Abstract
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol plus its glucuronides (total NNAL), metabolites of the lung carcinogen NNK, and total cotinine, metabolites of nicotine, are biomarkers of active and passive cigarette smoking. We calculated the total NNAL: total cotinine (× 103) ratio in 408 passive (infants, children, adults) and 1088 active smokers. The weighted averages were 0.73 (95% CI 0.71, 0.76) for passive smokers and 0.07 (0.06, 0.08) for active smokers (p<0.0001). These results demonstrate that cotinine measurements may underestimate exposure of passive smokers to the lung carcinogen NNK in secondhand cigarette smoke. The total NNAL:total cotinine (× 103) ratio may provide an improved biomarker for evaluating the health effects of passive smoking.
Keywords: cotinine, NNAL, passive smoking
Introduction
The urinary biomarker total NNAL, comprised of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides, is consistently elevated in non-smokers exposed to secondhand tobacco smoke (SHS) (Hecht, 2003; Hecht, 2006; Stark et al., 2007; Jensen et al., 2010; Thomas et al., 2011; Benowitz et al., 2010). These exposed non-smokers are referred to in this paper as passive smokers. NNAL and its glucuronides are metabolites of the potent tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (Hecht, 1998). The power of this biomarker relates to the tobacco-specificity and lung carcinogenicity of NNK. When NNAL is detected in the urine of a non-smoker, it is highly probable that its origin was passive smoking because NNK is not found in the diet or any other environment unpolluted by tobacco smoke (Hecht, 2008). In a series of studies beginning in the 1990s, and carried out in infants, children, and adult non-smokers, our group has demonstrated the presence of total NNAL in the urine of passive smokers, thus supporting the relationship between SHS exposure and lung cancer as observed in epidemiologic studies (Hecht et al., 1993; Parsons et al., 1998; Anderson et al., 2001; Hecht et al., 2001; Anderson et al., 2003; Tulunay et al., 2005; Hecht et al., 2006; Stepanov et al., 2006; Stark et al., 2007; Jensen et al., 2010; Thomas et al., 2011; International Agency for Research on Cancer, 2004). Consistent with our findings, total NNAL has been detected in the urine of 44% of reported non-smokers in a recent report of 6599 U.S. residents in the NHANES study (Bernert et al., 2010).
Another widely used biomarker of SHS exposure is cotinine, a major metabolite of nicotine, the main known addictive constituent of tobacco (U.S. Department of Health and Human Services, 2006). Levels of urinary cotinine are elevated in passive smokers, and correlate with levels of total NNAL (Hecht, 2006; Bernert et al., 2010). Cotinine and nicotine, however, are not carcinogens. Thus, the relationship between urinary cotinine and lung cancer in passive smokers is indirect. The amount of cotinine in the urine of a passive smoker is typically 1% or less than that of a smoker (Benowitz et al., 2010). As we have previously discussed, the amount of total NNAL in the urine of a non-smoker exposed to SHS is typically 1–6% of that in a smoker (Anderson et al., 2001; Hecht, 2006). These observations suggest that total NNAL may be a relatively more persistent biomarker of SHS exposure than is cotinine. This is plausible because the terminal half-life of NNAL is far longer than that of cotinine (Hecht et al., 1999). Furthermore, nicotine is known to readily adsorb to surfaces, thus dissipating from ambient air in environments polluted with tobacco smoke, while concentrations of NNK could increase (Jenkins, et al, 2000; Schick and Glantz, 2007; Sleiman et al., 2010).
Consistent with these observations, Benowitz et al. (2010) have recently reported that the ratio of urinary total NNAL to cotinine (× 103) in adults is significantly greater in passive smokers than in active smokers. This is a potentially important observation because it indicates that total NNAL may be a better biomarker of passive smoking than cotinine. Furthermore, the ratio possibly could be used to correctly classify light active smokers versus heavily exposed passive smokers (Lee, 1999). The Benowitz et al. study, based on 373 active smokers and 228 passive smokers, had some limitations. In the study reported here, we have examined the ratio of urinary total NNAL to total cotinine (× 103) in 1088 active smokers and 408 passive smokers who participated in our published studies. Using these data, obtained not only from studies of adults, but also passively exposed infants and children and actively exposed adolescents, we sought to confirm a significantly higher ratio of urinary total NNAL to total cotinine (× 103) in passive smokers than in active smokers.
Materials and Methods
Twenty studies were used to create five populations exposed to cigarette smoke: 1) infant passive smokers (Study 1); 2) child passive smokers (Studies 2–4); 3) adult passive smokers (Studies 5–9); 4) adolescent active smokers (Study 10); and 5) adult active smokers (Studies 11–20). The study designs and locations with references that describe further details of each are presented in Table 1. Only baseline data from all studies were used in this analysis.
Table 1.
Demographic characteristics of study participants (N = 1496)
| Study | Study Group |
Study Design | Study Location |
N3 | Age, years Mean±SD |
% Female1 | % White, Non- Hispanic1 |
Cigarettes per day |
Years Regular Smoking |
|---|---|---|---|---|---|---|---|---|---|
| Passive Smokers | |||||||||
| 1. (Hecht et al., 2006) | Infants | Intervention | Minnesota | 77 | 0.6±0.2 | 50 | 72 | ||
| 2. (Hecht et al., 2001) | Children | Observational | Minnesota | 56 | 10.8±1.2 | 52 | 7 | ||
| 3. (Stepanov et al., 2006) | Children | Observational | Moldova | 68 | 7.6±1.3 | 43 | 100 | ||
| 4. (Thomas et al., 2011) | Children | Randomized trial | Minnesota | 59 | 3.8±2.6 | 51 | 20 | ||
| 5. (Tulunay et al., 2005) | Adults | Observational | Minnesota | 15 | 26.5±8.1 | 73 | 33 | ||
| 6. (Anderson et al., 2001) | Adults | Observational | Minnesota | 16 | N/A2 | 100 | 87 | ||
| 7. (Anderson et al., 2003) | Adults | Observational | Minnesota | 16 | 37.6±14.3 | 78 | 94 | ||
| 8. (Stark et al., 2007) | Adults | Observational | Oregon | 86 | N/A | 67 | N/A | ||
| 9. (Jensen et al., 2010) | Adults | Observational | Minnesota | 15 | 30.9±13.3 | 53 | 100 | ||
| Active Smokers | |||||||||
| 10. (Hanson et al., 2011) | Adolescents | Randomized trial | Minnesota | 108 | 16.6±1.2 | 59 | 90 | 13.4±6.3 | 3.4±2.0 |
| 11. (Kotlyar et al., 2011) | Adults | Randomized trial | Minnesota | 81 | 42.8±11.5 | 43 | 80 | 20.5±7.4 | 25.6±11.6 |
| 12. (Church et al., 2010) | Adults | Observational | Minnesota | 66 | 50.6±11.9 | 48 | 59 | 24.3±10.7 | 32.2±12.4 |
| 13. (Joseph et al., 2005) | Adults | Observational | Minnesota | 86 | 45.0±11.9 | 59 | 63 | 10.4±12.2 | 25.1±11.4 |
| 14. (Mendoza-Baumgart et al., 2007) | Adults | Randomized trial | Minnesota | 40 | 38.6±11.9 | 55 | 92 | 21.3±5.0 | 22.2±12.1 |
| 15. (Le Marchand et al., 2008) | Adults | Observational | Hawaii | 297 | 61.4±6.2 | 50 | 33 | 24.2±10.2 | 40.3±8.1 |
| 16. (Hatsukami et al., 2007) | Adults | Dose-Ranging | Minnesota | 17 | 38.6±9.1 | 35 | 53 | 25.5±5.9 | 22.6±9.7 |
| 17. (Hatsukami et al., 2004) | Adults | Randomized trial | Minnesota | 38 | 40.9±9.7 | 0 | 95 | 23.7±7.3 | 23.3±10.2 |
| 18. (Hatsukami et al., 2010) | Adults | Randomized trial | Minnesota | 98 | 42.0±13.9 | 48 | 87 | 20.8±8.3 | 24.2±13.8 |
| 19. (Joseph et al., 2008) | Adults | Randomized trial | Minnesota | 139 | 58.5±9.3 | 12 | 93 | 27.6±10.9 | 41.3±9.8 |
| 20. (Hecht et al., 2004) | Adults | Randomized trial | Minnesota | 118 | 45.7±10.4 | 51 | 96 | 26.1±7.1 | 27.8±10.7 |
Some values are approximate
N/A, not available
Sample size included in analysis; not necessarily the sample size of the original study
The analysis was restricted to participants with both total cotinine (cotinine plus cotinine glucuronide) and total NNAL (NNAL plus NNAL-glucuronides) values which were above their respective detection limits in each study. The methods for total NNAL and total cotinine have been described (Hecht et al., 1999; Carmella et al., 2003).
The outcome of interest for this study was the ratio of total NNAL (pmol/mL urine) to total cotinine (nmol/mL urine) × 103. Between-study heterogeneity was assessed by testing the differences across studies within each of the groups (infant passive smoking, child passive smoking, adult passive smoking, adolescent active smokers, adult active smokers) using Kruskal-Wallis ANOVA. A mixed effects ANOVA model was fit with each group as a fixed effect and a random effect for individual study to adjust for between-study heterogeneity. To ensure the validity of the analyses, values for both biomarkers and their ratios, which had skewed distributions, were transformed using the natural logarithm to approximate normality and were summarized using geometric means. Group specific weighted averages were calculated using the inverse variance of each study within the group. All reported p-values were conservatively adjusted for multiple comparisons using a Bonferroni correction. Analyses were carried out in SAS Version 9.2 (SAS Institute, Inc., Cary, NC) and all significance levels were set at 0.05.
Results
Demographic characteristics of all subjects and tobacco use data for the smokers are summarized by study in Table 1. Geometric means of total NNAL, total cotinine, and their ratios (× 103) by study are summarized in Table 2 and Figure 1. Among the 408 passive smokers, total NNAL and total cotinine, both presented as geometric means, ranged across studies from 0.03–0.14 pmol/mL and 0.03–0.18 nmol/mL, respectively. The corresponding figures in 1088 active smokers were 0.61–2.95 pmol/mL total NNAL and 7.92–39.99 nmol/mL total cotinine.
Table 2.
Geometric means, 95% confidence intervals for total NNAL, total cotinine and their ratio in study participants by individual study and study group.
| Study | Study Group | N | Total NNAL (pmol/mL) |
Total Cotinine (nmol/mL) |
NNAL/Cotinine Ratio (x103) |
|---|---|---|---|---|---|
| Passive Smokers | |||||
| 1. (Hecht et al., 2006) | Infants | 77 | 0.14 (0.12, 0.17) | 0.18 (0.15, 0.22) | 0.78 (0.67, 0.91) |
| 2. (Hecht et al., 2001) | Children | 56 | 0.08 (0.06, 0.11) | 0.10 (0.07, 0.12) | 0.85 (0.66, 1.08) |
| 3. (Stepanov et al., 2006) | Children | 68 | 0.07 (0.06, 0.08) | 0.06 (0.05, 0.08) | 1.22 (1.01, 1.48) |
| 4. (Thomas et al., 2011) | Children | 59 | 0.10 (0.08, 0.13) | 0.10 (0.07, 0.14) | 1.02 (0.78, 1.32) |
| 5. (Tulunay et al., 2005) | Adults | 15 | 0.06 (0.05, 0.09) | 0.06 (0.03, 0.13) | 1.01 (0.51, 1.97) |
| 6. (Anderson et al., 2001) | Adults | 16 | 0.04 (0.02, 0.06) | 0.03 (0.02, 0.07) | 1.04 (0.52, 2.07) |
| 7. (Anderson et al., 2003) | Adults | 16 | 0.03 (0.02, 0.04) | 0.04 (0.03, 0.06) | 0.61 (0.41, 0.89) |
| 8. (Stark et al., 2007) | Adults | 86 | 0.04 (0.03, 0.05) | 0.12 (0.09, 0.15) | 0.33 (0.28, 0.38) |
| 9. (Jensen et al., 2010) | Adults | 15 | 0.06 (0.04, 0.10) | 0.09 (0.04, 0.21) | 0.67 (0.37, 1.24) |
| Active Smokers | |||||
| 10. (Hanson et al., 2011) | Adolescents | 108 | 0.61 (0.50, 0.74) | 13.48 (11.15, 16.29) | 0.05 (0.04, 0.05) |
| 11. (Kotlyar et al., 2011) | Adults | 81 | 0.92 (0.77, 1.11) | 16.98 (14.82, 19.45) | 0.05 (0.05, 0.06) |
| 12. (Church et al., 2010) | Adults | 66 | 1.25 (1.07, 1.45) | 21.53 (18.59, 24.92) | 0.06 (0.05, 0.07) |
| 13. (Joseph et al., 2005) | Adults | 86 | 0.81 (0.64, 1.02) | 7.92 (6.13, 10.23) | 0.10 (0.09, 0.12) |
| 14. (Mendoza-Baumgart et al., 2007) | Adults | 40 | 1.36 (1.08, 1.72) | 28.06 (22.96, 34.31) | 0.05 (0.04, 0.06) |
| 15. (Le Marchand et al., 2008) | Adults | 297 | 0.82 (0.75, 0.89) | 12.04 (11.00, 13.17) | 0.07 (0.06, 0.07) |
| 16. (Hatsukami et al., 2007) | Adults | 17 | 2.95 (2.12, 4.11) | 39.99 (31.08, 51.45) | 0.07 (0.06, 0.09) |
| 17. (Hatsukami et al., 2004) | Adults | 38 | 2.33 (1.78, 3.05) | 24.92 (20.09, 30.91) | 0.09 (0.08, 0.11) |
| 18. (Hatsukami et al., 2010) | Adults | 98 | 1.18 (1.00, 1.39) | 23.17 (19.93, 26.93) | 0.05 (0.04, 0.06) |
| 19. (Joseph et al., 2008) | Adults | 139 | 2.06 (1.83, 2.32) | 19.61 (17.01, 22.62) | 0.11 (0.09, 0.12) |
| 20. (Hecht et al., 2004) | Adults | 118 | 2.14 (1.91, 2.41) | 25.36 (22.56, 28.52) | 0.08 (0.08, 0.09) |
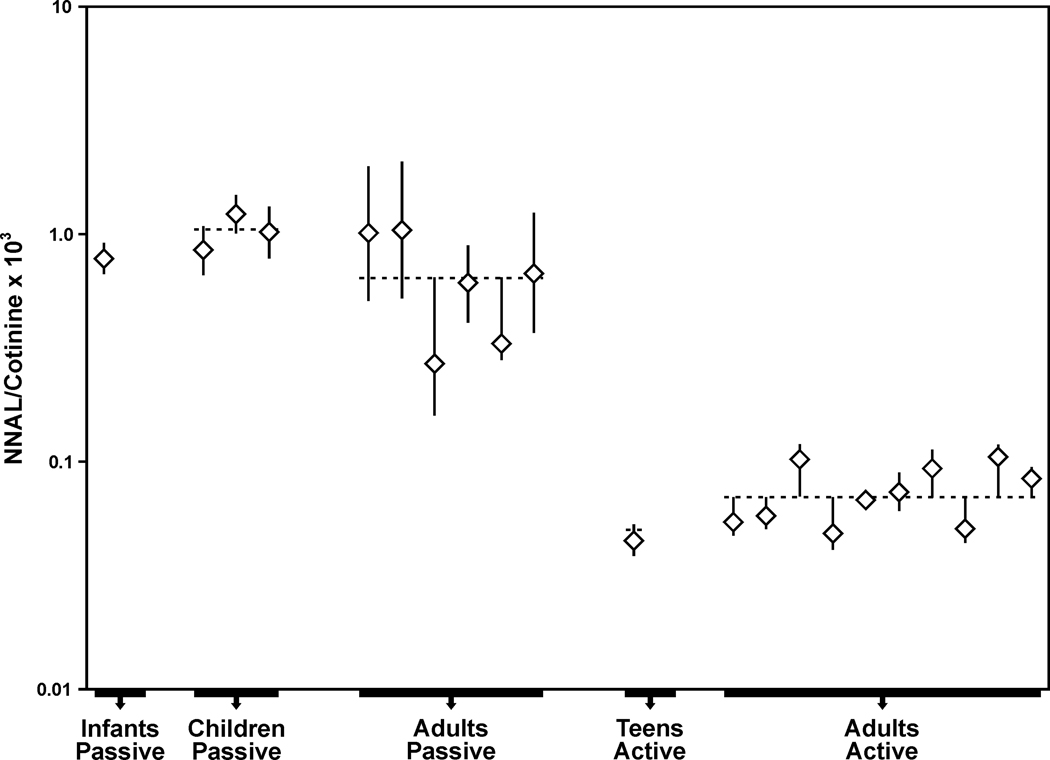
Figure 1.
Study-specific total NNAL:total cotinine ratios (× 103), presented as geometric means and 95% confidence intervals, by study group of passive and active smokers. The dotted line represents the weighted average of the combined groups.
The weighted averages of the total NNAL:total cotinine ratios (× 103), presented as the geometric means and 95% confidence intervals (CI), for the different groups were: infant passive smokers, 0.78 (0.67, 0.91); child passive smokers, 1.05 (0.98, 1.12); adult passive smokers, 0.69 (0.62, 0.75); adolescent active smokers, 0.05 (0.04, 0.05); adult active smokers 0.07 (0.06, 0.08) (Figure 1). The results of the mixed effect ANOVA model demonstrated that there were significant differences (all adjusted p<0.0001) between the total NNAL:total cotinine ratios (× 103) in each of the passive smoker vs. each of the active smoker groups.
We were unable to conclude that infants, children and adult passive smokers differed in their ratios, or that adolescent and adult smokers differed in their ratios (all p-values >0.50). Ignoring age group, the overall weighted averages of total NNAL and total cotinine in passive smokers, presented as geometric means and 95% CI, were 0.07 (0.07, 0.07) pmol/ml and 0.04 (0.04. 0.05) nmol/ml, respectively. The overall weighted averages for active smokers were 1.12 (1.10, 1.14) pmol/ml for total NNAL and 18.53 (18.26, 18.80) nmol/ml for total cotinine. The overall weighted averages of the total NNAL to total cotinine ratios (× 103) were 0.73 (0.71, 0.76) for passive smokers and 0.07 (0.06, 0.08) for active smokers (p<0.0001).
Discussion
The results of this study clearly demonstrate that total NNAL:total cotinine ratios (× 103) are significantly higher in passive smokers than in active smokers. The ratio was higher in each of the 9 passive smoking studies than in each of the 11 active smoking studies, as seen in Table 2. Overall, we observed a significant 10-fold higher ratio in passive smokers than in active smokers. Our results are consistent with those of Benowitz et al. (2010) who reported an approximate 18-fold higher ratio in passive vs. active smokers (2.85 × 103 vs. 0.16 × 103). Their total NNAL:cotinine ratios were 4 times higher than ours in passive smokers and about twice as high in active smokers. This results partially from their denominator being free cotinine whereas ours was total cotinine, the levels of which in urine are generally about twice as great (Hukkanen, et al, 2005).
Benowitz et al. (2010) reported limitations in the generalizability of their study. Their active smokers came from the U.S. and Poland and there were unexplained differences in the ratios between the two groups. Their passive smokers were also multinational, with vastly different levels of SHS exposure. Furthermore, some of the subjects had chronic obstructive pulmonary disease (COPD). While one of our studies (Study 19) enrolled cigarette smokers with cardiovascular disease, the remaining participants in our studies were outwardly healthy active and passive smokers. Additionally, all of our studies were conducted in the U.S. with the exception of Study 3, which observed SHS exposure among children in Moldova. One advantage of this analysis is the number of studies included, representing passive smokers with both intermittent and chronic exposure and active smokers with varying levels of exposure. The large sample size allowed us to pursue more complicated statistical models to incorporate these differences between studies in order to adequately address the differences in total NNAL:cotinine ratios between active and passive smokers. Most importantly, the participants in these studies represented a range of age levels, from infants to adults among the passive smokers, along with adolescent and adult active smokers.
The higher total NNAL: total cotinine ratio in passive compared to active smokers may result from differences in exposure. Thus, nicotine, a strongly basic semi-volatile compound, dissipates rapidly from ambient air (Jenkins, et al, 2000). This results in part from nicotine’s high affinity for surfaces. NNK, on the other hand, is less basic and less volatile than nicotine and may not dissipate rapidly from the air. One review of tobacco industry documents even suggests that more NNK is formed during the aging of air polluted with SHS (Schick and Glantz, 2007). There are few measurements of both nicotine and NNK in air polluted with SHS, but one study gives values of 17.5 ng/m3 NNK and 12.4 µg/m3 nicotine [a molar NNK:nicotine ratio (× 103) of 1.1] (Meger et al., 2000). This molar ratio is about 10 times and 50 times greater than the corresponding molar NNK:nicotine (× 103) ratios in mainstream and sidestream cigarette smoke, respectively (Roemer et al., 2004; International Agency for Research on Cancer, 2004). The shorter persistence of nicotine than NNK in the air could thus account for the higher total NNAL: total cotinine ratios in passive than in active smokers. Therefore, urinary cotinine measurements could be underestimating exposure to NNK.
The longer half-life of NNAL in the body (terminal phase reported in different studies as 10–18 days or 40–45 days in smokers) compared to that of cotinine (13 – 19 h) could also contribute to the different ratios (Hecht et al., 1999; Hukkanen, et al, 2005; Goniewicz et al., 2009). Exposure to NNK and nicotine in SHS would often be intermittent. The longer half-life of NNAL than cotinine could thus lead to higher NNAL:cotinine ratios under these intermittent exposure conditions. Exposure to NNK and nicotine in smokers would tend to be more constant as smokers attempt to maintain their desired nicotine level.
Both of these explanations for higher total NNAL:total cotinine ratios (× 103) in passive compared to active smokers assume that both the metabolism of NNK to NNAL and the half-life of NNAL are similar in passive and active smokers. The dose of NNK is lower in passive than in active smokers, and further studies would be necessary to determine the conversion of NNK to NNAL in humans at these differing doses as well as the half-life of NNAL in passive smokers. In rats and mice, the conversion of NNK to NNAL is greater at higher than at lower doses, which is not consistent with a dose-dependent difference in NNK metabolism explaining the results presented here (Morse et al., 1990). Differences in conversion of cotinine to 3′-hydroxycotinine in active vs. passive smokers might also influence the ratios, but only limited data are available (Lowe, et al, 2009).
Two of our early studies were not included in this analysis. One involved exposure of 5 male non-smokers to SHS and was not included because it was carried out in an exposure chamber, thus not reflecting natural conditions (Hecht et al., 1993). The second involved 9 workers in a hospital where smoking was allowed, but only NNAL-glucuronide data were reported (Parsons et al., 1998). In both of these studies as well as an early study of 17 non-smokers exposed to SHS by Meger et al. (2000), the NNAL:cotinine ratios (× 103) were lower than reported here: 0.25 in the chamber study, 0.26 in the hospital study, and 0.11 in Meger et al. In all three studies, these lower ratios resulted from higher cotinine levels than reported here. The higher cotinine level in the Meger et al. study may have resulted from the use of radioimmunoassay for its measurement. The reasons for the higher cotinine values in the other two studies are not clear.
The lower levels of total NNAL than of total cotinine in the urine of smokers are fully consistent with the levels of their precursors NNK and nicotine in cigarette smoke. Typical mainstream smoke levels of NNK and nicotine are about 100 ng and 800 µg, respectively, a ratio of 0.13 × 103, as noted above (Roemer et al., 2004), Total NNAL comprises about 15% of the NNK dose in smokers while total cotinine in urine comprises approximately 30% of the nicotine dose (Stepanov et al., 2008; Hukkanen, et al, 2005). Combining these data, the theoretical total NNAL:total cotinine ratio is 0.07 × 103, consistent with our observations in smokers. Thus, the ratio in smokers is reflecting constant exposure to both NNK and nicotine. It is more difficult to make this estimate for passive smokers because multiple factors are involved, and less is known about NNK metabolism at low doses. However, the relatively rapid dissipation of nicotine from the air is probably a major factor in the higher ratios, as mentioned above.
The NNAL:cotinine ratio might be useful in classifying a subject as a passive smoker versus a light active smoker in molecular epidemiology studies of passive smoking and cancer. The distinction between heavily exposed passive smokers and light active smokers is important and has given rise to some uncertainty in previous studies (Lee, 1999). The study reported here involved individuals smoking, on average, 10–30 cigarettes per day, so further data from light active smokers (less than 10 cigarettes per day) would be needed. Goniewicz et al. (2011) have recently concluded that the NNAL:cotinine ratio provides similar sensitivity but poorer specificity at discriminating passive versus active smokers when compared with NNAL alone, but their study included only 59 light active smokers (6.9 cigarettes per day) (Goniewicz et al., 2011). Further studies are required to clarify this issue.
Our study has potential limitations. It was retrospective in nature and the individual studies were not designed to investigate this particular question. The adults in the passive smoking groups tended to be female and white, thus not representing an accurate cross-section of gender and ethnicity. We did not find significant differences in biomarker levels between infant, children and adult passive smokers and therefore also presented combined data. There may, however, be some differences in NNK and nicotine metabolism among these groups which could affect the results. The smokers in our study were also predominantly white, thus potential influences of racial differences in metabolism, established for nicotine (Berg et al., 2010a; Berg et al., 2010b), were not taken into account.
Conclusions
Based on data from multiple studies of active and passive smokers carried out by our group, in which urine samples were analyzed in a consistent manner using well established and validated methods, we confirm the significantly higher ratio of total NNAL:total cotinine (× 103) in passive smokers than in active smokers. These results are significant because they may provide a potentially improved biomarker for evaluation of the health effects of passive smoking.
Acknowledgements
This study was supported by grants no CA-81301, DA-01333, and DA-014538 from the U.S. National Institutes of Health.
Footnotes
Declaration of interest
The authors report no declarations of interest.
Reference List
- Anderson KE, Carmella SG, Ye M, Bliss R, Le C, Murphy L, Hecht SS. Metabolites of a tobacco-specific lung carcinogen in the urine of nonsmoking women exposed to environmental tobacco smoke in their homes. J Natl Cancer Inst. 2001;93:378–381. doi: 10.1093/jnci/93.5.378. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Kliris J, Murphy L, Carmella SG, Han S, Link C, Bliss RL, Puumala S, Murphy SE, Hecht SS. Metabolites of a tobacco-specific lung carcinogen in nonsmoking casino patrons. Cancer Epidemiol, Biomarkers & Prev. 2003;12:1544–1546. [PubMed] [Google Scholar]
- Benowitz N, Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P., III Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiol Biomarkers Prev. 2010;19:2795–2800. doi: 10.1158/1055-9965.EPI-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JZ, Mason J, Boettcher AJ, Hatsukami DK, Murphy SE. Nicotine metabolism in African Americans and European Americans: variation in glucuronidation by ethnicity and UGT2B10 haplotype. J Pharmacol Exp Ther. 2010a;332:202–209. doi: 10.1124/jpet.109.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JZ, von Weymarn LB, Thompson EA, Wickham KM, Weisensel NA, Hatsukami DK, Murphy SE. UGT2B10 genotype influences nicotine glucuronidation, oxidation, and consumption. Cancer Epidemiol, Biomarkers & Prev. 2010b;19:1423–1431. doi: 10.1158/1055-9965.EPI-09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol, Biomarkers & Prev. 2010;19:2969–2977. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol, Biomarkers & Prev. 2003;12:1257–1261. [PubMed] [Google Scholar]
- Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, Yoder AR, Carmella SG, Hecht SS. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers. 2010;15:345–352. doi: 10.3109/13547501003753881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P, Benowitz NL. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13:202–208. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Havel CM, Peng MW, Jacob P, III, Dempsey D, Yu L, Zielinska-Danch W, Koszowski B, Czogala J, Sobczak A, Benowitz NL. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol, Biomarkers & Prev. 2009;18:3421–3425. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Allen S, Dayton R, Cao Q, Hatsukami DK. Smoking reduction after a failed quit attempt among adolescents. 2011 submitted. [Google Scholar]
- Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86:132–139. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used "reduced exposure" tobacco products. J Natl Cancer Inst. 2004;96:844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control. 2003;13 Suppl 1:i48–i56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. A biomarker of exposure to environmental tobacco smoke (ETS) and Ernst Wynder's opinion about ETS and lung cancer. Prev Med. 2006;43:256–260. doi: 10.1016/j.ypmed.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Le K, Murphy SE, Boettcher AJ, Le C, Koopmeiners J, Lee K-A, Hennrikus DJ. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol, Biomarkers & Prev. 2006;15:988–992. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. N Engl J Med. 1993;329:1543–1546. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–115. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Ye M, Carmella SG, Fredrickson A, Adgate JL, Greaves IA, Church TR, Ryan AD, Mongin SJ, Sexton K. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol, Biomarkers & Prev. 2001;10:1109–1116. [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 1191–1413. [PMC free article] [PubMed] [Google Scholar]
- Jenkins RA, Guerin MR, Tomkins BA. The Chemistry of Environmental Tobacco Smoke: Composition and Measurement. Second Edition. Boca Raton, FL: Lewis Publishers; 2000. pp. 77–106. [Google Scholar]
- Jensen JA, Schillo BA, Moilanen MM, Lindgren BR, Murphy S, Carmella S, Hecht SS, Hatsukami DK. Tobacco smoke exposure in nonsmoking hospitality workers before and after a state smoking ban. Cancer Epidemiol, Biomarkers & Prev. 2010;19:1016–1021. doi: 10.1158/1055-9965.EPI-09-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, Han S, Hatsukami DK. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol, Biomarkers & Prev. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Lando H, Carmella SG, Han S, Gross M, Bliss R, Le CT, Hatsukami DK. Smoking reduction fails to improve clinical and biological markers of cardiac disease: a randomized controlled trial. Nicotine Tob Res. 2008;10:471–481. doi: 10.1080/14622200801901948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, Murphy SE, Hecht SS, Hatsukami DK. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:91–100. doi: 10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, Tiirikainen M, Wang H. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PN. Uses and abuses of cotinine as a marker of tobacco smoke exposure. In: Gorrod JW, Jacob P III, editors. Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Amsterdam: Elsevier; 1999. pp. 669–719. [Google Scholar]
- Lowe FJ, Gregg EO, McEwan M. Evaluation of biomarkers of exposure and potential harm in smokers, former smokers and never-smokers. Clin Chem Lab Med. 2009;47:311–320. doi: 10.1515/CCLM.2009.069. [DOI] [PubMed] [Google Scholar]
- Meger M, Meger-Kossien I, Riedel K, Scherer G. Biomonitoring of environmental tobacco smoke (ETS)-related exposure to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Biomarkers. 2000;5:33–45. doi: 10.1080/135475000230523. [DOI] [PubMed] [Google Scholar]
- Mendoza-Baumgart MI, Tulunay OE, Hecht SS, Zhang Y, Murphy S, Le C, Jensen J, Hatsukami DK. Pilot study on lower nitrosamine smokeless tobacco products compared with medicinal nicotine. Nicotine Tob Res. 2007;9:1309–1323. doi: 10.1080/14622200701704228. [DOI] [PubMed] [Google Scholar]
- Morse MA, Eklind KI, Toussaint M, Amin SG, Chung F-L. Characterization of a glucuronide metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its dose-dependent excretion in the urine of mice and rats. Carcinogenesis. 1990;11:1819–1823. doi: 10.1093/carcin/11.10.1819. [DOI] [PubMed] [Google Scholar]
- Parsons WD, Carmella SG, Akerkar S, Bonilla LE, Hecht SS. A metabolite of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in the urine of hospital workers exposed to environmental tobacco smoke. Cancer Epidemiol, Biomarkers & Prev. 1998;7:257–260. [PubMed] [Google Scholar]
- Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Schick SF, Glantz S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: results from unpublished tobacco industry research. Cancer Epidemiol, Biomarkers & Prev. 2007;16:1547–1553. doi: 10.1158/1055-9965.EPI-07-0210. [DOI] [PubMed] [Google Scholar]
- Sleiman M, Gundel LA, Pankow JF, Jacob P, III, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci USA. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MJ, Rohde K, Maher JE, Pizacani BA, Dent CW, Bard R, Carmella SG, Benoit AR, Thomson NM, Hecht SS. The impact of clean indoor air exemptions and preemption policies on the prevalence of a tobacco-specific lung carcinogen among nonsmoking bar and restaurant workers. Am J Public Health. 2007;97:1457–1463. doi: 10.2105/AJPH.2006.094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS, Duca G, Mardari I. Uptake of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by Moldovan children. Cancer Epidemiol, Biomarkers & Prev. 2006;15:7–11. doi: 10.1158/1055-9965.EPI-05-0293. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Upadhyaya P, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol, Biomarkers & Prev. 2008;17:1764–1773. doi: 10.1158/1055-9965.EPI-07-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Guo H, Carmella SG, Balbo S, Han S, Davis AB, Yoder AR, Murphy SE, An LC, Ahluwalia JS, Hecht SS. Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-10-1027. Epub ahead of print 2011 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulunay O, Hecht SS, Carmella SG, Zhang Y, Lemmonds C, Murphy S, Hatsukami DK. Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol, Biomarkers & Prev. 2005;14:1283–1286. doi: 10.1158/1055-9965.EPI-04-0570. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington, D.C.: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health; 2006. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [Google Scholar]