Abstract
Treatment of glioblastoma remains a challenge in neuro-oncology. We investigated if treatment with neural stem cells engineered to express membrane-bound TRAIL (NSCs-mTRAIL) alone or in combination with proteasome inhibitors is a feasible therapeutic approach for experimental glioma. Glioma cells showed resistance to soluble TRAIL and proteasome inhibitors alone, but responded well to their combined treatment. In co-culture with NSCs-mTRAIL, glioma cells appeared to be more prone to apoptosis than to treatment with soluble TRAIL, which was enhanced by proteasome inhibitor bortezomib. In vivo, the survival of animals bearing intracranial glial xenografts was significantly improved by NSCs-mTRAIL. The addition of bortezomib further enhanced the efficacy of NSCs-TRAIL treated group in one of examined tumor models. These data demonstrate that therapy with NSCs-mTRAIL is a potent cell based approach for treatment of glioma. Such an approach warrants further search for therapeutics capable of increasing sensitivity of glioma cells to mTRAIL in vivo.
Keywords: neural stem cells, TRAIL, bortezomib, glioma, brain cancer
1. INTRODUCTION
Glioblastoma multiforme (GBM) represents a unique challenge in neuro-oncology. These tumors are characterized by their resistance to apoptotic mechanisms and their ability to widely disseminate throughout normal brain parenchyma, thus rendering a surgical solution virtually impossible and contributing to high recurrence rate of tumors. Advances in adjuvant therapies (chemotherapy and radiotherapy) have indeed impacted patient outcome [1], but the median survival after diagnosis of GBM is still only 14 to 15 months [1–3]. With this in mind, new therapies are urgently needed to target glioma cells in the primary mass as well those that accomplish distant migration.
An emerging means of meeting this challenge is the use of neural stem cells (NSCs). It has been well established that stem cells show tropism for glioma cells and will migrate to tumors in a predictable fashion after both intracranial and systemic administration [4–6]. Additionally, this migratory ability has been experimentally exploited for the delivery of cytotoxic therapeutics [7–9]. The aforementioned studies confirmed the strong tropism of NSCs for intracranial glioma, making them attractive vehicles for the delivery of a wide variety of therapeutic gene products directly to tumor cells.
One promising therapeutic agent is the TNF-related apoptosis-inducing ligand (TRAIL). It is well established that TRAIL can selectively induce apoptosis in a variety of neoplastic cells, including glioma cells [10–12]. TRAIL interacts with 5 receptors, 4 of which are located at the cell surface: (i) TRAIL-R1/death receptor 4 (DR4); (ii) TRAIL-R2/death receptor 5 (DR5); (iii) TRAIL-R3/decoy receptor 1(DcR 1) and (iv) TRAIL-R4/decoy receptor 2 (DcR2) [13]. The binding of TRAIL to TRAIL-R1 and TRAIL-R2 results in recruitment of the Fas-associated death domain (FADD), which recruits initiator caspases and triggers the formation of the Apo2L/TRAIL Death Inducing Signaling Complex (DISC). The formation of this complex culminates in downstream activation of the pathways of apoptosis [14, 15]. TRAIL-R1 and TRAIL-R2 differentiate between soluble (sTRAIL) and membrane-bound TRAIL (mTRAIL) for their activation [16, 17]. The decoy receptors, on the other hand, are unable to transmit an apoptotic signal: DcR1 lacks cytoplasmic and transmembrane domains, while DcR2 has a truncated death domain and hence is unable to trigger downstream signaling. DcR1 and DcR2 are thought to act as decoys, antagonizing TRAIL-apoptotic signaling. Decoy receptor expression is higher in normal tissue than in the majority of tumors [18] and can provide protection from TRAIL-mediated apoptosis [19, 20].
Despite the fact that many glioma cells exhibit TRAIL receptors, some have proven resistant to sTRAIL [15, 21]. Recently, much effort has been made to determine new methods for combined treatments with TRAIL to improve the efficacy TRAIL-based therapeutic approaches. Hetschko and others demonstrated that TRAIL-induced apoptosis could be enhanced in both TRAIL-sensitive and TRAIL-resistant glioma cell lines by combined treatment with proteasome inhibitors. The role of DR5 upregulation is implicated in this TRAIL sensitization [15]. Subsequent studies have confirmed that a combination of the proteasome inhibitor bortezomib with sTRAIL can overcome TRAIL-resistance in vitro in a wide variety of cancer cells [22–25] including glioma [26]. In addition, it has been reported that resistance of other cancer cells to sTRAIL can also be overcome by treatment with full size TRAIL expressed on the cell surface [27, 28]. Although the therapeutic efficacy of NSCs modified to secrete TRAIL was previously established in vitro and in vivo [29], nothing is known about the efficacy of NSCs expressing mTRAIL. In this study, we took a novel approach using NSCs-mTRAIL alone and in combination with proteasome inhibitors for treatment of intracranial experimental glioma in mice, which to our best knowledge has never been examined in vivo before.
2. MATERIALS AND METHODS
2.1. Reagents
DMEM, antibiotic-antimycotic were purchased from Invitrogen (Carlsbad, CA). Trypsin-EDTA was purchased from Mediatech Inc. (Manassas, VA). Bortezomib (LC laboratories, Woburn, MA), epoxomicin (EMD, Gibbstown, NJ), recombinant human SuperKillerTRAIL (sTRAIL) (Alexis Biochemicals, Lausen, Switzerland), rabbit anti-cleaved caspase-3 antibody (Cell Signaling, Danvers, MA), mouse anti-human DR5 conjugated with phycoerythrin (PE) antibody (eBioscience, San Diego, CA), anti-rabbit AlexaFluor 647, lipofectamin 2000, competent One Shot Top10 cells (Invitrogen, Carlsbad, CA), laminin (Sigma-Aldrich, St. Louis, CA), human neural progenitor cells (RenCells), RenCell media, human epidermal growth factor (EGF) (Millipore, Temecula, CA), human basic fibroblast growth factor (bFGF) (Sigma-Aldrich, St. Louis, Mo), Caspase-Glo 3/7 kit and CytotoxOne Homogeneous membrane integrity Assay kit (Promega, Madison, WI), fluoromount-G (Southern Biotech, Birmingham, Alabama). Quantikine ELISA based immunoassay of human TRAIL was obtained from R&D Systems (Minneapolis, MN). iScript cDNA kit was purchased from Bio-Rad (Hercules, CA). Athymic nu/nu mice were purchased from Harlan Laboratories (Indianapolis, IN). Human oligodendroglioma cell line (HOG) was kindly provided by Dr. Campagnoni (UCLA Semel Institute for Neuroscience, Los Angeles, USA). Plasmid pLERNL encoding EGFRvIII was kindly provided by Dr. Furnari (University of California, San Diego, USA).
2.2. Cell cultures
It is established that gliomas expressing EGFRvIII are more invasive and more resistant to chemoradiation therapy [30]. Taking into consideration that (i) the EGFRvIII mutation is present up to 60% of EGFR-overexpressing GBMs [31, 32], and (ii) human GBMs are highly invasive and show resistance to soluble TRAIL [15, 21], we chose EGFRvIII expressing cells as a model relevant to human GBMs for our in vitro and in vivo studies. The U87MG glioma cells expressing EGFRvIII were grown in MEM media supplemented with 10% heat inactivated FBS and 200 μg/ml of geneticin. Neural progenitor cells (NSCs) were grown on laminin coated plates in RenCell media supplemented with 20 ng/ml of bFGF and 20 ng/ml of EGF. Accutase was used to disintegrate cell monolayer for their passaging. HOG cell line was transfected with a plasmid pLERNL encoding EGFRVIII using lipofectamin 2000 and selected with antibiotic geneticin to generate population of cells stably expressing EGFRvIII.
2.3. Cloning of human TRAIL
Jurkat cells were used to obtain total RNA for subsequent cloning of human full size TRAIL cDNA. Total RNA was obtained from 1×106 cells and converted in cDNA using iScript cDNA kit. The cDNA for full size TRAIL was generated by PCR using following primers: forward 5′-GCACGTCGACCAGGATCATGGCTATGATGG-3′ and reverse 5′-CGTGAGCGGCCGCCAGGTCAGTTAGCCAACT-3′ [33]. Resulted PCR product was amplified with pair of primers for its directional topo cloning in pLenti6/V5 vector: forward 5′-CACCATGGCTATGATGGAGGTCCAG-3′ and reverse 5′-CAG TTAGCCAACTAAAAAGGCCCC-3′. Obtained cDNA was sub-cloned into pLenti6/V5 vector per manufactures recommendation. Chemically competent cells were transformed and bacterial clones were screened by PCR for the correct size insert. In-house sequencing of selected clones was performed using CMV forward 5′-CGCAAATGGGCGGTAGGCGTG-3′ and V5 reverse 5′-ACCGAGGAGAGGGTTAGGGAT-3′ primers provided by manufacturer.
2.4. Generation of lentiviral particles
HEK293T cells (ATCC) were transfected with a mixture of plasmids encoding β-galactosidase protein (control) or TRAIL, viral gag-, pol- and VSV-G envelope protein using lipofectamine 2000. After 48 hours, supernatants were collected, centrifuged and filtered for subsequent infection of NSCs carried out for 6 hours. NSCs, which stably incorporated TRAIL or β-galactosidase cDNAs were selected with the antibiotic blasticidin.
2.5. Analysis of TRAIL and TRAIL receptor’s expression
Expression profile of mRNA for TRAIL receptors in glioma cells, primary GBM tissues, or in U87-EGFRvIII xenograft tissues were evaluated by PCR or qPCR using a set of previously published primers [34]. Expression of DR5, on the surface of control and 24 hours bortezomib treated U87-EGFRvIII glioma cells, was analyzed using isotype control mouse IgG1-PE and anti-human DR5-PE antibody by flow cytometry. In order to determine level of TRAIL protein expression, control and modified NSCs cells (1×106) were plated in a T25 flask. After 48 hours, supernatant and cells were collected and processed for measurement of TRAIL protein using quantitative sandwich enzyme immunoassay technique (kit from R&D Systems). All measurements were performed per manufacturer’s recommendations.
2.6. Cytotoxicity assay
Control and mTRAIL expressing NSCs, U87 or HOG glioma cells were plated at 5 ×103 cells per well in 96 well plates. The following day, media was replaced with fresh media containing sTRAIL alone or in combination with proteasome inhibitors in glioma cells or with media containing bortezomib in NSCs. After 24 hours, a cytotoxicity assay was carried out using CytotoxOne Homogeneous membrane integrity assay kit according to the manufacturer’s recommendations and measured using Tecan Safari 2 microplate reader.
2.7. Immunocytochemistry
U87-EGFRvIII cells alone or in co-culture with NSCs were grown on glass cover slips. Methanol-fixed cells were stained for human cleaved caspase-3 using rabbit polyclonal antibodies and goat anti-rabbit AlexaFluor 647 secondary antibodies at dilution 1:500. Cover slips were mounted in fluoromount-G containing 1 μg/ml DAPI. Cleaved caspase-3 expression was analyzed using Olympus IX70 inverted microscope and MetaMorph software. Flash frozen brain tumor tissues were cut to a thickness of 8 μm and stained with hematoxylin and eosin for their histological evaluation.
2.8. Flow cytometry
U87-EGFRvIII cells expressing GFP were co-cultured with control NSCs or NSCs expressing mTRAIL (NSCs-mTRAIL). After for 48 hours cells were collected, fixed, permeabilized using BD cytotox/cytoperm kit and stained with rabbit antibodies raised against cleaved caspase-3 and goat anti-rabbit AlexaFluor 647 secondary antibodies at dilution 1:500. Samples were analyzed using BD FACSCanto flow cytometer and FACSDiVa™ software.
2.9. Caspase-3/-7 activity assay
U87-EGFRvIII cells were plated at density of 10×103 cells per well in 96 well plate. Next day, 10×103 of control NSCs and NSCs-mTRAIL or sTRAIL were added to each well. Caspase-3/-7 activity was measured at 24 hour time point by adding equal volume of Caspase-Glo 3/7 substrate in each well per manufacture’s recommendations. Luciferase activity was recorded in each sample using GliomaX Lumonometer 20/20. Background of caspase-3/-7 activity in media and in control NSCs and NSCs-TRAIL was subtracted from each experimental measurement and from the data collected from co-cultures of U87 glioma cells and NSCs, respectively.
2.10. Animal studies
All animals were maintained and cared for in accordance with the Institutional Animal Care and Use Committee protocol and regulations. The animals used in the experiments were 6–8 weeks old male athymic nu/nu mice. Mice were anaesthetized with an intraperitoneal injection of ketamine hydrochloride (25 mg/ml)/xylazine (2.5 mg/ml) cocktail.
To establish intracranial tumors, a midline cranial incision was made and a right-sided burr hole was placed 2 mm lateral to the sagittal sinus and approximately 2 mm superior to the lambda. Animals were positioned in stereotactic frame and a Hamilton needle was inserted into the burr hole and advanced 3 mm. Intracranial penetration was followed by injection of 1×105 U87-EGFRvIII glioma cells in 2.5 μl of sterile PBS. In one set of experiments, U87-EGFRvIII cells were injected alone or mixed with 0.5 ×105 or 1×105 NSCs. Two weeks later, bortezomib (in sterile PBS or respective amount of solvent) was injected into the tail vein at 0.8 mg/kg (equal to 20 μg per 25 g of animal weight) twice a week for of four weeks. All mice were followed to assess survival. Animal’s brains were harvested for microscopic analysis.
In order to assess the therapeutic effect of NSCs-mTRAIL on established tumors, 5×105 control NSCs or NSCs-mTRAIL were injected in the same burr hole 7 days after inoculation of 1×105 U87-EGFRvIII glioma cells. Bortezomib injections were performed as described above. All mice were followed to assess survival. The brains were harvested for analysis of RNA or frozen sectioning.
2.11. Statistical analysis
The differences between groups were evaluated by calculating Student’s t-value, one way ANOVA with post-hoc comparison Tukey’s test. For the in vivo survival data, a Kaplan-Meier survival analysis was used and statistical analysis was performed using a Logrank test. P< 0.05 was considered statistically significant.
3. RESULTS
3.1. Sensitivity of glioma cell lines to sTRAIL, proteasome inhibitors and combination treatment
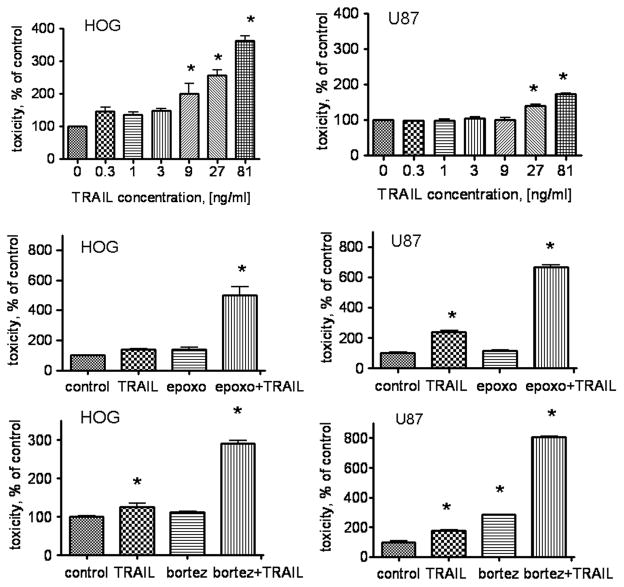
We investigated the sensitivity of two glioma cell lines, U87 and HOG (both expressing EGFRvIII), to hrTRAIL. Fig. 1 (upper panel) shows that sTRAIL causes toxicity in the HOG cell line at a concentration of 0.3 ng/ml (146±11.5 % of untreated control, p<0.001, n=3). In U87 cells, a similar effect was observed at an sTRAIL concentration of 27 ng/ml (139± 4 percent of untreated control, p<0.001, n=3). The HOG cell line demonstrated a substantial increase in toxicity (361± 14.7% of control) compared to U87 cell line (172± 3.2% of control) at the maximum concentration of sTRAIL used in this assay (81ng/ml).
Fig. 1. Combined cytotoxic effect of sTRAIL and proteasome inhibitors in human glioma cell lines.
U87 and HOG glioma cell lines were treated with hrTRAIL for 24 hours followed by measurement of cytotoxicity (upper panel). The combined cytotoxic effects of epoxomicin and bortezomib and sTRAIL are presented in middle and lower panel, respectively. Data are shown as mean±SD, * p<0.05.
In response to the proteasome inhibitor epoxomicin, the HOG cell line showed only a slight (39% of control cells) increase in toxicity, whereas U87 cells did not respond at all. However, the combination of epoxomicin at 50nM and sTRAIL at low concentration (1 ng/ml) drastically increased the toxicity in HOG cell line in comparison with sTRAIL or epoxomicin alone (499± 56 % of untreated control, p<0.001, n=3). No combined cytotoxic effect of sTRAIL at 1 ng/ml and epoxomicin was observed in U87 cells (data not shown). However, when we used the minimum effective dose of sTRAIL (27 ng/ml) in combination with epoxomicin, significant increase in cytotoxicity was observed (669± 32 % of untreated control, p<0.001, n=3) (Fig. 1 middle panel). Cytotoxic effect of combined treatment significantly exceeded the effect observed with each of these agents alone (290±20 and 808± 8 % of untreated control, p<0.001 for HOG and U87 glioma cell lines respectively) (Fig 1 lower panel)
These data demonstrate that the U87 glioma cell line is more resistant to sTRAIL than HOG. However, combined treatment with sTRAIL and proteasome inhibitors overcome this resistance in U87 glioma cells.
3.2. Proteasome inhibitors cause increase in DR5 expression in glioma cell lines
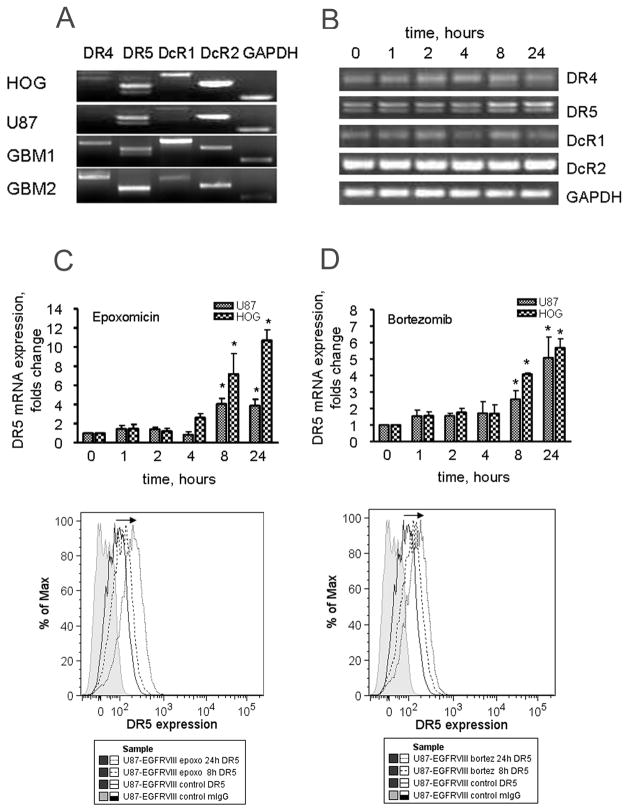
Next, we investigated if the combined effect of proteasome inhibitors and sTRAIL is mediated by an increase in the expression of TRAIL receptors in HOG and U87 glioma cell lines. Fig 2A shows the expression profile of mRNA for TRAIL receptors in U87 and HOG glioma cell lines as wells as in two GBM samples. The expression of DR5 and DcR2 mRNA prevailed in both U87 and HOG glioma cell lines. In addition, the HOG cell line showed a relatively high expression of DcR1. The level of mRNA expression for DR4 was weak in both cell lines. In primary GBMs, the expression of mRNA for DR4, DR5, DcR1 and DcR2 was detected at various levels. Both, U87 and HOG cell lines responded to treatment with epoxomicin at a concentration 50 nM and bortezomib at 25 nM by time dependent up-regulation of DR5. This was not observed in DR4, DcR1 and DcR2 receptors. Fig 2B shows a sample PCR of mRNA expression of TRAIL receptors in HOG cell line treated with epoxomicin. Changes in the mRNA level and DR5 protein expression on the cell surface in response to proteasome inhibitors were confirmed by quantitative PCR and flow cytometry. Both, U87 and HOG glioma cell lines responded to treatment with epoxomicin (Fig 2C) and bortezomib (Fig 2D) by up-regulation of mRNA (upper panel) for DR5 and the increase in the expression DR5 on the cells surface of U87 glioma cells (lower panel). The mRNA levels for DR5 were significantly increased at 8 hours and continued to increase at 24 hours post-treatment. The increase in expression of DR5 on the cell surface (expressed as percent of positive cells) in response to proteasome inhibitors was recorded at 8 hours and was 2.7 and 3 times higher in bortezomib and epoxomicin treated cells than in control cells at 24 hours, respectively.
Fig. 2. Expression of TRAIL receptors in glioma cell lines. Response to proteasome inhibitors.
A. Expression of mRNA for TRAIL receptors in U87, HOG glioma cell lines and samples from patients with primary GBM. B. Changes in expression of TRAIL receptors at the mRNA level in response to treatment with proteasome inhibitors was evaluated by PCR. C and D. Upper panels show changes in the expression mRNA of DR5 in U87 cells treated with proteasome inhibitors, evaluated by qPCR using pairs of specific primers. Lower panels show changes in the expression of DR5 on the surface of U87 cells treated with epoxomicin (C) and bortezomib (D) respectively, evaluated by flow cytometry. Data are shown as mean±SD, * p<0.05.
3.3. Expression of mTRAIL by NSCs
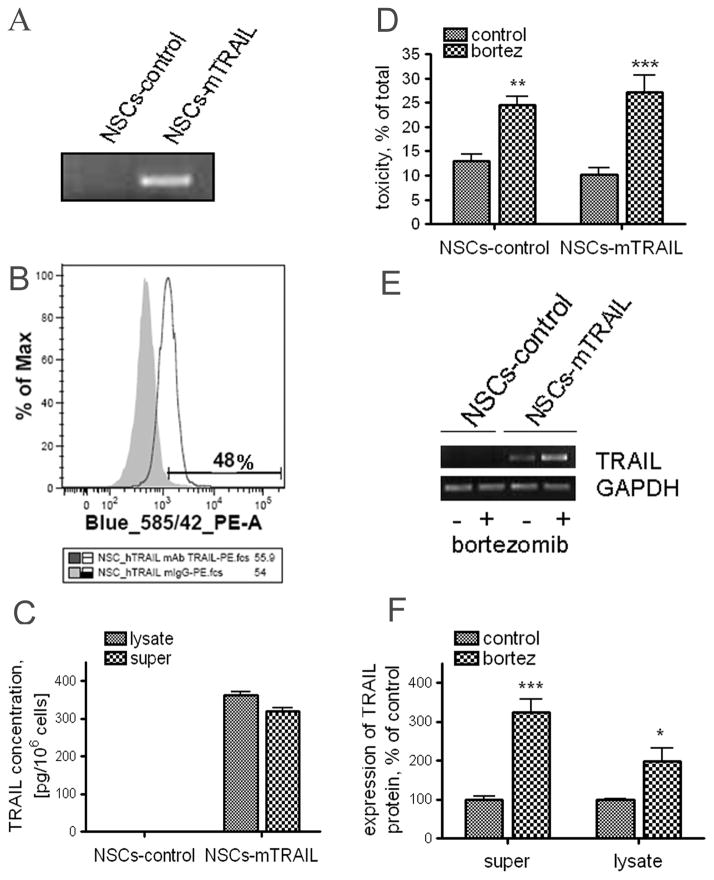
In order to achieve stable expression of mTRAIL in NSCs, cells were infected with VSV-G pseudotyped lentivirus encoding either LacZ gene or full-size TRAIL. After infection, NSCs expressing mTRAIL were further selected with antibiotic blasticidin. The presence of TRAIL transcripts in NSCs was confirmed by PCR reaction with TRAIL specific primers. Fig 3A shows the absence of TRAIL in control NSCs, whereas a band corresponding to full size TRAIL was detected in NSCs-mTRAIL cells. Next, we evaluated the expression of mTRAIL on the surface of NSCs by flow cytometry using anti-TRAIL-PE antibody. A detectable expression of TRAIL on the cell surface was revealed in about 50% of NSCs (Fig. 3B). We also estimated the actual amount of TRAIL expressed by NSCs by quantitative ELISA. Fig. 3C shows that TRAIL is detected in cell fraction at about 340 pg per 1×106 cells and about 47% of total TRAIL (320 pg) was detected in supernatant collected over 48 hours. These experiments indicate that infection of NSCs with lentiviral particles encoded mTRAIL, followed by subsequent antibiotic selection, allowed generation of NSCs stably expressing mTRAIL. Both, control and NSCs-mTRAIL demonstrated similar sensitivity to bortezomib at concentration 25nM (Fig 3D). Intriguingly, we observed up-regulation of TRAIL expression in NSCs-mTRAIL induced by bortezomib at both mRNA and protein levels (Fig 3E, F). The increase of TRAIL expression in cell fraction was accompanied by an increase of soluble TRAIL fraction in the culture media.
Fig. 3. Expression of mTRAIL by NSCs.
A. Control and NSCs-mTRAIL were analyzed for TRAIL mRNA expression by PCR of cDNA with a pair of specific primers. B-Surface expression of mTRAIL by NSCs was evaluated by flow cytometry. C. Quantitative measurement of mTRAIL expression in NSCs was analyzed in plate ELISA. D. Effect of bortezomib on toxicity of NSCs was studied using membrane integrity permeability assay. E–F. Changes in the expression of mTRAIL in NSCs-mTRAIL cells treated with bortezomib for 24 hours at mRNA (E) and protein level (F) analyzed by RT-PCR and quantitative ELISA. Data are shown as mean±SD, * p<0.05.
3.4. NSCs-mTRAIL cause apoptosis in U87-EGFRvIII cells
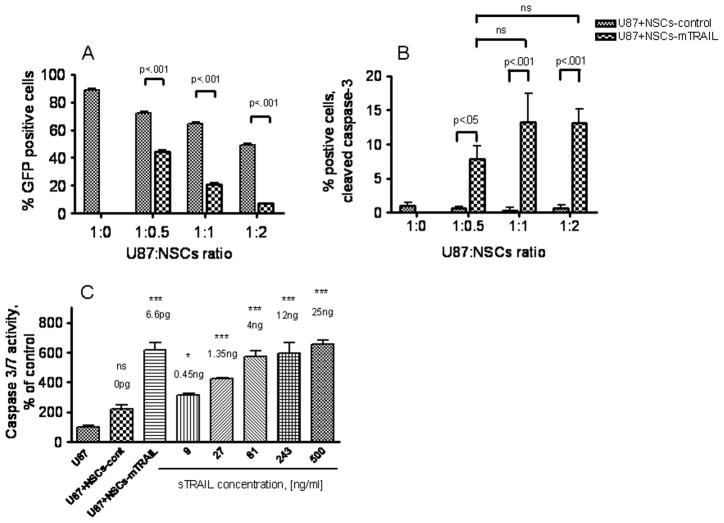
We next evaluated the therapeutic potential of NSCs-mTRAIL on glioma cells in co-culture experiments. For all following in vitro and in vivo experiments, U87-EGFRvIII glioma cell line was chosen because of its greater resistances to sTRAIL in comparison with HOG cells (Fig 1). U87-EGFRvIII cells previously modified to express GFP [32] were co-cultured with control or NSCs-mTRAIL at various ratios for 48 hours. In order to distinguish between U87-EGFRvIII cells and NSCs, only the GFP positive fraction of cells was analyzed for the expression of cleaved caspase-3 by flow cytometry (Fig 4). Interestingly, we observed a drastic decrease in the GFP signal in U87-EGFRvIII cells co-cultured with NSCs-mTRAIL, which indirectly indicates an apoptotic process in glioma cells (Fig 4A). The number of GFP positive U87-EGFRvIII glioma cells in co-culture with NSCs-mTRAIL was 1.5, 3 and 7 times less than the number of GFP positive glioma cells in co-culture with control NSCs at 1:0.5, 1:1 and 1:2 glioma cell to NSCs ratios, respectively. In GFP positive fraction of U87-EGFRvIII cells, the number of cleaved caspase-3 positive cells was about 8 to 13 times higher than that in U87-EGFRvIII cells cultured alone or in presence of control NSCs at various glioma-to-NSCs ratios (Fig 4B). These experiments indicate that NSCs- mTRAIL possess therapeutic potential toward U87-EGFRvIII glioma cell line defined here as loss of GFP signal in glioma cells and the expression of cleaved caspase-3.
Fig. 4. Therapeutic effect NSCs-mTRAIL in U87-EGFRvIII glioma cells line.
U87 cells expressing GFP were co-cultured with control NSCs or NSCs-mTRAIL at various ratios. At 48 hours, U87 cells were analyzed for: A. GFP expression; B. cleaved caspase-3 expression by flow cytometry. C. Activity of caspase-3/-7 in U87 cells co-cultured with NSCs-mTRAIL or treated with sTRAIL. Data are shown as mean±SD, n=3.
Next, we directly compared the effects of mTRAIL expressed by NSCs and sTRAIL in inducing apoptosis in U87-EGFRvIII glioma cells. For that reason, the activity of caspase-3/-7 was measured in U87-EGFRvIII cells treated for 24 hour either with sTRAIL or control NSCs and NSCs-mTRAIL. Fig. 4C shows that 1×104NSCs-mTRAIL (which equals to 6.6 pg of TRAIL protein measured by quantitative TRAIL ELISA, Fig 3C) were as efficient as 12 ng of sTRAIL in activating caspase-3/-7 in glioma cells. These data indicate that about 1800 times less mTRAIL expressed on the cell surface is required to induce apoptosis in glioma cells in comparison with sTRAIL.
All together, our data demonstrate that NSCs-mTRAIL are potent cell-based apoptosis-inducing reagent in glioma model cell line.
3.5. Combined therapeutic effect of NSCs-mTRAIL and bortezomib in vitro
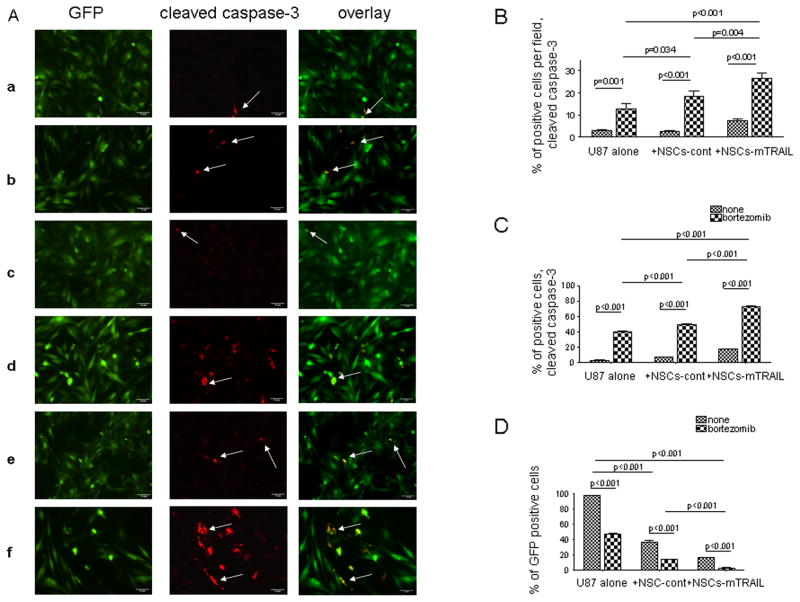
Next, we investigated whether the combined treatment of NSCs-mTRAIL and bortezomib would result in enhanced apoptosis in U87-EGFRvIII glioma cells. For these experiments, U87 cells were co-cultured with NSCs at 1:1 ratio for 18 hours followed by 24 hours treatment with 25nM bortezomib. The presence of cleaved caspase-3 in the GFP-positive fraction of U87 cells was analyzed. The number of cleaved caspase-3 positive cells per several fields of view was calculated and divided by the total number of cells in each field (Fig 5A and B). A very low number of cleaved caspase-3 positive cells were detected in culture of U87 glioma cells alone (panel a in Fig 5A). Treatment with bortezomib significantly increased (about four times) the number of apoptotic U87 cells (panel b in Fig 5A and Fig 5B). Note that U87 glioma cells were not affected by control NSCs (panel c in Fig 5A and Fig 5B), however the number of apoptotic cells was significantly increased after the treatment with bortezomib (panel d in Fig 5A and Fig 5B). Co-culture with NSCs-mTRAIL cells alone tripled the number of apoptotic U87 glioma cells (panel e in Fig 5A and Fig 5B). Lastly, the combined treatment of glioma cells with NSC-mTRAIL and bortezomib together significantly increased a number of cleaved caspase-3 positive cells (panel f in Fig 5A and Fig 5B). These data were further confirmed by flow cytometry (Fig 5C–D). Importantly, treatment of U87 glioma cells with bortezomib alone resulted in a significant loss of the population GFP positive glioma cells (Fig. 4D). The effect of bortezomib was amplified in glioma cells co-cultured with control NSCs and NSCs-mTRAIL. Thus, our in vitro experiments demonstrated the ability of NSCs-mTRAIL alone and in combination with bortezomib to cause the apoptosis in U87 glioma cell line. Therefore, in our following experiments we set to investigate if the combined treatment of NSCs expressing mTRAIL and bortezomib could improve the survival of mice with intracranial glioma xenografts.
Fig. 5. Co-culture of NSCs-mTRAIL and U87 cells treated with bortezomib results in apoptosis in glioma cells.
A. NSCs were co-cultured overnight with U87 (GFP) cells followed by treatment with bortezomib and stained to detect cleaved caspase-3. Images of GFP (green) and cleaved caspase-3 expression (red) were overlayed. Arrows indicate cleaved caspase-3 positive cells in the GFP-positive fraction of U87 cells. B. The number of cleaved caspase-3 positive cells was counted in 5 fields and equilibrated per total cell number present in field. C. U87(GFP) cells alone or in co-culture with NSCs were treated with bortezomib and analyzed for the number of cleaved caspase-3 positive or GFP positive cells by flow cytometry. D. Changes in the population of GFP-positive U87 cells treated bortezomib and NSCs-mTRAIL. Data are presented as mean± SEM.
3.6. Combined therapeutic effect of NSCs-mTRAIL and bortezomib on survival of mice with intracranial glial tumors
First, we evaluated the toxicity of bortezomib in animals at 0.2–1.6 mg/kg of body weight. Bortezomib at 0.8 mg/kg (20 μg per 25 g of animal weight) was chosen for all in vivo experiments. At this dose bortezomib did not negatively affect the survival of animals and no changes in the body weight were noted in comparison with animals injected with PBS (data not shown). Treatment of tumor bearing animals with bortezomib alone at 0.2–0.8 mg/kg did not improve animal survival. Thus, we next questioned if bortezomib can produce therapeutic effect in combined therapy with NSCs-mTRAIL in animals with intracranial glial tumors.
Co-injection model
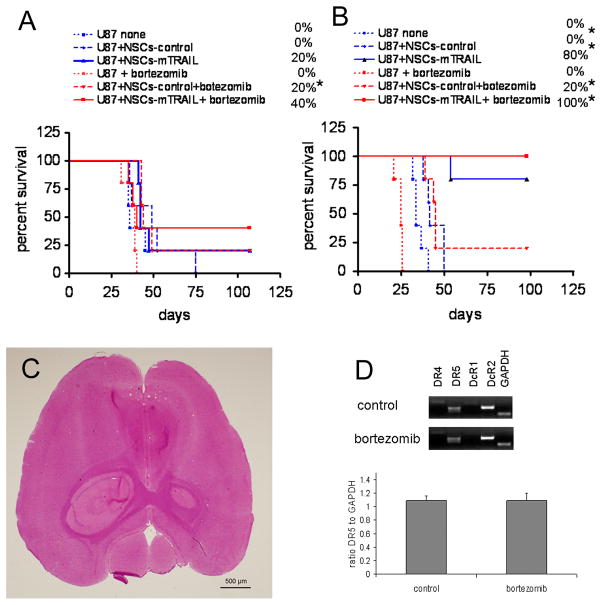
In the first experiment, U87 glioma cells were co-injected with NSCs at a ratio of 1:0.5. Animals treated with control or NSCs-mTRAIL demonstrated an improvement in survival of the animals versus the PBS only group. Specifically, in the NSCs-mTRAIL group, 20% of animals survived over 100 days. Treatment with bortezomib had no effect on animal survival in the PBS group, but 20 and 40% animals in control NSCs and NSCs-mTRAIL groups respectively survived over 100 days (Fig 6A). Similar improvement in survival of animals treated with bortezomib was observed in the experiment where glioma cells were co-injected with NSCs at a 1:1 ratio (Fig. 6B). In NSCs-mTRAIL group, 80% of animals survived over 100 days. Again, treatment with bortezomib did not result in improvement of survival in the PBS group of animals, but 20% of animals in control NSCs group survived over 100 day period. A similar 20% improvement was observed in the NSCs-mTRAIL group treated with bortezomib, thus increasing survival of animals from 80% to 100% (Fig. 6B). Again, none of the survived animals showed any sign of developing tumors as evaluated by H&E staining (Fig. 6C).
Fig. 6. The effect of combined therapy of NSCs-mTRAIL and bortezomib on the survival of mice with intracranial glioma in a co-injection model.
A. U87 cells (1×105) were co-injected with 5×104 of control NSCs or NSCs-mTRAIL. B. U87 cells alone (1×105) were co-injected with equal amount of control NSCs or NSCs-mTRAIL (n=5). C. A representative microphotograph of an 8μm tissue section from a surviving animal that received NSCs-mTRAIL therapy. H&E staining. D. Profile of mRNA expression for TRAIL receptors from tumor tissues of control and bortezomib treated animals. * p<0.05.
Taken together, these survival experiments demonstrate that (i) the effect of NSCs-mTRAIL on the survival of nude mice with intracranial glioma xenografts is specific and concentration dependent; (ii) the effect of bortezomib is additive to the treatment of animals with NSCs and independent of effect of mTRAIL.
Based on the obtained data, we questioned if bortezomib up-regulated the expression of DR5 in vivo. For that reason, mRNA obtained from the tumors of control animals and treated with bortezomib was analyzed for TRAIL receptors expression profile (Fig. 6D). The expression of TRAIL receptors from control and bortezomib treated glioma was similar to that in cultured U87-EGFRvIII cells (Fig. 2A). Specifically, no change in the expression of DR5, which is known to interact with mTRAIL, was observed in tumor samples from mice treated with bortezomib (Fig. 6D).
Established tumors
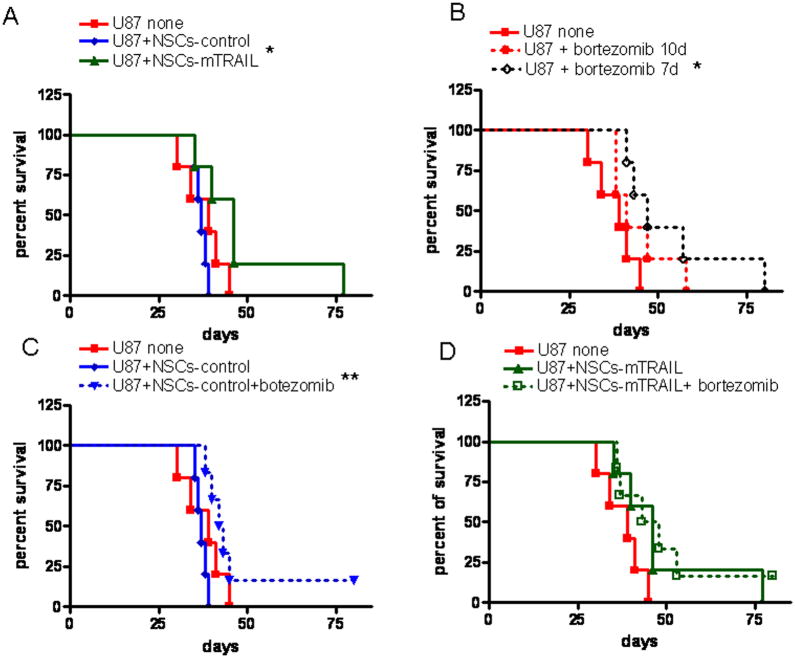
To investigate the effect of NSCs-mTRAIL and bortezomib in the setting of an established intracranial xenograft, 1×105 of U87-EGFRvIII glioma cells were injected into the brain of nude mice as described above. PBS or NSCs (5×105) were inoculated into the same burr hole 7 days later (n= 5 per group) followed by treatment with bortezomib in another 3 days. Based on obtained data, we hypothesized that the therapeutic effect of bortezomib could be achieved when injected early in tumor development. Therefore, in an additional PBS group, treatment with bortezomib was initiated in 7 days after inoculation of glioma cells. Mice in the NSCs-mTRAIL group displayed an increased median survival in comparison with the control NSCs group (37 days vs 46 days, p=0.027) and 20% of mice survived for 77 days (Fig 7A). Treatment with bortezomib improved the median survival of animals in all groups. Moreover, mice in the group in which bortezomib injection was initiated at day 7 showed additional improvement in median survival from 39 to 47 days (p=0.0256)(Fig 7B). The effect of NSCs-mTRAIL was not further amplified in bortezomib treated mice, however, bortezomib prolonged the survival of animals in all experimental groups (Fig. 7C–D). Thus, these experiments confirmed results obtained in co-injection model and demonstrated the improvement in survival of animals treated with NSCs-mTRAIL.
Fig. 7. The effect of combined therapy of NSC-mTRAIL and bortezomib on the survival of mice with intracranial glioma in established tumor model.
A. The effect of NSCs-mTRAIL on the survival of animals. B. The effect of bortezomib on the survival of tumor bearing animals. C. The effect of bortezomib on the survival of animals treated either with control NSCs or D. NSCs-mTRAIL. * p<0.05, ** p<0.005.
4. DISCUSSION
Resistance of human GBMs to radiation and chemotherapy treatments (1, 34, 35) demands development of novel approaches that increase the sensitivity of glioma cells to apoptosis. Human primary astrocytoma cells, WHO grade I to IV, have been shown to be resistant to sTRAIL but demonstrate increased sensitivity after treatment with bortezomib [26]. Moreover, several studies indicate that resistance of other cancer cells to sTRAIL can also be overcome by treatment with full size TRAIL expressed on the cell surface [27, 28]. In this study, we investigated the effect of NSCs expressing mTRAIL alone or in combination with proteasome inhibitors on glioma cells in vitro and in vivo.
In our study, two glioma cell lines demonstrated different sensitivity to sTRAIL in agreement with previous studies [26, 36]. RNA interference has shown that proteasome inhibitors are able to reactivate sensitivity of glioma cells in vitro to sTRAIL by up-regulation of DR5 [15]. Similarly, in our study treatment of glioma cells with proteasome inhibitors up-regulated the expression of DR5 at both mRNA and protein levels and drastically enhanced the response of glioma cells to both sTRAIL and mTRAIL.
Neural and mesenchymal stem cells have been explored as a carriers of therapeutic TRAIL for treatment of glial tumors [29, 37–40]. In this study, we engineered NSCs to express full size TRAIL on the cell surface. The expression of TRAIL in NSCs was confirmed on mRNA and protein level and was in agreement with previously published data [36]. Due to the preferential expression of DR5 in U87 glioma cells line, the interaction of mTRAIL with DR5 most likely accounts for the therapeutic effect of NSCs-mTRAIL on U87 glioma cells in vitro and in vivo. Our data is in agreement with previously published reports regarding the ability of mTRAIL to delay the progression of glial and other tumors [27, 28, 39, 41]. Moreover, direct comparison of ability of soluble and mTRAIL to activate apoptosis in U87 glioma cells demonstrated high potency of mTRAIL expressed by NSCs over the sTRAIL. In support, it has been shown that resistance of prostate cancer cells to sTRAIL is overcome after expression of full-size TRAIL in cancer cells [27]. CD34+ cells expressing mTRAIL demonstrated cytotoxicity against tumor cells that were resistant to soluble TRAIL [28]. One possibility is that stem or other cell types expressing TRAIL on the surface are highly avid, and this high avidity might be crucial for activation its receptors. Recently, Kohlhaas and co-authors reported that no receptor mediated endocytosis is required for TRAIL-induced apoptosis. Moreover, blockade of receptor internalization amplified TRAIL-induced apoptosis signal [42]. It is likely that TRAIL expressed on the surface of NSCs may generate amplified apoptotic signal due to the absence or lower internalization in comparison with sTRAIL. Interestingly, we observed upregulation of TRAIL expression on the mRNA and protein levels in NSCs-mTRAIL treated with bortezomib. This effect, together with upregulation of DR5 in U87 glioma cells, can also contribute to enhanced toxicity in glioma cells in combined treatment. However, the exact mechanism by which mTRAIL overcomes the resistance of cancer cells to soluble TRAIL remains to be elucidated.
Analysis of TRAIL receptor expression in primary GBMs found that DR4 and DR5 are expressed in 75% and 95% of tumor samples [43]. In our study, the expression of DR5 at the mRNA level surpassed DR4 expression in glioma cell lines, but transcripts for both DR4 and DR5 were found in primary GBM samples. Taking in consideration that in contrast to sTRAIL, mTRAIL can activate both DR4 and DR5 [16, 17], further investigations of therapeutic effect of NSCs-mTRAIL alone and in combination with other therapies on primary GMB samples in vivo are warranted. At this point however, very little is known about the effect of combined therapy mTRAIL and bortezomib in vivo. In our study, NSCs-mTRAIL alone significantly improved the survival of animals in both co-injection and established intracranial glioma models. However, studies optimizing the level of expression of mTRAIL on the surface of NSCs, time of inoculation and number of injected NSCs in established glioma model are warranted for further investigations of therapeutic applicability of NSCs-mTRAIL.
Originally, bortezomib (Velcade) was approved by Food and Drug administration for treatment of multiple myeloma [44]. Currently, there are completed and ongoing clinical trails investigating combined therapy of bortezomib with temozolomide [45] or avastin in patients with a new or recurrent malignant glioma. No effect of bortezomib on tumor growth in experimental flank glioma and renal cell adenocarcinoma models has been previously reported [46, 47], but it improved the survival of rats with established intracranial gliosarcoma [48]. In our study, mice with established intracranial xenografts demonstrated an increase in median survival only when therapy with bortezomib was initiated on day 7 following inoculation of glioma cells into the brain. Therapy initiated on day 10 or 14 was beneficial only to mice treated with control or mTRAIL expressing NSCs and resulted in 20% increase of survival in both groups. The fact that treatment with bortezomib resulted in therapeutic effect suggests that bortezomib was able to reach tumor site. We observed no increase in DR5 expression in tumor samples from mice treated with bortezomib, which could be accounted for low intra-tumoral concentration of bortezomib. In support, low intra-tumoral and normal brain concentration of 14C-labeled bortezomib (0.074 ± 0.015 nM and 0.021 ± 0.02 nM, respectively) has been found in rats with intracranial gliosarcoma 1 hour after systemic administration at a dose of 0.2 mg/kg [48]. It also can not be excluded that the expression of DR5 in tumor cells from the brain samples was not affected by bortezomib treatment at the time when samples were harvested for analysis. Therefore, the kinetics of DR5 expression and other downstream effectors [26, 36, 49, 50] involved in the increased sensitivity glioma cells to TRAIL in brain tumors from animals treated with bortezomib warrants further investigation. The absence of up-regulation of DR5 in vivo may be responsible in part for the absence of TRAIL specific effect of bortezomib, which in contrast to in vitro data, was rather additive in animals bearing intracranial gliomas treated with NSCs. Perhaps an intra-tumoral route of administration may overcome bortezomib’s limited penetration of the blood-brain barrier, toxicity and enhance its local concentration hence improving its therapeutic effects.
In summary, our data indicate that therapy of glioma cells with NSCs-mTRAIL is potent cell based approach for treatment of U87 glioma in vitro and in vivo. Soluble TRAIL as well as NSCs expressing mTRAIL generated a synergistic effect in combined therapy with proteasome inhibitor bortezomib in vitro. Treatment with NSCs-mTRAIL significantly improved the survival of animals with intracranial glioma. In vivo, bortezomib failed to up-regulate expression of DR5, and improved survival of animals without restriction to NSCs-mTRAIL treated groups. Our in vitro data indicate that TRAIL expressed on the cell surface of NSCs is a more potent reagent than soluble TRAIL. Taking in consideration that a limited number of NSCs expressing either soluble or membrane-bound TRAIL can be injected into the mouse brain, this approach using NSCs-mTRAIL seems to be preferential to soluble TRAIL. These data suggest that further search for agents that increase the sensitivity of glioma cells to mTRAIL in vivo is necessary in order to further validate this approach.
Research highlights.
NSCs engineered to express membrane-bound TRAIL induced apoptosis in glioma cells.
Bortezomib enhanced response of glioma cells to NSCs expressing TRAIL.
TRAIL expressed on the cell surface of NSCs is a more potent reagent than soluble TRAIL.
Bortezomib upregulated expression of DR5 in glioma cells in vitro but not in vivo.
Survival of animals with intracranial glioma was improved in combined treatment.
Acknowledgments
This research was supported by the NCI (R01CA122930, R01CA138587), the National Institute of Neurological Disorders and Stroke (U01NS069997), and the American Cancer Society (RSG-07-276-01-MGO). We thank Bart Thaci, MD for his assistance in performing animal surgery.
Footnotes
CONFLICT OF INTEREST
The authors do not have any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 3.Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 4.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J, Zhu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 7.Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 9.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 10.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 12.Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, Parney IF, Roa WH, Petruk KC. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- 13.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 14.Bouralexis S, Findlay DM, Evdokiou A. Death to the bad guys: targeting cancer via Apo2L/TRAIL. Apoptosis. 2005;10:35–51. doi: 10.1007/s10495-005-6060-0. [DOI] [PubMed] [Google Scholar]
- 15.Hetschko H, Voss V, Seifert V, Prehn JH, Kogel D. Upregulation of DR5 by proteasome inhibitors potently sensitizes glioma cells to TRAIL-induced apoptosis. Febs J. 2008;275:1925–1936. doi: 10.1111/j.1742-4658.2008.06351.x. [DOI] [PubMed] [Google Scholar]
- 16.Muhlenbeck F, Schneider P, Bodmer JL, Schwenzer R, Hauser A, Schubert G, Scheurich P, Moosmayer D, Tschopp J, Wajant H. The tumor necrosis factor-related apoptosis-inducing ligand receptors TRAIL-R1 and TRAIL-R2 have distinct cross-linking requirements for initiation of apoptosis and are non-redundant in JNK activation. J Biol Chem. 2000;275:32208–32213. doi: 10.1074/jbc.M000482200. [DOI] [PubMed] [Google Scholar]
- 17.Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, Peters N, Scheurich P, Pfizenmaier K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20:4101–4106. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- 18.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 19.Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 20.Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41–45. doi: 10.1016/s0014-5793(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 21.Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C. TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol. 2003;13:539–553. doi: 10.1111/j.1750-3639.2003.tb00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nencioni A, Wille L, Dal Bello G, Boy D, Cirmena G, Wesselborg S, Belka C, Brossart P, Patrone F, Ballestrero A. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res. 2005;11:4259–4265. doi: 10.1158/1078-0432.CCR-04-2496. [DOI] [PubMed] [Google Scholar]
- 23.Conticello C, Adamo L, Vicari L, Giuffrida R, Iannolo G, Anastasi G, Caruso L, Moschetti G, Cupri A, Palumbo GA, Gulisano M, De Maria R, Giustolisi R, Di Raimondo F. Antitumor activity of bortezomib alone and in combination with TRAIL in human acute myeloid leukemia. Acta Haematol. 2008;120:19–30. doi: 10.1159/000151511. [DOI] [PubMed] [Google Scholar]
- 24.Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CP, McConkey DJ. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res. 2005;65:4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 25.Saulle E, Petronelli A, Pasquini L, Petrucci E, Mariani G, Biffoni M, Ferretti G, Scambia G, Benedetti-Panici P, Cognetti F, Humphreys R, Peschle C, Testa U. Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis. Apoptosis. 2007;12:635–655. doi: 10.1007/s10495-006-0025-9. [DOI] [PubMed] [Google Scholar]
- 26.Koschny R, Holland H, Sykora J, Haas TL, Sprick MR, Ganten TM, Krupp W, Bauer M, Ahnert P, Meixensberger J, Walczak H. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13:3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 27.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164–172. doi: 10.1038/sj.cgt.7700420. [DOI] [PubMed] [Google Scholar]
- 28.Carlo-Stella C, Lavazza C, Di Nicola M, Cleris L, Longoni P, Milanesi M, Magni M, Morelli D, Gloghini A, Carbone A, Gianni AM. Antitumor activity of human CD34+ cells expressing membrane-bound tumor necrosis factor-related apoptosis-inducing ligand. Hum Gene Ther. 2006;17:1225–1240. doi: 10.1089/hum.2006.17.1225. [DOI] [PubMed] [Google Scholar]
- 29.Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, Yu JS. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 30.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 31.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 32.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;27:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 33.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 34.Abdollahi T, Robertson NM, Abdollahi A, Litwack G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003;63:4521–4526. [PubMed] [Google Scholar]
- 35.Balyasnikova IV, Franco-Gou R, Mathis JM, Lesniak MS. Genetic modification of mesenchymal stem cells to express a single-chain antibody against EGFRvIII on the cell surface. J Tissue Eng Regen Med. 2009;4:247–258. doi: 10.1002/term.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetschko H, Voss V, Horn S, Seifert V, Prehn JH, Kogel D. Pharmacological inhibition of Bcl-2 family members reactivates TRAIL-induced apoptosis in malignant glioma. J Neurooncol. 2008;86:265–272. doi: 10.1007/s11060-007-9472-6. [DOI] [PubMed] [Google Scholar]
- 37.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SA, Hwang SK, Wang KC, Cho BK, Phi JH, Lee JY, Jung HW, Lee DH, Kim SK. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol. 2010;13:61–69. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B, Wu X, Mao Y, Bao W, Gao L, Zhou P, Xie R, Zhou L, Zhu J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. discussion 624. [DOI] [PubMed] [Google Scholar]
- 40.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell delivered TRAIL in experimental glioma models. Cancer Res. 2010;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 41.Luetzkendorf J, Mueller LP, Mueller T, Caysa H, Nerger K, Schmoll HJ. Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J Cell Mol Med. 2010;14:2292–2304. doi: 10.1111/j.1582-4934.2009.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–12841. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- 43.Kuijlen JM, Mooij JJ, Platteel I, Hoving EW, van der Graaf WT, Span MM, Hollema H, den Dunnen WF. TRAIL-receptor expression is an independent prognostic factor for survival in patients with a primary glioblastoma multiforme. J Neurooncol. 2006;78:161–171. doi: 10.1007/s11060-005-9081-1. [DOI] [PubMed] [Google Scholar]
- 44.Joazeiro CA, Anderson KC, Hunter T. Proteasome inhibitor drugs on the rise. Cancer Res. 2006;66:7840–7842. doi: 10.1158/0008-5472.CAN-06-2033. [DOI] [PubMed] [Google Scholar]
- 45.Kubicek GJ, Werner-Wasik M, Machtay M, Mallon G, Myers T, Ramirez M, Andrews D, Curran WJ, Jr, Dicker AP. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2009;74:433–439. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labussiere M, Pinel S, Delfortrie S, Plenat F, Chastagner P. Proteasome inhibition by bortezomib does not translate into efficacy on two malignant glioma xenografts. Oncol Rep. 2008;20:1283–1287. [PubMed] [Google Scholar]
- 47.Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, Takeda K, Smyth MJ, Murphy WJ, Sayers TJ. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100:649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson JJ, Bowers G, Zhang Z. Proteasome inhibitor therapy in a brain tumor model. In: Adams J, editor. Cancer Drug Discovery and Development: Proteasome Inhibitors in Cancer Therapy. Totowa (NJ): Humana Press, Inc; 2004. pp. 161–170. [Google Scholar]
- 49.Tamm I. AEG-35156, an antisense oligonucleotide against X-linked inhibitor of apoptosis for the potential treatment of cancer. Curr Opin Investig Drugs. 2008;9:638–646. [PubMed] [Google Scholar]
- 50.Oltersdorf T, Elmore SW, Shoemaker A, Armstrong R, Augeri D, Belli B, Bruncko M, Deckwerth T, Dinges J, Hajduk P, Joseph M, Kitada S, Korsmeyer S, Kunzer A, Letai A, Li C, Mitten M, Nettesheim D, Ng S, Nimmer P, O’Connor J, Oleksijew A, Petros A, Reed J, Shen W, Tahir S, Thompson C, Tomaselli K, Wang B, Wendt M, Zhang H, Fesik S, Rosenberg S. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]