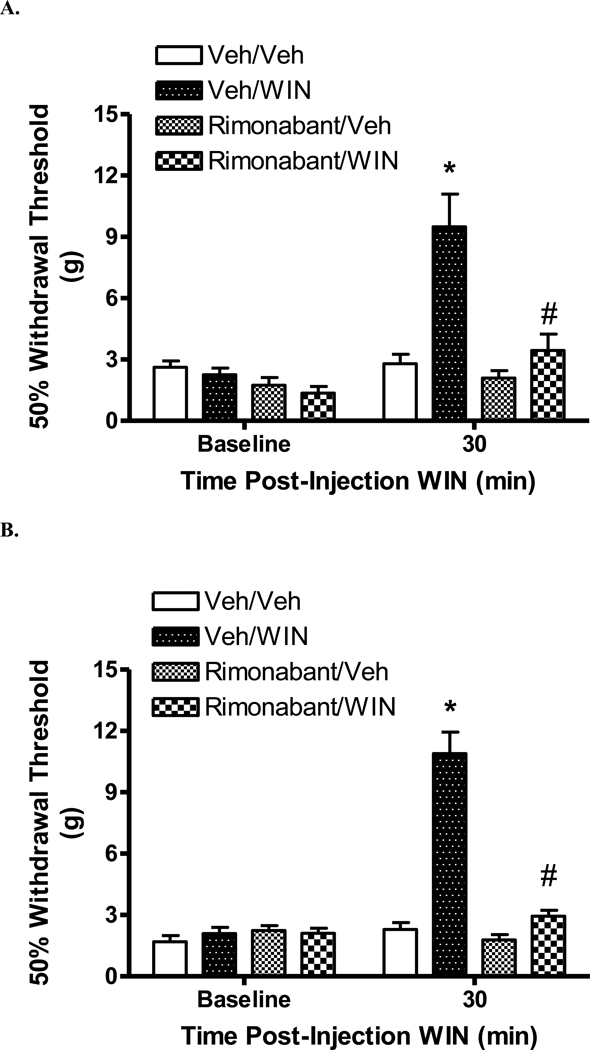
By contrast, i.t. pretreatment with rimonabant, but not vehicle, blocked the antinociceptive effect of i.t. WIN 55,212-2 (Fig. 4A; p < 0.05, Rimonabant/WIN 55,212-2 vs. Vehicle/WIN 55,212-2).
Figure 4.
Effect of rimonabant pretreatment on the antinociceptive effect of WIN 55,212-2 in rats with a spinal cord injury. Rats were pretreated with either rimonabant or vehicle (Veh). Thirty min later, rats were injected with either WIN 55,212-2 (WIN) or vehicle and tested 30 min thereafter. The four groups are (pretreatment/post-treatment): Veh/Veh; Veh/WIN; Rimonabant/Veh and Rimonabant/WIN. A. Intrathecal pretreatment with 64.7 nmol rimonabant blocked the antinociceptive effect of 57.4 nmol WIN 55,212-2. B. Subcutaneous pretreatment with 6.5 µmol/kg (s.c.) rimonabant blocked the antinociceptive effect of i.c.v 19.1 nmol WIN 55,212-2. Data are expressed as mean ± S.E.M. N = 6/group. * p < 0.05 vs. Veh/Veh, #p < 0.05 vs. Veh/WIN 55,212-2.
Because of a problem with solubility, rimonabant was injected systemically rather than as an i.c.v. co-treatment with WIN 55,212-2 (See Experimental Procedures). Pretreatment with s.c. rimonabant (6.47 µmol/kg), but not vehicle, blocked the antinociceptive effect of i.c.v. WIN 55,212-2 (Fig. 4B; p < 0.05, Rimonabant/WIN 55,212-2 vs. Vehicle/WIN 55,212-2). In the Rimonabant/Vehicle group, no significant alteration of withdrawal threshold from baseline threshold was noted following rimonabant treatment.
3. Discussion
There were two main findings of the current study. First, the nonapeptide CB1 receptor ligand HE demonstrated no antinociceptive effect on below-level neuropathic SCI pain in rats, whether injected i.t. or i.c.v. However, activation of either lumbar spinal dorsal horn or supraspinal CB receptors with the non-subtype selective CB receptor agonist WIN 55,212-2 significantly ameliorated neuropathic SCI pain. Hemopressin has been described as a CB1 receptor antagonist, so its potential as a CB1 receptor antagonist in vivo was tested. The second finding was that pretreatment with HE, either i.t. or i.c.v., did not block the antinociceptive effect of centrally administered WIN 55,212-2. By contrast, pretreatment with the CB1 receptor antagonist rimonabant completely blocked the onset of the antinociceptive effect of WIN 55,212-2. Hemopressin has previously demonstrated a prominent antinociceptive effect in a model of inflammatory pain. However, the underlying mechanism of HE’s efficacy in inflammatory pain could be markedly attenuated or lacking in neuropathic SCI pain. The current data extend a previous finding of a CB1 receptor-mediated antinociception following systemic administration of WIN 55,212-2, that activation of either spinal or supraspinal CB1 receptors leads to a significant amelioration of below-level neuropathic pain SCI rats.
The current study utilized a rat model of acute spinal compression which leads to a robust and chronic cutaneous hypersensitivity below the level of injury (Bruce et al., 2002; Hama and Sagen, 2007b). Abnormal spontaneous and evoked neural activity in spinal dorsal horn and supraspinal nuclei, such as the thalamus, have been reported in both patients and rats following SCI (Drew et al., 2001; Falci et al., 2002; Gerke et al., 2003; Gorecki et al., 1989; Hubscher and Johnson, 2006; Loeser et al., 1968; Pattany et al., 2002). The alterations in basal and evoked neural activity and their persistence long after injury are due to a number of injury-mediated changes, including glial activation, cytokine production and synaptic plasticity, which have also been reported in the CNS following peripheral nerve injury (Gwak and Hulsebosch, 2011; Millan, 1999). Striking changes to neural function can be found throughout the neuraxis, rostral and at-level as well as caudal to the injury (Carlton et al., 2009; Hubscher and Johnson, 2006; Hulsebosch et al., 2000; Kloos et al., 2005; Siddall et al., 1995; Xu et al., 1992). These changes in turn are behaviorally expressed as neuropathic pain symptoms such as spontaneous pain and cutaneous hypersensitivity.
The hind paw responses of SCI rats to innocuous mechanical stimulation are more than segmentally-mediated reflexes. Thalamic neurons exhibit significant hyper-responsiveness to stimulation of the dermatome below the level of the injury (Hains et al., 2005). Behaviors such as post-stimulation tending to the hind paw and hind paw licking reflect activity of supraspinal neural circuitry (Christensen et al., 1996; Detloff et al., 2008). The hind paw withdrawal response to stimulation with von Frey filaments in sciatic nerve-injured rats also involves a significant supraspinal component (Ossipov et al., 2000). In fact, the hind paw response to von Frey filament probing, but not noxious heating, is completely abolished after a spinal transaction (Bian et al., 1998). Both central and peripheral neuropathic pain patients report mechanical hypersensitivity within the dermatome of spontaneous pain, and in SCI patients, both evoked and spontaneous pains can be found within the same region below the level of the injury (Chaplan et al., 1994; Finnerup et al., 2007; Rowbotham and Fields, 1996). Thus, the presence of hind paw mechanical hypersensitivity could serve as a surrogate for below-level spontaneous pain in SCI rats.
A previous study demonstrated robust CB1 receptor-mediated antinociception following systemic dosing of WIN 55,212-2 in SCI rats (Hama and Sagen, 2007a). The current study expands on those findings, demonstrating that activation of either lumbar spinal or supraspinal CB1 receptors leads to significant antinociception. Interestingly, i.t. injection of WIN 55,212-2 was not as efficacious or potent as i.c.v injection. A similar finding of differential efficacy between spinal and supraspinal CB1 receptors was noted in a model of cutaneous hypersensitivity following hind paw tissue injury (Zhu et al., 2009). The diminished efficacy following i.t. compared to i.c.v. injection could be due to a functional change or deceased expression of CB1 receptors following a SCI. Four weeks after SCI, a 50 percent decrease in CB1 receptor mRNA at the level of injury has been observed, but expression at the lumbar spinal cord has yet to be reported (Garcia-Ovejero et al., 2009). A possible explanation underlying the greater potency of i.c.v. compared to i.t. WIN 55,212-2 in the current study is that CB receptors have been identified in numerous brain areas that modulate nociception (Horti et al., 2006; Howlett et al., 2004). Thus, high efficacy could be due to diffusion of WIN 55,212-2 from the lateral ventricle to these nuclei (Zhu et al., 2009). Since tactile mechanical hypersensitivity is significantly supraspinally mediated, a robust efficacy following i.c.v. injection should not be surprising (Ossipov et al., 2000). At the same time, however, many brain nuclei also mediate the side-effects typically obtained with CB1 receptor agonists, including catalepsy, hypothermia and motor dysfunction (Pertwee, 1997).
Although preclinical and limited clinical data suggest that CB receptor agonists are promising analgesics, there are serious limitations associated with this drug class that impedes widespread usage (Martin-Sanchez et al., 2009; Pertwee, 2005a). One possible method of minimizing supraspinally mediated side-effects while continuously delivering CB ligands is via the i.t. route (Pertwee, 2005a). In spinal dorsal horn, CB1 receptors are found mainly on dorsal horn neurons and possibly on central terminals of primary afferents (Agarwal et al., 2007; Farquhar-Smith et al., 2000; Ross et al., 2001). The antinociceptive effect of CB1 receptor agonists applied at the spinal level, then, arises from inhibition of spinal nociceptive neurons and the inhibition of excitatory neurotransmitter release from primary afferent terminals. Long-term clinical pain relief has been demonstrated via i.t. infusion pumps utilizing various analgesics but has yet to be demonstrated for CB receptor ligands, so the long-term safety and efficacy of CBs delivered by such a method is unknown (Bennett et al., 2000). The i.t. space could also be utilized for the implantation of genetically engineered cells, acting as bio-pumps to continuously exude naturally derived analgesic substances, including peptides such as HE (Eaton, 2006). Such a strategy could be useful for SCI patients in particular, for whom few analgesic therapies are effective (Warms et al., 2002).
Hemopressin binds to rat brain CB1 receptors with sub-nanomolar affinity (Heimann et al., 2007). In in vitro functional assays, HE blocks intracellular signaling induced by the non-selective CB receptor agonist HU-210 in a manner similar to that of rimonabant. Furthermore, in the absence of an agonist, HE demonstrates activity as a potent CB1 receptor inverse agonist. In light of its in vitro characteristics, most intriguing is HE’s potent antinociceptive effect (Dale et al., 2005; Heimann et al., 2007). In a rat model of unilateral hind paw inflammation, i.t. HE (at doses of 0.09 and 0.92 nmol per rat) significantly attenuated hind paw hypersensitivity and did not affect withdrawal responses of the contralateral uninflamed hind paw, an effect that mirrors the effect of 19.1 nmol WIN 55,212-2 (Martin et al., 1999). In non-inflamed rats, HE did not alter withdrawal thresholds, indicating that the in vivo effect of HE is limited to the hypersensitive state. Importantly, no side-effects associated with CB receptor activation were reported. Given the favorable in vivo effects of HE, the antinociceptive potential of HE was evaluated in below-level cutaneous hypersensitivity in SCI rats.
Several mechanisms have been proposed to underlie the antinociceptive effect of CB1 receptor block, with either HE, rimonabant or rimonabant-like compounds, in previous studies. Increases in spinal concentrations of endocannabinoids have been reported following peripheral tissue injury (Guasti et al., 2009; Petrosino et al., 2007). Endocannabinoid activation of CB1 receptors on spinal dorsal horn inhibitory interneurons leads to inhibition of these neurons, which in turn leads to disinhibition of spinal nociceptive neurons and increased activity of these neurons (Pernia-Andrade et al., 2009). Thus, blocking CB1 receptors with either rimonabant or HE prevents endocannabinoids from shutting down segmental spinal inhibition. Alternatively, blocking CB1 receptors could “induce” endocannabinoids to activate CB2 receptors or desensitize TRPV1 receptors, leading to antinociception (Costa, 2007). Another proposed mechanism is the inhibition of inflammatory processes that are CB1 receptor-sensitive such as cytokine production. Pro-inflammatory and pro-nociceptive cytokines, such as TNF-α and interleukin-6, have been shown to be up-regulated in the spinal cord following SCI as well as peripheral tissue injury (Detloff et al., 2008; Peng et al., 2006; Raghavendra et al., 2003; Raghavendra et al., 2004). Rimonabant treatment decreased spinal cord concentrations of TNF-α (Costa et al., 2005). A similar effect of HE on tissue TNF-α has yet to be documented.
It is also possible that the antinociceptive effects of CB1 receptor antagonism was mediated through non-CB receptor mechanisms (Pertwee, 2005b; Pertwee, 2010). Rimonabant activity has been demonstrated at a number of pain-related G protein-coupled receptors and ion channels. No significant activity at CB2 and opioid receptors has been reported for HE—activity at ion channels has not been reported (Heimann et al., 2007). Although a non-CB receptor mechanism is possible, the absence of rimonabant’s antinociceptive effect in neuropathic CB1 receptor knockout mice, however, strengthens the contention rimonabant’s effect is CB1 receptor dependent (Costa et al., 2005).
In the current study, there was an absence of an antinociceptive effect following i.t. injection of 9.2 nmol HE in SCI rats, even though this dose was one hundred-fold higher than the dose that demonstrated a significant amelioration of inflammation-induced hind paw hypersensitivity (Heimann et al., 2007). Also, i.t. rimonabant (64.7 nmol) did not demonstrate an antinociceptive effect in SCI rats. Other studies (Fox et al., 2001; Zhu et al., 2009) did not reveal an antinociceptive effect of i.t. rimonabant, though those pain models differed from the one used by Costa et al. (2005). The lack of antinociceptive efficacy of HE and rimonabant in the current study suggests that mechanisms that exist in the inflamed or nerve-injured state are either absent or greatly attenuated in the SCI state. For example, in those models in which no effect of rimonabant was observed, it is possible that there was no significant injury-induced increase in pro-nociceptive endocannabinoids in the spinal cord. While an increase in endocannabinoid has been observed at the level of SCI, the level of endocannabinoids caudal to the SCI—in lumbar spinal cord—has not been reported (Garcia-Ovejero et al., 2009). Although spinal tissue concentrations of cytokines increase following SCI, these increases could be insensitive to CB receptor modulation. Evaluation of tissue cytokine levels following CB1 receptor modulation will be needed to confirm this.
Since greater potency and efficacy of WIN 55,212-2 was obtained following i.c.v. injection and that cytokines were previously found to be elevated in brain following SCI, it was anticipated that i.c.v. injection of HE would lead to antinociception (Brewer and Nolan, 2007; Zhao et al., 2007). In the current study, the highest tested dose of HE (2.8 nmol) did not increase hind paw withdrawal thresholds. Although rimonabant was not i.c.v. injected in SCI rats, antinociceptive effects have not been demonstrated following i.c.v. injection in other pain models, including 150 nmol in rats with hind paw tissue injury and 647 nmol in uninjured rats (Lichtman and Martin, 1997; Zhu et al., 2009). The effects of HE following supraspinal injection in inflammatory and other injury models of pain have yet to be thoroughly characterized.
Given the lack of an antinociceptive effect alone and HE’s in vitro effect as an antagonist, HE was tested in vivo as a CB1 receptor antagonist. Antagonism of WIN 55,212-2 was not observed with pretreatments of HE, whether injected i.t. or i.c.v. It is possible that the doses of HE used were insufficient to block all CB1 receptors. However, in vivo effects were observed at higher doses of HE in other pain models (manuscript in preparation), which limited the maximum usable doses for the current study. (For example, high doses of HE lead to increased pain-related behavior, whereas low doses were antinociceptive, in the formalin test.) Nonetheless, the doses of HE tested in SCI rats were much greater than that used by Heimann et al. (2007) to induce a CB1 receptor-mediated response and the in vitro data indicated that HE is a highly potent CB receptor ligand.
Perhaps the lack of an acute antinociceptive effect in SCI rats of CB1 receptor ligands like HE and rimonabant should not be surprising. In a previous study, a systemic pretreatment with CB1 receptor antagonist AM251, a rimonabant analogue, at a dose (5.4 µmol/kg) which was sufficient to block the antinociceptive effect of WIN 55,212-2, was not antinociceptive in SCI rats (Hama and Sagen, 2007a). Likewise, in the current study, pretreatment with rimonabant (6.5 µmol/kg), which was sufficient to block the antinociceptive effect of i.c.v. WIN 55,212-2, did not significantly alter withdrawal thresholds. Other in vivo studies reported no significant alterations in cutaneous hypersensitivity following acute CB1 receptor antagonist treatment at doses which were sufficient to fully suppress the effect of a CB receptor agonist and block CB1 receptors to prevent the pro-nociceptive effect of endocannabinoids described earlier (Bridges et al., 2001; Choong et al., 2007; Hohmann et al., 1999; Scott et al., 2004; Zhu et al., 2009). The current data indirectly indicate that activation of CB1 receptors by endocannabinoids produced by SCI is not a crucial mechanisms underlying below-level cutaneous hypersensitivity. Also, it does not appear that cytokine-mediated below-level hypersensitivity is sensitive to CB1 receptor blockade. However, these processes could be important in the mechanism of at-level or above-level neuropathic SCI pain—the effects of CB receptor antagonists on these pains have yet to be determined.
Common features of studies in which significant antinociception was observed following CB1 receptor block include the use of a peripherally mediated inflammation and noxious stimulation. Croci and Zarini (2007) and Heimann et al. (2007) injected inflammogens into the hind paw. Costa et al. (2005) induced a unilateral chronic constriction injury, which leads to a marked perineural inflammation of the sciatic nerve. Hind paw injection of capsaicin in mice induces a neurogenic inflammation and exaggerated responsiveness to peripheral stimulation of dorsal horn neurons (Lin et al., 1999; Pernia-Andrade et al., 2009; Saade et al., 2002). Activity of dorsal horn neurons evoked by noxious stimulation was significantly attenuated following AM251 treatment (Pernia-Andrade et al., 2009). It is possible that stimulation of SCI rats with a noxious stimulus, rather than with innocuous von Frey filaments used in the current study, could have uncovered an antinociceptive effect of CB1 receptor block. However, Pernia-Andrade et al. (2009) also demonstrated that AM251 reduced dorsal horn neuron responses to innocuous stimuli. The effect of either rimonabant or HE in other chronic pain models to innocuous stimuli is not known.
Another factor that could influence the expression of an antinociceptive effect of CB1 receptor antagonism is repeated dosing (Costa et al., 2005; Croci and Zarini, 2007). Costa et al. (2005) dosed neuropathic rats with either vehicle or rimonabant for seven days; antinociception at the highest tested dose was maintained for at least 28 days after the last dose of rimonabant. Croci and Zarini (2007) used a similar treatment protocol. Repeated, rather than acute, CB1 receptor block could be required for amelioration of hind paw mechanical and thermal hypersensitivity. However, Heimann et al. (2007) and Pernia-Andrade et al. (2009) demonstrated antinociception after a single i.t. treatment. If the spinal cord dorsal horn is a key site in mediating the effect of CB1 receptor antagonists, perhaps a single, direct injection is sufficient to evoke antinociception in particular models. It is possible that repeated dosing or chronic exposure to HE may lead to antinociception in SCI rats. One way to accomplish long-term spinal dosing is to engineer cells to express HE and implant them into the lumbar subarachnoid space (Hentall and Sagen, 2000).
In fact, such a delivery system may be ideal given that the ultimate objective is a long-term treatment strategy for neuropathic SCI pain. Moreover, if expression is accomplished in cells that secrete antinociceptive neurotransmitters, such as GABA or catecholamines, HE could further increase the antinociceptive effect of these transmitters, through the mechanism of synergy, even though HE alone has no antinociceptive effect (Eaton, 2006; Tallarida, 2007). Hemopressin could enhance the efficacy of known analgesics that have previously demonstrated efficacy in SCI rats (Hama and Sagen, 2010; Mao et al., 2011).
The lack of an acute antinociceptive effect of HE in the neuropathic SCI pain state but efficacy in the inflamed state indicates that the putative mechanism of action of HE is either attenuated or not critical to the maintenance of neuropathic SCI pain. It appears that the functionality of central CB1 receptors depends on the pain state, the details of which will need further elaboration. A divergence in receptor function suggests that treatment required for one pain state could be counter to what is needed for the other pain state, which raises the prospect of novel therapeutic interventions but at the same time the need to treat pain states individually rather than collectively (Yun et al., 2005).
4. Experimental Procedures
Procedures were reviewed and approved by the University of Miami Animal Care and Use Committee and followed recommendations of the National Research Council’s Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (100–150 g at the time of SCI surgery; Harlan, IN) were used for these experiments. Rats were acclimated to the animal facility for 5–7 days prior to surgery and housed two per cage. However, following cannulation surgeries rats were singly housed. Rats were allowed free access to food and water before and after surgical procedures. At the end of the studies, rats were euthanized with CO2.
4.1 Surgical procedures
Rats were anesthetized and maintained on isoflurane in O2 for the duration of the surgical procedures. Aseptic surgical techniques were used.
4.12 Spinal cord injury
The procedure to induce a mid-thoracic SCI has been described elsewhere (Hama and Sagen, 2007b). Briefly, a laminectomy was performed to expose spinal cord segment T6–T7. A micro-vascular clip (Harvard Apparatus, Holliston, MA) was placed vertically on the exposed thoracic spinal cord and then left in place for 60 sec. Following removal of the vascular clip, the muscles were sutured shut and the skin closed with wound clips. Three weeks following compression SCI surgery, rats underwent i.t. catheter or i.c.v. surgery; rats were tested four weeks following SCI surgery.
4.13 Intrathecal catheters
The method of implanting an i.t. catheter in rats has been described elsewhere (Yaksh and Rudy, 1976). Briefly, rats were anesthetized and the head secured in a stereotaxic unit. The atlanto-occipital membrane was exposed and cut and an i.t. catheter (ReCathCo, Allison Park, PA), internalized length about 8.5 cm, was threaded down the intrathecal space and the tip terminated at the lumbar enlargement (below the level of the SCI). The catheter was secured to the neck musculature with sutures and the skin incision was closed with cyanoacrylate. After flushing the catheter with 10 µl saline, the externalized catheter was melted shut.
At the end of testing, prior to euthanasia, 5 µl of 1.5% lidocaine was i.t. injected to confirm that the catheter terminated at the lumbar enlargement. An acute bilateral flaccid paralysis of the hind limbs indicated that the catheter tip was in the correct spinal location.
4.14 Intracerebroventricular surgery
Implantation of i.c.v. cannulae into the right ventricular space and the stereotaxic coordinates (Anterior-Posterior: −0.7 mm from bregma; Medial-Lateral: −1.5 mm from bregma; Dorsal-Ventral: −3.5 from the top of the skull) were adopted from a method described elsewhere (Taylor et al., 1994). The guide cannula was secured in place with screws and dental cement. (Cannula parts obtained from Plastics One, Inc., Roanoke, VA. Dental cement obtained from Stoelting, Wood Dale, IL.) Five µl of either drug or vehicle was injected into the ventricular space with an injection cannula that extended 1 mm below the guide cannula.
At the end of the experiment, prior to euthanasia, proper placement of the guide cannula was confirmed by visualization of 5 µl methylene blue injected into the right ventricular space.
4.2 Testing procedures
4.21 Mechanical sensitivity
Hind paw sensitivity to innocuous mechanical stimulation was evaluated with von Frey filaments. Rats were placed on an elevated wire mesh surface and enclosed in a Plexiglas chamber. Using the up-down method, filaments were pressed on the plantar hind paw until they slightly bent (Chaplan et al., 1994). The pattern of responses to the filaments determined the 50% withdrawal threshold (in grams, g). In uninjured rats, the highest force filament, 15 g, did not evoke a response. To be included in the study, rats needed a withdrawal threshold of 4 g or less. Thresholds were measured prior to i.t. or i.c.v. injection and once every 30 min up to 2 hrs post-injection.
Four weeks after mid-thoracic SCI, decreased bilateral hind limb function was observed (Bruce et al., 2002; Hama and Sagen, 2007b). Despite decreased hind limb function, rats were able to respond to plantar hind paw probing with von Frey filaments.
4.22 Antagonism of WIN 55,212-2 in SCI rats
To antagonize the antinociceptive effect of i.t. WIN 55,212-2, either 64.7 nmol rimonabant or 2.8 nmol HE (or vehicle) was i.t. injected 30 min prior to i.t. injection of 57.4 nmol WIN 55,212-2 (or vehicle). Rats were tested 30 min following the second i.t. injection.
The doses of HE chosen for the current study were based in part on studies by Heimann et al. (2007) and from in-house data. Heimann et al. found that i.t. doses of 0.09 nmol and 0.92 nmol were antinociceptive. In the formalin test, we found that an i.t. dose of 2.8 nmol was antinociceptive (manuscript in preparation). Since this dose of HE demonstrated in vivo activity, we used this dose as the “antagonist dose”.
Because of solubility issues, for the i.c.v.-antagonism study, rimonabant and WIN 55,212-2 were injected separately. Three mg/kg (6.47 µmol/kg) rimonabant (or vehicle) was injected s.c. in a volume of 2 ml/kg 30 min prior to i.c.v. injection of 19.1 nmol WIN 55,212-2 (or vehicle). Rats were tested 30 min after i.c.v. injection. Hemopressin (2.8 nmol), however, was freely soluble, so it was co-injected i.c.v. with 19.1 nmol WIN 55,212-2 in a volume of 5 µl (or an equal volume of vehicle) and rats were tested 30 min after i.c.v. injection.
The same “antagonist dose” of HE was chosen for i.c.v. injection. Heimann et al. (2007) did not test i.c.v. injection of HE. In contrast to the antinociceptive effect of i.t. HE in the formalin test, i.c.v. injection of 2.8 nmol HE increased formalin-evoked behaviors (i.e. hyperalgesia; manuscript in preparation).
To summarize, there were four treatment groups in the antagonist arm of the study (pretreatment/post-treatment): i) Vehicle/Vehicle, ii) Vehicle/WIN 55,212-2, iii) antagonist/Vehicle and iv) antagonist/WIN 55,212-2.
4.3 Drugs
Intracerebroventricular and i.t. injections of drugs were done in a volume of 5 µl. A 5 µl vehicle flush followed i.t. drug injection. WIN 55,212-2 mesylate was obtained from Sigma-Aldrich, Inc. (St. Louis, MO) and was dissolved in a vehicle of 45% 2-hydroxypropyl-β-cyclodextrin in saline. Rimonabant was obtained from Cayman Chemical Co. (Ann Arbor, MI) and dissolved in a vehicle of 10% DMSO: 10% Tween-80: 80% saline. Hemopressin (PVNFKFLSH) was obtained from 21st Century Biochemicals (Marlboro, MA) and dissolved in saline.
The highest tested i.t. dose of WIN 55,212-2 was 57.4 nmol and no noticeable adverse side-effects were observed following injection. A higher i.t. dose (191.4 nmol), however, lead to hind limb flaccid paralysis. In addition, it has been reported elsewhere that hind paw withdrawal thresholds are elevated beyond normal levels, suggesting a possible anesthetic rather than antinociceptive effect of a high dose of WIN 55,212-2 (Martin et al., 1999). Therefore, the maximum tested dose of i.t. WIN 55,212-2 in these studies was 57.4 nmol. Because of solubility issues in formulating a mixture of rimonabant and WIN 55,212 for i.c.v. injection, these drugs were injected separately via different routes. The doses of rimonabant (i.t., 64.7 nmol; s.c., 6.47 µmol/kg) were chosen from studies that reported no observable side-effects and demonstrated in vivo antagonism of CB receptor agonists (Martin et al., 1999; Rinaldi-Carmona et al., 1996; Welch et al., 1998).
4.4 Statistical analysis
The drug effect on withdrawal threshold was converted to a percent maximum possible effect (MPE):
MPE % = (Drug threshold – Baseline threshold) ÷ (15 g – Baseline threshold)*100.
The A50 (50% antinociceptive dose) and 95% confidence limits of the drugs were calculated from the linear portions of the log dose-response curves using a web-based program (Tallarida and Murray, 1981). The program can be found on the Web at: http://www.u.arizona.edu/~michaelo/
Comparisons between treatment groups over time were performed using a repeated-measure two-way ANOVA with Student-Newman-Keuls test for post hoc comparisons. Statistical analysis of antagonist pretreatment on the antinociceptive effect of WIN 55,212-2 was performed using a two-way ANOVA. Statistical significance was taken at p < 0.05. Data are expressed as mean ± S.E.M.
Highlights.
WIN 55,212-2 is antinociceptive in spinal cord injured rats.
Brain and spinal cannabinoid receptors mediate the antinociceptive effect of WIN 55,212-2.
The CB receptor peptide hemopressin is not antinociceptive in spinal cord injured rats.
Acknowledgements
Supported by NIH grant NS61172. We thank Ms. Ann W. Plum and Mr. Paul Shekane for excellent technical support and Dr. Shyam Gajavelli for helpful discussions.
Abbreviations
- A50
50% antinociceptive dose
- CB
cannabinoid
- HE
hemopressin
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- MPE
maximum possible effect
- SCI
spinal cord injury
- s.c.
subcutaneous
- THC
Δ9-tetrahydrocannabinol
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors disclose no conflict of interests.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G, Serafini M, Burchiel K, Buchser E, Classen A, Deer T, Du Pen S, Ferrante FM, Hassenbusch SJ, Lou L, Maeyaert J, Penn R, Portenoy RK, Rauck R, Willis KD, Yaksh T. Evidence-based review of the literature on intrathecal delivery of pain medication. J Pain Symptom Manage. 2000;20:S12–S36. doi: 10.1016/s0885-3924(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Bian D, Ossipov MH, Zhong C, Malan TP, Jr, Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury. Neurosci Lett. 1998;241:79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Nolan TA. Spinal and supraspinal changes in tumor necrosis factor-alpha expression following excitotoxic spinal cord injury. J Mol Neurosci. 2007;31:13–21. doi: 10.1007/BF02686114. [DOI] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Exp Neurol. 2002;178:33–48. doi: 10.1006/exnr.2002.8026. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: A survey study. J Spinal Cord Med. 2006;29:109–117. doi: 10.1080/10790268.2006.11753864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choong KC, Su X, Urban MO. Effect of CP55,940 on mechanosensory spinal neurons following chronic inflammation. Neurosci Lett. 2007;414:105–109. doi: 10.1016/j.neulet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Colleoni M, Giagnoni G, Zarini E, Croci T. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain. 2005;116:52–61. doi: 10.1016/j.pain.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Costa B. Rimonabant: more than an anti-obesity drug? Br J Pharmacol. 2007;150:535–537. doi: 10.1038/sj.bjp.0707139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci T, Zarini E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br J Pharmacol. 2007;150:559–566. doi: 10.1038/sj.bjp.0707138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CS, Pagano Rde L, Rioli V, Hyslop S, Giorgi R, Ferro ES. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides. 2005;26:431–436. doi: 10.1016/j.peptides.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Siddall PJ, Duggan AW. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 2001;893:59–69. doi: 10.1016/s0006-8993(00)03288-1. [DOI] [PubMed] [Google Scholar]
- Eaton MJ. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma. 2006;23:549–559. doi: 10.1089/neu.2006.23.549. [DOI] [PubMed] [Google Scholar]
- Falci S, Best L, Bayles R, Lammertse D, Starnes C. Dorsal root entry zone microcoagulation for spinal cord injury-related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J Neurosurg. 2002;97:193–200. doi: 10.3171/spi.2002.97.2.0193. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Jensen TS. Spinal cord injury pain--mechanisms and treatment. Eur J Neurol. 2004;11:73–82. doi: 10.1046/j.1351-5101.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sorensen L, Biering-Sorensen F, Johannesen IL, Jensen TS. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp Neurol. 2007;207:139–149. doi: 10.1016/j.expneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C, Di Marzo V, Molina-Holgado E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis. 2009;33:57–71. doi: 10.1016/j.nbd.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–722. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- Gorecki J, Hirayama T, Dostrovsky JO, Tasker RR, Lenz FA. Thalamic stimulation and recording in patients with deafferentation and central pain. Stereotact Funct Neurosurg. 1989;52:219–226. doi: 10.1159/000099504. [DOI] [PubMed] [Google Scholar]
- Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, Alexander SP, Kendall D, Michael GJ, Chapman V. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol Pain. 2009;5:35. doi: 10.1186/1744-8069-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr Pain Headache Rep. 2011;15:215–222. doi: 10.1007/s11916-011-0186-2. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128:2359–2371. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp Neurol. 2007a;204:454–457. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007b;1185:117–128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hama AT, Sagen J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology. 2010;58:758–766. doi: 10.1016/j.neuropharm.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Sagen J. The alleviation of pain by cell transplantation. Prog Brain Res. 2000;127:535–550. doi: 10.1016/s0079-6123(00)27027-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J Neurophysiol. 1999;81:575–583. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, Alexander M, Kumar A, Rahmim A, Scheffel U, Wong DF, Dannals RF. 11CJHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47:1689–1696. [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47 Suppl 1:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol. 2006;197:177–188. doi: 10.1016/j.expneurol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol Biochem Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Ward AA, Jr, White LE., Jr Chronic deafferentation of human spinal cord neurons. J Neurosurg. 1968;29:48–50. doi: 10.3171/jns.1968.29.1.0048. [DOI] [PubMed] [Google Scholar]
- Mao J, Gold MS, Backonja MM. Combination drug therapy for chronic pain: a call for more clinical studies. J Pain. 2011;12:157–166. doi: 10.1016/j.jpain.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353–1368. doi: 10.1111/j.1526-4637.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Loo CM, Basbaum AI. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–764. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005a;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005b;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog Brain Res. 2006;152:265–274. doi: 10.1016/S0079-6123(05)52017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, Breliere JC, Soubrie P, Le Fur G. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996;58:1239–1247. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- Rioli V, Gozzo FC, Heimann AS, Linardi A, Krieger JE, Shida CS, Almeida PC, Hyslop S, Eberlin MN, Ferro ES. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. J Biol Chem. 2003;278:8547–8555. doi: 10.1074/jbc.M212030200. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Fields HL. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain. 1996;119(Pt 2):347–354. doi: 10.1093/brain/119.2.347. [DOI] [PubMed] [Google Scholar]
- Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers. 2007;4:1729–1743. doi: 10.1002/cbdv.200790150. [DOI] [PubMed] [Google Scholar]
- Saade NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol. 2002;545:241–253. doi: 10.1113/jphysiol.2002.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport. 1995;6:1241–1244. doi: 10.1097/00001756-199506090-00003. [DOI] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. Bmj. 2004;329:253. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacological Calculations with Computer Programs. New York, NY: Springer-Verlag; 1981. Vol. [Google Scholar]
- Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacol Ther. 2007;113:197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Holloway D, Printz MP. A unique central cholinergic deficit in the spontaneously hypertensive rat: physostigmine reveals a bradycardia associated with sensory stimulation. J Pharmacol Exp Ther. 1994;268:1081–1090. [PubMed] [Google Scholar]
- Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Welch SP, Huffman JW, Lowe J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J Pharmacol Exp Ther. 1998;286:1301–1308. [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Finnerup NB, Siddall PJ. Biopsychosocial perspective on a mechanisms-based approach to assessment and treatment of pain following spinal cord injury. J Rehabil Res Dev. 2009;46:1–12. [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Lee PY, Bazar KA. Paradoxical strategy for treating chronic diseases where the therapeutic effect is derived from compensatory response rather than drug effect. Med Hypotheses. 2005;64:1050–1059. doi: 10.1016/j.mehy.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Mikusa JP, Fan Y, Hollingsworth PR, Pai M, Chandran P, Daza AV, Yao BB, Dart MJ, Meyer MD, Decker MW, Hsieh GC, Honore P. Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain. Br J Pharmacol. 2009;157:645–655. doi: 10.1111/j.1476-5381.2009.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]