Abstract
Previous studies have shown the superiority of nanofibrous (NF) poly(L-lactic acid) (PLLA) scaffolds in supporting the osteogenic differentiation of a few cell types and bone regeneration. The aim of the current study was to investigate whether NF-PLLA scaffolds are advantageous for the odontogenic differentiation and mineralization of human dental pulp stem cells (DPSCs) over solid-walled (SW) PLLA scaffolds. The vitro studies demonstrated that, compared with SW scaffolds, NF scaffolds enhanced attachment and proliferation as well as odontogenic differentiation of human DPSCs. The alkaline phosphatase (ALP) activity and the expression of odontogenic genes of human DPSCs was increased on NF scaffolds compared with that on SW scaffolds. In addition, more mineral deposition was observed on the NF scaffolds as demonstrated by von Kossa staining, calcium content measurement and scanning electron microscopy. Consistent with the in vitro studies, NF scaffolds promoted odontogenic differentiation and hard tissue formation compared with SW scaffolds after 8 weeks of ectopic transplantation in nude mice as confirmed by von Kossa staining, Masson’s trichrome staining and immunohistochemical staining for dentin sialoprotein. In conclusion, NF-PLLA scaffolds enhanced the odontogenic differentiation of human DPSCs and mineralization both in vitro and in vivo, and are promising scaffolds for dentin regeneration.
Keywords: Dental pulp stem cells (DPSCs), Nanofibrous poly(L-lactic acid) (NF-PLLA) scaffold, Solid-walled (SW-PLLA) scaffold, Odontogenic differentiation, Mineralization
Introduction
Tissue engineering technology provides an approach to achieve dentin regeneration to potentially replace or repair the impaired dentin-pulp tissues. Although there are three key components (cells, signals and scaffolds) for tooth tissue engineering, much of previous efforts have focused on investigating the suitable cells and signals [1–3]. Dental pulp stem cells (DPSCs) have been demonstrated to be a suitable cell source for dental tissue regeneration because of the clonogenic ability, rapid proliferation rate and multiple differentiation potentials [4, 5]. The inductive factors for the odontogenic differentiation of DPSCs have also been investigated. However, there is relatively little knowledge on a suitable scaffold to support DPSCs to regenerate dental tissues. To achieve successful dentin tissue regeneration, a highly porous scaffold that mimics the natural extracellular microenvironment of dentin tissue is likely critical for cell attachment, proliferation, differentiation, and neo tissue genesis [6].
Collagen type I (Col I), is the dominant fibrous protein in hard tissues, including bone and dentin. In dentin, collagen comprises about 80–90% of the organic matrix and is present as nano-sized fibers [7]. Previous studies indicated that Col I accelerated odontogenic differentiation and mineralization of dental pulp cells [8–10]. Therefore, collagen foam has been used as a scaffold in tooth tissue engineering [11–13]. As an extracellular matrix (ECM) component, collagen provides a natural environment for cells. However, there are concerns over the potential pathogen transmission, immune reactions and the poor mechanical properties of this material.
Compared to collagen, synthetic polymers have shown advantages in stability, controllable degradation rate, and flexible molecular/structural design, and therefore have been used extensively as scaffolds for the engineering of various tissues, including teeth [14, 15]. To imitate the natural extracellular matrix (ECM), nanofibrous polymer scaffolds can be fabricated by using a few different processing techniques. Electrospinning is one of the most common techniques that can process polymers into nanofibrous nonwoven scaffolds for tissue engineering [16, 17]. However, one of the limitations of electronspinning is that designed three-dimensional pore structures are difficult to achieve in the resulting scaffolds. In our laboratory, a phase separation technique has been developed to fabricate scaffolds with highly interconnected spherical macroporous structures with a nanofibrous pore wall architecture [18–20]. By using this fabrication process, the physical architecture of the scaffolds can be more accurately controlled at several size scales, including the macroscopic shape of the scaffold, the spherical pore size and the nano scale fiber diameter.
Our previous studies have shown that NF scaffolds were superior to solid-walled (SW) scaffolds in promoting osteoblast differentiation and bone formation [21–25]. Since osteoblasts and odontoblasts are closely related lineages [4], and bone and dentin are similar in their matrix protein composition, we hypothesized that NF scaffolds were superior to SW scaffolds for the odontogenic differentiation of DPSCs and their matrix mineralization. To test this hypothesis, the odontogenic differentiation and dentin tissue regeneration were evaluated using both types of scaffolds with similar macroscopic shape (circular disks with a diameter of 5.2 mm and a thickness of 1.5 mm), macroporous structure (interconnected spherical pores), pore size (250–420 µm) and porosities (96%). The only difference was the wall architecture of the macropores, which is either smooth or nanofibrous.
Materials and Methods
Preparation of NF-PLLA scaffolds and SW-PLLA scaffolds
PLLA with an inherent viscosity of approximately 1.6 dl/g was purchased from Boehringer Ingelheim (Ingelheim, Germany). The fabrication methods of NF-PLLA and SW-PLLA scaffolds have been reported previously [20, 21], with some modifications. Briefly, for NF-PLLA scaffolds, PLLA was dissolved in 4/1 (v/v) dioxane/methanol solvent mixture and was cast onto paraffin sphere assemblies. The polymer/paraffin composite was transferred into a freezer (−80°C) for the polymer solution to phase separate overnight. Hexane was used to extract the solvent and leach the paraffin spheres for a total of 4 days. Hexane in the scaffolds was then exchanged with cyclohexane. For SW-PLLA scaffolds, a 10% (w/v) solution of PLLA was prepared in dioxane until a homogeneous solution was obtained. The paraffin sphere mold preparation and the polymer casting procedures were performed in the same way as for the NF-PLLA scaffolds [23]. Paraffin leaching with hexane and solvent exchange with cyclohexane were also proceeded in the same manner as those for NF-PLLA scaffolds. Finally both the NF-PLLA and the SW-PLLA scaffolds were lyophilized and cut into disks 5.2 mm in diameter and 1.5 mm in thickness. The scaffolds were sterilized with ethylene oxide.
Cell culture and seeding
Human DPSCs were purchased from the Center of Craniofacial Molecular Biology, School of Dentistry, University of Southern California and the isolation method was reported previously [4]. The thawed DPSCs were cultured in α-modified essential medium (α-MEM) (GIBCO, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (GIBCO) and 1% Pen Strep (GIBCO) in a humidified incubator at 37°C with 5% CO2. The medium was changed every two days and DPSCs of passages 3–6 were used in the following studies.
The NF and SW scaffolds were soaked in 70% ethanol to prewet for 30 min and then were exchanged with phosphate-buffered saline (PBS) from GIBCO for three times (30 min each). The scaffolds were then washed twice with α-MEM containing 10% FBS (1 hour each). One million human DPSCs were seeded on each scaffold and the cell-scaffold constructs were cultured in 2 mL culture medium for 24 hours; and then changed into “odontogenic” medium (containing 50 µg/mL ascorbic acid, 5 mM β-glycerophosphate, and 10 nM dexamethasone). All the cell-scaffold constructs were cultured on an orbital shaker in an incubator at 37°C with 5% CO2. The medium was changed every two days.
DNA assay
To examine the proliferation of DPSCs on NF or SW scaffolds at different time intervals, the cell-scaffold constructs were homogenized in 1× DNA assay buffer and lysis buffer (Sigma, St. Louis, MO), and then were incubated at 37°C for 1 hour. Lysate was centrifuged at 500 g at room temperature for 5 min. The supernatant was collected for DNA content determination using fluorescence assay with Hoechst 33258 dye (Sigma) [26].
Scanning electron microscopy (SEM) observation
The cell-scaffold constructs cultured on both scaffolds were rinsed in PBS, fixed in 2.5% glutaraldehyde, and post-fixed in 1% osmium tetroxide. Samples were dehydrated in a series of increasing concentrations of ethanol, and hexamethyldisilizane (HMDS). The samples were then sputter-coated with gold using a sputter coater (Desk-II, Denton Vacuum Inc., Moorstown, NJ) and observed under a scanning electron microscope (Philips XL30 FEG) at 10 kV. The elemental contents on the surface of the scaffolds were measured by energy dispersive x-ray microanalysis (EDX) (Phoenix XEDS system).
Alkaline phosphatase (ALP) activity
ALP activity was detected using SensoLyte™ pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA) according to the manufacturer’s protocol. Briefly, the cell-scaffold constructs were homogenized in 1 mL lysis buffer provided with the kit. Lysate was centrifuged at 10000 g at 4°C for 15 min. Supernatant was collected for ALP assay using p-nitrophenyl phosphate (p-NPP) as a phosphatase substrate and alkaline phosphatase standard supplied with the kit. The absorbance was measured at 405 nm and the amount of ALP in the cells was normalized against total protein content.
Real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen) and the first-strand cDNA was reverse transcribed using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) from each sample after odontogenic induction for 1, 2 and 4 weeks. Real-time PCR quantitation of the mRNA of odontogenic marker genes was performed in a 7500 Real-Time PCR System (Applied Biosystems) by using Power SYBR-Green (Applied Biosystems). The sequence of the specific primer sets are listed below: Col I (sense 5'-AAAAGGAAGCTTGGTCCACT-3'; antisense 5'-GTGTGGAGAAAGGAGCAGAA-3'); osteocalcin (OCN) (sense 5’-ACTGTGACGAGTTGGCTGAC-3’; antisense 5’-CAAGGGCAAGAGGAAAGA AG-3’); dentin sialophosphoprotein (DSPP) (sense 5’-TTAAATGCCAGTGGAACCAT-3’; antisense 5’-ATTCCCTTCTCCCTTGTGAC-3’); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sense 5’-GAGTCAACGGATTTGGTCGT-3’; antisense 5’-GACAAGCTTCCCGTTCTGAG-3’).
Calcium quantification
The calcium content of cell-scaffold constructs was determined by using a calcium assay kit (Stanbio laboratory, Boerne, TX) according to the manufacturer’s instruction. The absorbance was measured at 550 nm and the calcium content was calculated using the standard provided in the kit.
Histological and immunohistochemical analyses
For histological analysis, the cell-scaffold constructs were washed in PBS, fixed with 10% (wt/v) formalin in PBS overnight, dehydrated through a graded series of ethanol, embedded in paraffin, and sectioned at a thickness of 5 µm. The sections were then deparaffinized, rehydrated and stained with hemotoxylin and eosin (H&E), Von Kossa method for calcium deposits, and Masson’s trichrome method for collagen cumulation. For immunohistochemical analysis, sections were deparaffinized with xylene and ethanol, reacted with primary dentin sialoprotein (DSP) antibody (1:100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA). A Cell and Tissue Staining Kit (HRP-AEC System, from R&D Systems, Minneapolis, MN) was used to stain the prepared slides according to the manufacturer’s instruction.
Subcutaneous implantation
The animal surgical procedure was approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan. For implantation, six nude (nu/nu) mice with an age range of 6–8 weeks (Charles River Laboratories) were used in this study. Surgery was performed under general inhalation anesthesia with isofluorane. One midsagittal incision was made on the dorsa and two subcutaneous pockets were created using blunt dissection. The cell-scaffold constructs were induced in odontogenic media for 2 weeks before subcutaneously implantation, and the cell-scaffold constructs cultured in media without odontogenic inducers were used as the control group. One construct of each group was implanted into each pocket at random. Three samples were implanted for each group (n = 3). After placement of the constructs, the incisions were closed with staples. Animals were euthanized and samples were retrieved after 8 weeks of implantation. Harvested specimens were immediately fixed in 10% (wt/v) formalin for 24 hours, and then processed for histological examination using hematoxylin-eosin (H&E), von Kossa, Masson’s trichrome, and immunohistochemical staining for DSP.
Statistical Analysis
Data were reported using the means ± standard deviations (n=3). Statistical analysis was carried out using Student’s t-test for differences among groups and a value of p<0.05 was considered to be statistically significant.
Results
Proliferation and matrix production of DPSCs on scaffolds
Both the NF-PLLA (Fig. 1A) and SW-PLLA (Fig. 1C) scaffolds had similar interconnected macroporous structures created using spherical paraffin porogen (diameter: 250–420 µm) and similar porosities of about 96%. The only difference was in the wall architectures of macropores, being either smooth (Fig. 1B) or nanofibrous (Fig. 1D). The diameter of the nanofibers ranged from 50 to 500 nm, which is the same as the scale of natural collagen fibers.
Fig. 1.
SEM images of SW-PLLA and NF-PLLA scaffolds. (A) Macroporous structure of SW-PLLA scaffolds at low magnification; (B) Solid-walled architecture of SW-PLLA scaffolds at high magnification; (C) Macroporous structure of NF-PLLA scaffolds at low magnification; and (D) Nanofibrous architecture of NF-PLLA scaffolds at high magnification.
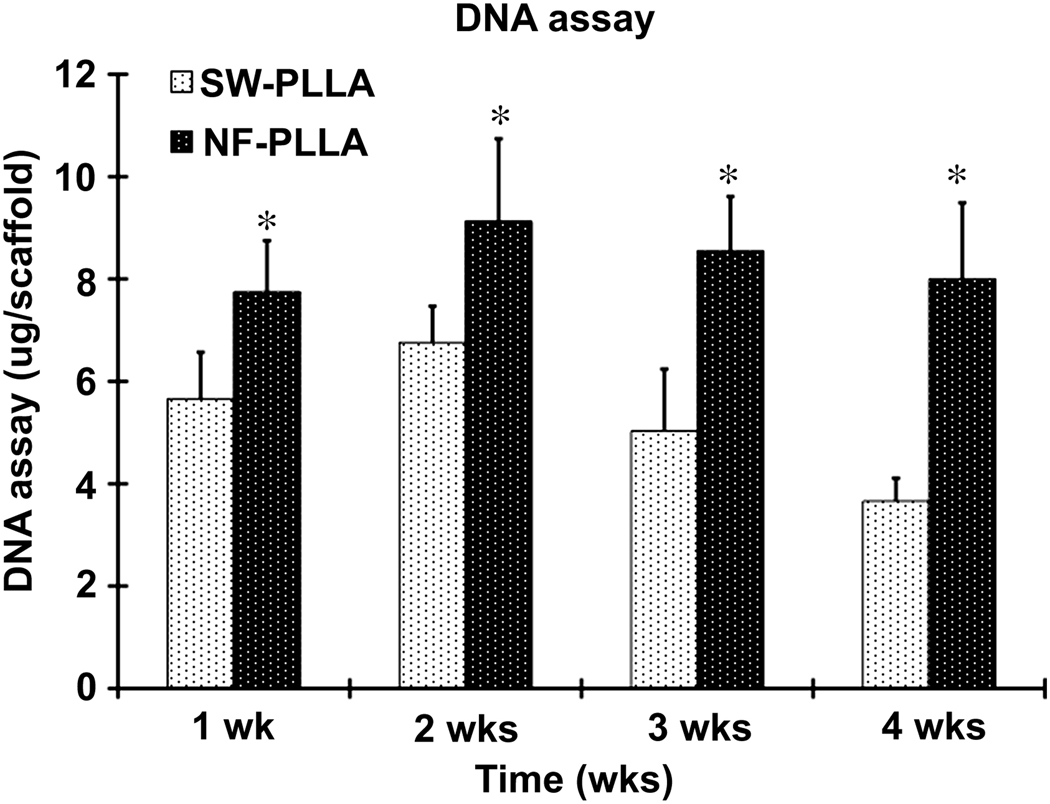
The total DNA assay revealed that cells proliferated faster on NF scaffolds than on SW scaffolds during the first 2 weeks (Fig. 2). The DNA content (number of DPSCs) on SW scaffolds decreased after 2 weeks of culture in odontogenic media, but not on the NF-PLLA scaffolds (Fig. 2). Histological sections of the samples showed more cells and ECM secretion in the macropores of NF scaffolds than in those of SW scaffolds after 4 weeks of culture in odontogenic media (Fig. 3A&B). This trend continued after 8 weeks of culture (Fig. 3C&D).
Fig. 2.
The proliferation of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds. The bars represent means ± SD (n = 3). Comparison between NF-PLLA group and SW-PLLA group: *p<0.05.
Fig. 3.
H&E staining of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds in odontogenic media. (A) on SW-PLLA scaffolds for 4 weeks; (B) on NF-PLLA scaffolds for 4 weeks; (C) on SW-PLLA scaffolds for 8 weeks; and (D) on NF-PLLA scaffolds for 8 weeks. Scale bars represent 100 µm.
Odontogenic differentiation and mineralization in vitro
The ALP activity increased with culture time in both NF-PLLA and SW-PLLA scaffolds and peaked at 3 wks, after which the activity decreased in both groups. There were significantly higher ALP levels in cells cultured on NF scaffolds than those on SW scaffolds during the first 3 weeks, whereas the difference in ALP level was not statistically significant at 4 wks (Fig. 4). Expression levels of genes related to the odontogenic differentiation of DPSCs, including Col I, OCN and DSPP, were detected using real-time PCR on both NF and SW scaffolds at 1, 2 and 4 weeks (Fig. 5). The expression levels of OCN and DSPP genes increased with culture time on both NF and SW scaffolds at different time points. While a similar trend was observed with the expression level of Co l I gene, but the increases were not statistically significant. The expression levels of OCN and DSPP in cells on NF scaffolds were significantly higher than those on SW scaffolds at all time points examined, while the difference in Col I expression level between the NF and SW scaffolds was significant only at 4 weeks.
Fig. 4.
ALP activity quantification of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds. The bars represent means ± SD (n = 3). Comparison between NF-PLLA group and SW-PLLA group: *p<0.05.
Fig. 5.
Real-time PCR quantification of gene expression of human DPSCs grown on the SW-PLLA and NF-PLLA scaffolds for 1, 2 and 4 weeks. The gene expression of Col I, ALP, OCN, DSPP and DMP-1 was upregulated on NF-PLLA scaffolds in comparison with SW-PLLA scaffolds at the examined time points. Comparison between NF-PLLA group and SW-PLLA group: *p<0.05.
The mineralization on scaffolds was examined by using von Kossa staining, calcium quantification assay, SEM and EDX. Von Kossa staining revealed substantial levels of mineral deposition (black color) after 4 and 8 weeks of culture on both types of scaffolds, and increased with increasing culture time (Fig. 6A-D). At the same culture times, there was more mineral generated on NF scaffolds than on SW scaffolds. This result was confirmed by calcium content quantification, which demonstrated greater amount of calcium deposition on NF scaffolds than on SW scaffolds at both 4 and 8 weeks (Fig. 7). The SEM images also revealed a noticeable amount of small globular mineral deposits on the surfaces of macropores in SW scaffolds (Fig. 8A), while an appreciably larger amount of mineral nodules was observed on NF scaffolds (Fig. 8B). This observation was verified by energy dispersive x-ray microanalysis, which detected higher calcium content (20.42%) on the NF scaffolds than on the SW scaffolds (9.10%) after 8 weeks of culture (Fig. 8C&D).
Fig. 6.
Von Kossa staining of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds in odontogenic media: (A) on SW-PLLA scaffolds for 4 weeks; (B) on NF-PLLA scaffolds for 4 weeks; (C) on SW-PLLA scaffolds for 8 weeks; (D) on NF-PLLA scaffolds for 8 weeks. Black color indicates mineral deposition. Scale bars represent 100 µm.
Fig. 7.
Calcium content quantification of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds for 4 or 8 weeks. The bars represent means ± SD (n = 3). Comparison between NF-PLLA group and SW-PLLA group: *p<0.05.
Fig. 8.
SEM observation of human DPSCs cultured on SW-PLLA and NF-PLLA scaffolds in odontogenic media for 8 weeks. (A) on SW-PLLA scaffolds and (B) on NF-PLLA scaffolds. Energy dispersive x-ray microanalysis of human DPSC-scaffold constructs cultured in odontogenic media for 8 weeks. (C) on SW-PLLA scaffolds and (D) on NF-PLLA scaffolds.
In vivo study
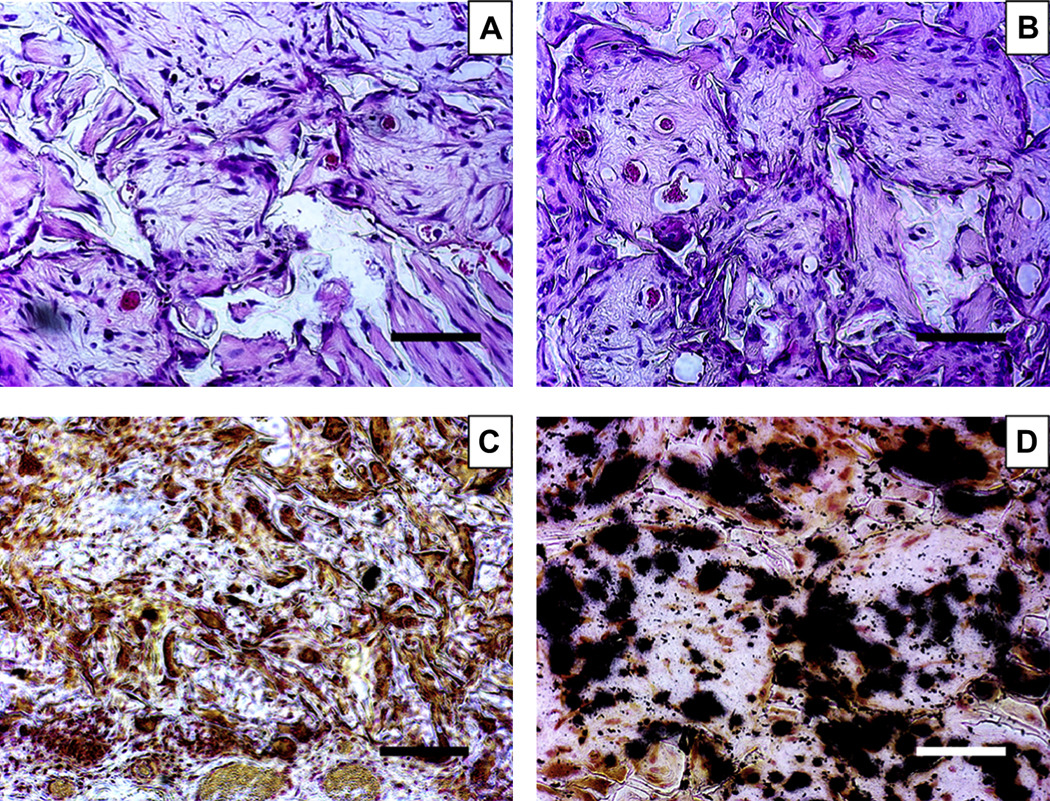
After each scaffold was seeded with one million human DPSCs, the cell-scaffold constructs were cultured in odontogenic media for 2 weeks to induce differentiation, where the cell-scaffold constructs cultured in media without odontogenic inducers were used as control. Subsequently, the constructs were implanted subcutaneously in nude mice for 8 weeks. The retrieved neo-tissue constructs were then examined. Macroscopically, all specimens were surrounded by vascularized thin fibrous connective tissue. The H&E staining illustrated that more cells, blood vessels and denser tissue were present in the macropores of the NF scaffolds than in those of SW scaffolds (Fig. 9 A&B). Moreover, von Kossa staining revealed greater mineralization in the NF scaffolds than in the SW scaffolds (Fig. 9 C&D). The collagen formation in different scaffolds was examined using Masson’s trichrome staining. Blue-stained dense collagen matrix was observed inside the macropores of the NF scaffolds, while sparse collagen matrix was observed in SW scaffolds (Fig. 10 A&B). The odontogenic differentiation of DPSCs on different scaffolds was examined through immunohistochemical staining for DSP. The tissue in NF scaffolds was strongly stained (dark brown) for DSP, while the tissue in SW scaffolds was weakly stained for DSP (Fig. 10C&D).
Fig. 9.
H&E and von Kossa staining of human DPSC-scaffold constructs subcutaneously implanted in nude mice for 8 weeks. (A) on SW-PLLA scaffolds (H&E staining); (B) on NF-PLLA scaffolds (H&E staining); (C) on SW-PLLA scaffolds (von Kossa staining); and (D) on NF-PLLA scaffolds (von Kossa staining). Black color indicates mineral deposition in C&D. Scale bars represent 100 µm.
Fig. 10.
Masson’s trichrome staining and immunohistochemical staining for DSP of human DPSC-scaffold constructs implanted subcutaneously in nude mice for 8 weeks. (A) on SW-PLLA scaffolds (Masson’s trichrome staining); (B) on NF-PLLA scaffolds (Masson’s trichrome staining); (C) o n S W-PLLA scaffolds (immunohistochemical staining for DSP); and (D) on NF-PLLA scaffolds (immunohistochemical staining for DSP). Blue color indicates collagen in A&B. Dark brown color indicates positive staining for DSP in C&D. Scale bars represent 100 µm.
Discussion
Scaffolds play a vital role in tissue engineering. They serve as three-dimensional tissue templates and are intended to provide synthetic ECM microenvironments for cell attachment, proliferation, differentiation, and neo tissue genesis. An advanced scaffold therefore may benefit from mimicking certain advantageous features of the natural ECM [6]. Collagen type I accounts for 80–90% of the organic substances of demineralized dentin ECM and is correlated closely with the dentin formation [7]. Previous studies demonstrated that collagen matrix promoted odontogenic differentiation and mineralization and has been used as a scaffold in tooth tissue engineering [8–10]; however, there are concerns over the potential pathogen transmission, immune reactions and the poor mechanical properties of collagen matrix. In our laboratory, NF-PLLA scaffolds were fabricated to emulate the nanofibrous architecture of collagen fibers [18–20]. Both SW and NF scaffolds with similar interconnected spherical pores were formed by using a paraffin sphere porogen to facilitate cell seeding, migration and growth. The only difference between the two types of scaffolds was in the architecture of the macropore walls of the scaffolds, which was either nanofibrous or solid-walled (smooth). Our previous studies showed that the nanofibrous architecture facilitated osteoblast proliferation, differentiation and mineralization compared to smooth pore wall architecture [21, 27, 28]. Since dentin is a tissue analogous to bone and shares many extracellular components with bone, we therefore hypothesized that NF scaffolds had better potential in promoting the proliferation and odontogenic differentiation of DPSCs, resulting in enhanced dentin-like tissue formation than SW scaffolds. This study was intended to test this hypothesis.
Histological staining and DNA assay in vitro demonstrated that NF scaffolds provided a better extracellular microenvironment for the proliferation of DPSCs and their ECM production than SW scaffolds. Since both scaffolds had similar macropore structures, the nanofibrous features seemed to play an important role in cell attachment and odontogenic differentiation. Our previous studies demonstrated that nanofibrous architecture enhanced protein adsorption, including fibronectin and vitronectin, contributing to pre-osteoblast cell attachment [26]. This was consistent with our observation that more human DPSCs were present throughout the macropores of NF scaffolds compared to those of SW scaffolds. Another possible interpretation of the enhanced cell attachment and growth could be that filopodia played an important role in biological processes [29, 30] and nanofibrous architecture could have altered the mode of anchorage, allowing filopodia to anchor more tightly. In addition, the local mass transport conditions in the nanofibrous scaffolds is likely better than those in solid-walled architecture. The nanofibrous pore walls might improve nutrient/oxygen supply to and metabolic waste removal from the attached cells, contributing to better extracellular environment for DPSCs growth and ECM production.
To investigate the ability of odontogenic differentiation and mineralization on NF and SW scaffolds, ALP activity quantification, real-time PCR, calcium content assay, SEM and histological analyses were carried out. ALP is regarded as an early marker of osteogenic differentiation and hard tissue formation [31]. Col I, OCN and DSPP were chosen as gene markers for the odontogenic phenotype. Collagen I is the predominant protein and the basis for dentin repair [7]. OCN, a vitamin K-dependent noncollagenous ECM protein, is generally regarded as a late marker for osteogenic and odontogenic differentiation [32]. DSPP is the major dentinal noncollagenous protein and plays a crucial role during dentinogenesis [33]. From our results, DPSCs cultured on NF scaffolds showed significantly higher ALP activity than those on SW scaffolds. In addition, the expression of the specific markers associated with odontogenic differentiation, especially OCN and DSPP, was upregulated on NF scaffolds compared with those on SW scaffolds. The high levels of OCN and DSPP mRNA in cells on NF scaffolds were consistent with more mineral formation observed in NF scaffolds. These findings suggest that DPSCs grown on NF scaffolds allowed for enhanced expression of the odontoblast phenotype compared to SW scaffolds. In terms of mineralization, von Kossa staining and calcium content assay revealed a greater amount of mineral generated in the NF scaffolds compared with that in the SW scaffolds. This was also confirmed by the SEM and EDX results. Consistent with the in vitro studies, results from in vivo studies showed that the newly formed ECM in NF scaffolds were strongly immunostained for DSP, which indicated that the DPSCs differentiated into odontoblast-like cells and regenerated dentin-like hard tissue in the NF scaffolds. Taken together, these outcomes provided strong evidence that NF scaffolds better promoted odontogenic differentiation and mineralization than SW scaffolds both in vitro and in vivo.
Several factors might have contributed to the enhanced odontogenic differentiation and mineralization of human DPSCs on the NF scaffolds. In dentin tissue, type I collagen is considered to provide initiation sites for calcification and have been verified to modulate odontogenic differentiation and mineralization [7]. These effects were mediated by the interaction of collagen with integrin receptors present on the cell membrane [8–10]. NF-PLLA scaffolds with interconnected macropores were fabricated in our laboratory to imitate type I collagen fibers [18–20]. Our previous results demonstrated that NF scaffolds adsorbed greater quantities of cell adhesion proteins (such as fibronectin) than SW scaffolds [26]. In dentin tissue, fibronectin enhances the differentiation of odontoblasts and dentine formation [34, 35]. A fibronectin-rich matrix may serve as a reservoir of growth factors, which have participated in the differentiation of odontoblasts [36, 37]. Our findings suggest that nanofibrous architecture of PLLA scaffolds exhibited certain characteristics similar to natural collagen fibers to facilitate the odontogenic differentiation and biomineralization of human DPSCs.
Although DSP-positive staining and hard tissue formation were confirmed, no typical palisade arrangement of the cells and dentin-pulp like tissue were identified in the implants in both of the scaffolds. In this study, we focused on investigating the influences of NF scaffolds on the differentiation of the cells. While not being the focus of this study, signaling molecules also play a vital role in tissue regeneration. More investigation on optimal odontogenic factors, their spatial and temporal application, and integration with scaffolds may lead to further improved microenvironments for high quality dentin regeneration, which will be explored in our future studies.
Conclusion
NF-PLLA scaffolds, mimicking the physical architecture of Col I, provided a favorable extracellular matrix microenvironment for the attachment and proliferation of human DPSCs. Moreover, NF-PLLA scaffolds better supported odontogenic differentiation of human DPSCs and dentin-like hard tissue formation compared to SW-PLLA scaffolds, demonstrating the advantages of NF-PLLA scaffolds in dental tissue engineering.
Acknowledgements
The authors would like to acknowledge the financial support from the National Institutes of Health (Research Grants DE015384 and DE017689: PXM).
References
- 1.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21(9):1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31(10):711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 3.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13(2):151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 6.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiesmann HP, Meyer U, Plate U, Hohling HJ. Aspects of collagen mineralization in hard tissue formation. Int Rev Cytol. 2005;242:121–156. doi: 10.1016/S0074-7696(04)42003-8. [DOI] [PubMed] [Google Scholar]
- 8.Mizuno M. KY. Type 1 collagen matrix and b-glycerophosphate facilitates mineralized tissue formation by rat dental pulp cells. Jpn J Oral Biol. 2000;42:102–108. [Google Scholar]
- 9.Mizuno M, Miyamoto T, Wada K, Watatani S, Zhang GX. Type I collagen regulated dentin matrix protein-1 (Dmp-1) and osteocalcin (OCN) gene expression of rat dental pulp cells. J Cell Biochem. 2003;88(6):1112–1119. doi: 10.1002/jcb.10466. [DOI] [PubMed] [Google Scholar]
- 10.Kim NR, Lee DH, Chung PH, Yang HC. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):e94–e100. doi: 10.1016/j.tripleo.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34(4):421–426. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27(17):3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Walboomers XF, van Kuppevelt TH, Daamen WF, Bian Z, Jansen JA. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials. 2006;27(33):5658–5668. doi: 10.1016/j.biomaterials.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83(7):523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 15.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11(9–10):1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 16.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 17.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 18.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7(1):23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 20.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Woo KM, Chen VJ, Jung HM, Kim TI, Shin HI, Baek JH, et al. Comparative evaluation of nanofibrous scaffolding for bone regeneration in critical-size calvarial defects. Tissue Eng Part A. 2009;15(8):2155–2162. doi: 10.1089/ten.tea.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 24.Smith LA, Liu X, Hu J, Ma PX. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30(13):2516–2522. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 31(6):1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Liu X, Ma PX. Induction of osteoblast differentiation phenotype on poly(L-lactic acid) nanofibrous matrix. Biomaterials. 2008;29(28):3815–3821. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng Part A. 2009;15(7):1855–1864. doi: 10.1089/ten.tea.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacinto A, Wolpert L. Filopodia. Curr Biol. 2001;11(16):R634. doi: 10.1016/s0960-9822(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 30.Rorth P. Communication by touch: role of cellular extensions in complex animals. Cell. 2003;112(5):595–598. doi: 10.1016/s0092-8674(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 31.Vakeva L, Mackie E, Kantomaa T, Thesleff I. Comparison of the distribution patterns of tenascin and alkaline phosphatase in developing teeth, cartilage, and bone of rats and mice. Anat Rec. 1990;228(1):69–76. doi: 10.1002/ar.1092280111. [DOI] [PubMed] [Google Scholar]
- 32.Viereck V, Siggelkow H, Tauber S, Raddatz D, Schutze N, Hufner M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86(2):348–356. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 33.Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273(16):9457–9464. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 34.Lesot H, Fausser JL, Akiyama SK, Staub A, Black D, Kubler MD, et al. The carboxy-terminal extension of the collagen binding domain of fibronectin mediates interaction with a 165 kDa membrane protein involved in odontoblast differentiation. Differentiation. 1992;49(2):109–118. doi: 10.1111/j.1432-0436.1992.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 35.Tziafas D, Alvanou A, Kaidoglou K. Dentinogenic activity of allogenic plasma fibronectin on dog dental pulp. J Dent Res. 1992;71(5):1189–1195. doi: 10.1177/00220345920710051101. [DOI] [PubMed] [Google Scholar]
- 36.Tziafas D, Panagiotakopoulos N, Komnenou A. Immunolocalization of fibronectin during the early response of dog dental pulp to demineralized dentine or calcium hydroxide-containing cement. Arch Oral Biol. 1995;40(1):23–31. doi: 10.1016/0003-9969(94)00148-5. [DOI] [PubMed] [Google Scholar]
- 37.Murray PE, About I, Franquin JC, Remusat M, Smith AJ. Restorative pulpal and repair responses. J Am Dent Assoc. 2001;132(4):482–491. doi: 10.14219/jada.archive.2001.0211. [DOI] [PubMed] [Google Scholar]