Abstract
The adverse skeletal effects of glucocorticoid excess are due to increased osteoclast survival, decreased production of osteoblasts, and increased apoptosis of osteoblasts and osteocytes, but it remains unknown which of these is the principle cause of the decrease in bone strength. Previous studies suggested that osteocytes contribute to bone strength independently of changes in bone mass. Administration of the receptor activator for nuclear factor κB ligand (RANKL) antagonist osteoprotegerin (OPG) rapidly decreases osteoclasts followed by a decrease in osteoblasts but should not affect the long-lived osteocytes. Therefore, to distinguish between glucocorticoid effects on osteoclasts, osteoblasts, or osteocytes, we administered glucocorticoids, alone or in combination with OPG with the fragment crystallizable region of Ig heavy chains (OPG-Fc), to mice. The suppressive effect of glucocorticoids on spinal bone mineral density, cortical thickness, and strength was prevented by OPG-Fc. OPG-Fc, with or without glucocorticoids, profoundly reduced osteoclasts, osteoblasts, and bone formation. Unexpectedly, OPG-Fc prevented the glucocorticoid-induced increase in osteocyte apoptosis and reduction in solute transport from the systemic circulation to the osteocyte-lacunar-canalicular network. The fluid in the osteocyte-lacunar-canalicular network was inversely related to osteocyte apoptosis and directly related to bone mineral density. Consistent with the in vivo findings, Both OPG-Fc and OPG decreased glucocorticoid-induced apoptosis of MLO-Y4 osteocytic cells. OPG can also bind and antagonizes the activity of the TNF-related apoptosis-inducing ligand (TRAIL), but glucocorticoids did not change TRAIL expression, and knockdown of TRAIL did not prevent OPG-Fc from reducing glucocorticoid-induced osteocyte apoptosis. Based on these results, we conclude that at least part of the OPG-induced preservation of bone strength is due to the maintenance of osteocyte viability and the lacunar-canalicular network.
The adverse effects of glucocorticoid excess on the skeleton have been recognized for more than 75 yr (1) and ascribed to both skeletal and extraskeletal mechanisms (2). However, it is now clear that the primary impact of excess glucocorticoids is directly on bone cells, accounting for the decrease in the production of osteoblasts and osteoclasts, an increase in osteoblast and osteocyte apoptosis, and prolongation of osteoclast lifespan (3, 4). These direct effects result in a disproportionately greater loss of bone strength than bone mass, but whether the loss of strength results from the adverse effects of glucocorticoids on all three cell types, osteoblasts, osteocytes, and osteoclasts, remains unknown.
We have previously reported that prevention of glucocorticoid-induced osteocyte apoptosis via transgenic expression of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), an enzyme that inactivates glucocorticoids, prevents glucocorticoid-induced loss of bone strength, independent of loss of bone mass (5). In addition, we showed that part of the explanation for the greater decline in bone strength than in loss of bone mass with glucocorticoid excess may be due to disruption of bone vasculature and diminished hydraulic support of bone (6). Furthermore, mice in which osteoblasts and osteocytes were shielded from glucocorticoids via cell-specific transgenic expression of 11β-HSD2 were protected from the adverse effects of glucocorticoids on vasculature volume, interstitial fluid, and bone strength. This protection may be due to preventing the adverse effects of glucocorticoids on bone angiogenesis caused by suppression of hypoxia-inducible factor 1α (HIF1α) transcription and vascular endothelial growth factor (VEGF) production in the osteoblasts and osteocytes themselves (6).
Osteoprotegerin (OPG) is a well-recognized decoy receptor for the receptor activator for nuclear factor κB ligand (RANKL), an essential factor for osteoclast development (7). OPG can also intercept and bind the TNF-related apoptosis-inducing ligand (TRAIL), which shares a 25% amino acid homology with RANKL (8). Thus, OPG acts as a soluble inhibitor of both TRAIL and RANKL. The biological importance of the former action is revealed by the evidence that OPG binding to TRAIL increases endothelial cell survival (9, 10).
Glucocorticoid administration enhances expression of RANKL while inhibiting production of OPG by stromal cells and osteoblasts in vitro (7). Blockade of RANKL using denosumab, a neutralizing monoclonal antibody against RANKL, has recently been reported to prevent glucocorticoid-induced loss of bone mass and strength in mice, but osteocyte viability and the lacunar-canalicular circulation were not examined in that report (11).
In the studies described herein, we investigated the effects of OPG administration on bone in the setting of glucocorticoid excess. We report that OPG administration prevents glucocorticoid-induced osteocyte apoptosis by a TRAIL-independent mechanism. Decreased osteocyte apoptosis was associated with preservation of the solute transport from the systemic circulation to the lacunar-canalicular system. These results provide additional insights into how RANKL inhibition prevents the loss of bone strength that results from excess glucocorticoids.
Materials and Methods
Animals
Mice were electronically tagged (Biomedic Data System Inc., Maywood, NJ) and kept in plastic cages (one animal per cage) under standard laboratory conditions with a 12-h dark, 12-h light cycle and a constant temperature of 20 C and humidity of 48%. Mice were fed a standard rodent diet (Agway RMH 3000, Arlington Heights, IL) containing 22% protein, 5% fat, 5% fiber, 6% ash, 3.5 kcal/g, 1.0 IU vitamin D3/g, 0.97% calcium, and 0.85% phosphorus with water ad libitum. The Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System approved these studies. Six-month-old C57BL/6 mice were purchased from Harlan Inc. (Indianapolis, IN). We implanted slow-release pellets (Innovative Research of America, Sarasota, FL) of placebo or 2.1 mg/kg·d of prednisolone for 28 d, a dose equivalent to 20 mg/d of prednisolone to a human according to the relationship with the metabolic weight (kilograms to the three-fourths power) (3–5, 12). We used a 90-d pellet to provide consistent prednisolone availability throughout the 28-d experiment. OPG with the fragment crystallizable region of Ig heavy chains (OPG-Fc), supplied by Amgen, Inc. (Thousand Oaks, CA), was given as sc injections at a dose of 10 μg/g, three times per week (13). There were four groups of mice (n = 9–15 per group): placebo and vehicle, OPG-Fc and vehicle, prednisolone and vehicle, and prednisolone and OPG-Fc.
Quantitative real-time PCR
Total mRNA was obtained from L5 using Ultraspec reagent (Biotecx Laboratories, Inc., Houston, TX) following instructions of the manufacturer. Equal amounts of RNA (2 μg) from each sample were reverse transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Aliquots of the cDNA were amplified by real-time PCR (RT-PCR) using TaqMan Universal PCR Master Mix (Applied Biosystems) on a 7300 Sequence Detection System (Applied Biosystems) as follows: 5 min denaturation at 95 C, 40 cycles of amplification including denaturation at 94 C for 15 sec, and annealing/extension at 60 C for 1 min. The following TaqMan primer-probe sets from Applied Biosystems were used: cathepsin-K (Mm00484036_m1); calcitonin receptor (Mm00432271_m1); TRAIL (Mm01283606_m1); osteocalcin forward 5′-GCTGCGCTCTGTCTCTCTGA-3′, reverse 5′-TGCTTGGACATGAAGGCTTTG-3′, and probe 5′-FAM-AAGCCCAGCGGCC-NFQ-3′; and ribosomal protein S2 (ChoB) forward 5′-CCCAGGATGGCGACGAT-3′, reverse 5′-CCGAATGCTGTAATGGCGTAT-3′, and probe 5′-FAM-TCCAGAG CAGGATCC-NFQ-3′. Gene expression was calculated using the comparative threshold cycle method using the housekeeping gene ChoB (14).
Bone mass measurements
Spinal bone mineral density (BMD) of L1–L4 was measured using a QDR-1000 with customized murine software (Hologic, Inc., Bedford, MA) as previously described (3, 5). Microcomputed tomography (μCT) analysis of the fifth lumbar vertebrae was done after the bones were dissected, cleaned, fixed in 10% Millonig's formalin and transferred to ethanol, loaded into 12.3-mm-diameter scanning tubes, and imaged (μCT40; Scanco Medical, Bassersdorf, Switzerland) as previously described (6). The vertebrae were aligned vertically and held in place using a broom straw passed through the vertebral foramen. In addition, specimens were separated from each other and firmly anchored by low-density sponge disks. Scans were integrated into three-dimensional voxel images (1024 × 1024 pixel matrices for each individual planar stack). A Gaussian filter (σ = 0.8, support = 1) was applied to all analyzed scans. Scans were done at 12-μm isotropic voxel size (x-ray energy = 55 kVp; x-ray intensity = 145 μA; integration time = 200 msec) using a threshold of 200. The entire vertebral body was scanned with a transverse orientation excluding any bone outside the vertebral body. Manual analysis excluded the cortical bone and the primary spongiosa from the analysis. All trabecular measurements were made by drawing contours every 10–20 slices and using voxel counting for bone volume per tissue volume and sphere filling distance transformation indices without assumptions about the bone shape as a rod or plate for trabecular microarchitecture. μCT measurements were expressed in three-dimensional nomenclature (15). After a 1-h warm-up period, calibration and quality control were done weekly using five density standards, and spatial resolution was verified monthly using a tungsten wire rod. Beam-hardening correction was based on the calibration records. Corrections were made for 200 mg hydroxyapatite for all energies. Over the past 3 yr, the coefficient of variation for the fifth density standard (mean five) was 1.28 (781 ± 10 sd mg hydroxyapatite/cm3) and for rod volume was 3.16 (0.0633 ± 0.002 sd cm3).
Bone strength measurements
The load-bearing properties of L6 were measured using a single column material testing machine and a calibrated tension/compression load cell (model 5542; Instron Corp., Canton, MA) as previously described (5, 6, 14). Load cell calibration was verified in accordance with American Society for Testing and Materials E74-02 standards and traceable to the National Institute of Standards and Technology. Data were recorded and analyzed using the Merlin IX software package (Instron). The L6 specimens were cleaned of surrounding soft tissue, wrapped in gauze soaked in 37 ± 0.5 C normal saline and tested on the day of killing. The length, width, and depth of the bones were recorded with a digital caliper at a resolution of 0.01 mm (Mitutoyo tool number 500-196; Ace Tools, Ft. Smith, AR). Standard materials (200-mg coated tablets of generic ibuprofen) for compression were run before each set of determinations. The maximum load at the breaking point was normalized for bone size and expressed in megapascals (or Newtons per square millimeter).
Bone histomorphometry
The lumbar vertebrae (L1–L4) were fixed and embedded undecalcified in methylmethacrylate and the histomorphometric examination was done on longitudinal sections with a digitizer tablet (OsteoMetrics, Inc., Decatur, GA) interfaced to a Zeiss Axioscope (Carl Zeiss, Thornwood, NY) with a drawing tube attachment, as previously described (3–5). Apoptosis of osteocytes was detected by in situ nick-end labeling using the Klenow enzyme (Oncogene Research Products, Cambridge, MA) in sections counterstained with 2% methyl green, as previously described (3, 6). Apoptotic osteocytes were detected as brown, pyknotic cells buried in lacunae within mineralized bone. To calculate the extent of apoptosis, an average of 2800 ± 340 osteocytes were counted per vertebral bone section (16). Vertebral cancellous bone measurements were restricted to the secondary spongiosa and expressed in two-dimensional nomenclature (15). Osteoclasts were identified by their morphology and positive tartrate-resistant acid phosphatase staining. Bone formation rate was defined as the distance between the double tetracycline labels multiplied by the cancellous mineralizing perimeter expressed as the sum of the double-labeled perimeter plus one half of the single-labeled perimeter.
Imaging of bone interstitial fluid
Solute transport through the osteocyte-lacunar-canalicular system was visualized using Procion Red MX-5B (Sigma-Aldridge, St. Louis, MO), a 200–300 molecular weight fluorescent tracer, which was injected before necropsy into the tail vein of anesthetized mice using a 0.8% aqueous solution (0.01 ml/g body weight at 0.1 ml/min), as previously described (6). In preliminary experiments, mice were killed at various intervals after injection to determine the time required for maximal filling of the lacunar-canalicular system independently of the perfusion rate through bone. Filling of the system could be seen as early as 5 min after injection, and fading of the Procion signal occurred by 20 min, so necropsy was done 15 min after injection. Lumbar vertebrae L1–L4 were prepared for embedding in methyl methacrylate. Three mice were injected from each group, three nonconsecutive sections were taken from each mouse, and three photomicrographs were made from each section. Quantification of the Procion Red in the lacunar-canalicular system of the cancellous bone was by image analysis using photomicrographs taken with structured epifluorescent illumination obtained by means of a tomographic grid projection technique (Zeiss Apotome system; Carl Zeiss) and analyzed by Image-Pro Plus software (Media Cybernetics, Silver Springs, MD) (6).
Cell culture and glucocorticoid-induced apoptosis
MLO-Y4 cells, a murine long bone-derived osteocytic cell line developed by Dr. Lynda Bonewald (University of Missouri, Kansas City, MO), were seeded in a collagen-coated 48-well tissue culture dish (1 × 104 per well) and cultured in α-MEM (Invitrogen, Carlsbad, CA) containing 2.5% fetal bovine serum (FBS), 2.5% bovine calf serum, and 1% penicillin, streptomycin, and glutamine, as previously described (5, 17, 18). After an overnight incubation, the medium was changed to 1% FBS in α-MEM and cultured for an additional 30 min. The cells were then pretreated for 1 h with vehicle or 300 ng/ml OPG-Fc, followed by 10−6 m dexamethasone for 6 h. Cells were harvested by trypsinization and resuspended with 25 μl 1% trypan blue in PBS. The live (clear) and dead (blue) cells were counted using a hemocytometer, and the results are expressed as the percentage of dead cells. At least 100 cells were counted.
To determine the effects of OPG-Fc as well as intact OPG without the Fc portion (R&D Systems, Minneapolis, MN) and confirm apoptosis, caspase-3 activity was measured by cleavage of the fluorogenic substrate Ac-DEVD-AFC (Biomol, Plymouth Meeting, PA). After treatment of the cells as described above, the cells were lysed in 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 10 mm NaF, 1 mm sodium orthovanadate, 5 μg/ml leupeptin, 0.14 U/ml aprotinin, 1 mm phenylmethylsulfonyl fluoride, and 1% Triton X-100. Protein concentration was measured using a Bio-Rad (Hercules, CA) detergent-compatible kit. Lysates (100 μg protein) were incubated with 50 μm DEVD-AFC in 50 mm HEPES (pH 7.4), 100 mm NaCl, 0.1% CHAPS, 10 mm dithiothreitol, 1 mm EDTA, and 10% glycerol, in the absence or presence of the irreversible inhibitor DEVD-CHO for 60 min. The fluorescence was measured in a microplate fluorescence reader with excitation/emission wavelengths of 340/542 nm.
In addition, to quantify apoptosis by nuclear morphology, cells were cotransfected with enhanced green fluorescent protein containing a nuclear localization sequence, using Lipofectamine Plus (Invitrogen). The day before transfection, cells were seeded in medium containing 10% FBS. The next day, the cells were washed once with serum-free medium, and each plate was incubated with serum-free medium containing the plasmids to be transfected and the Lipofectamine Plus reagent for 3 h. The medium was replaced with 1% FBS in α-MEM, and the cells were allowed to recover for 24 h before treatment of the cells as described above. Apoptotic cells were defined as those exhibiting nuclear fragmentation and/or chromatin condensation, a sine qua non of programmed cell death.
Knockdown of TRAIL expression by lentivirus-mediated short hairpin RNA (shRNA)
The shRNA sequence (5′-CGCTTCCAAGATGGTCTCAAA-3′) targeting the mRNA of murine TRAIL was purchased from Sigma and was inserted in the pLKO.1 vector (TRAIL-sh649). A firefly luciferase shRNA (5′-GCTTACGCTGAGTACTTCGA-3′) in pLKO.1 (Luc-sh) was used as a control. The 293-T cells were transfected with pLKO.1 and lentiviral packaging plasmids for 17 h after which 50–60% confluent MLO-Y4 cells were transduced with virus supernatants and selected in medium containing 10 μg/ml puromycin for 3 d. The knockdown efficiency of TRAIL expression was determined by real-time PCR.
Statistics
To investigate treatment effects for the various measurements between the groups, one-way ANOVA with Sidak's correction for multiple comparisons was used, and P values < 0.05 were considered significant (Stata Statistical Software release 8.2; StataCorp, College Station, TX). Comparisons of interest were specified a prioi. Bartlett's test was conducted to determine the homogeneity of the variance assumption. Pearson correlation coefficients were calculated to test for an association between two independently measured variables. The data are shown as the means ± sd.
Results
OPG-Fc prevents the decrease in BMD, cortical thickness, and strength in mice receiving prednisolone
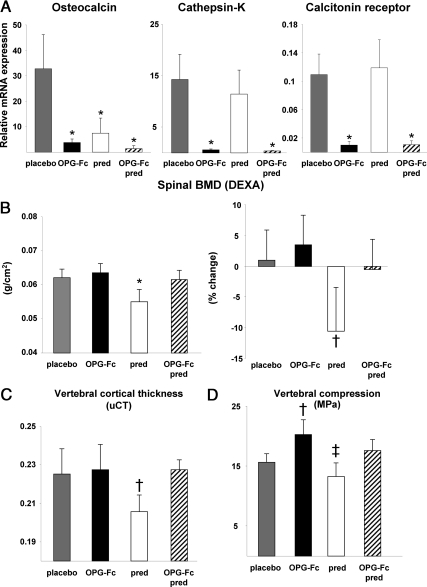
Osteocalcin expression, an index of osteoblast number (6), was markedly decreased in the mice receiving OPG-Fc, prednisolone, or both for 28 d as compared with the group receiving placebo (P < 0.001) (Fig. 1A). However, expression of cathepsin-K and calcitonin receptor, reflecting osteoclast number, was not altered by prednisolone administration alone, whereas OPG-Fc administration, with or without prednisolone, reduced the expression of both genes.
Fig. 1.
OPG-Fc prevents the decrease in BMD, cortical thickness, and strength in 6-month-old C57BL/6 male mice receiving prednisolone. A, Relative mRNA expression for osteocalcin, cathepsin-K, and the calcitonin receptor (from extracts of L5); B, spinal BMD at the end of the experiment and the percent change; C, vertebral cortical thickness as determined by μCT; D, vertebral compression strength. *, P < 0.001; †, P < 0.03; ‡, P < 0.05 vs. placebo. DEXA, Dual-energy x-ray absorptiometry; pred, prednisolone.
Male mice receiving prednisolone alone compared with the mice receiving placebo exhibited an 11% decrease in the final spinal BMD (grams per square centimeter) and an 8-fold greater decrease in the percent change in spinal BMD when compared with the baseline measurement (P < 0.001 and P < 0.03, respectively) (Fig. 1B) (n = 5–7 per group). OPG-Fc administration, with or without prednisolone, preserved spinal BMD at values similar to those in the group receiving placebo. Vertebral cortical thickness as determined by μCT was also preserved by OPG-Fc, with or without prednisolone, whereas prednisolone alone decreased cortical thickness by 9% (P < 0.03) (Fig. 1C). As expected, cortical thickness was directly related to vertebral strength and BMD (r = 0.81, P < 0.001 and r = 0.38, P < 0.02, respectively). There were no significant changes in vertebral cancellous bone volume by μCT due to increased variance in the OPG-Fc alone group (data not shown). Prednisolone administration alone also caused a 12% decrease in vertebral compression strength (P < 0.05) (Fig. 1D). Compared with the placebo group, the administration of OPG-Fc alone resulted in a 29% increase in vertebral strength (P < 0.03). When both prednisolone and OPG-Fc were administered, bone strength was similar to the placebo group. The same trends shown here in males were found in the female mice, but the changes were not significant.
OPG-Fc decreases osteoid, osteoblasts, osteoclasts, and bone formation more than in mice receiving prednisolone
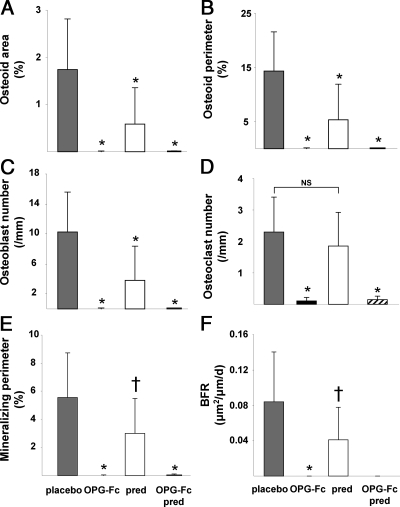
In both male and female mice receiving prednisolone alone compared with the animals receiving placebo, osteoid area and perimeter and osteoblast number were decreased by 34, 37, and 37%, respectively (P < 0.001). However, OPG-Fc, without or with prednisolone, profoundly reduced these measurements of osteoid formation (Fig. 2, B and C). Osteoclast number in the mice receiving prednisolone was not significantly different from that in the placebo group, but OPG-Fc, without or with prednisolone, reduced osteoclast numbers to only 7 and 5%, respectively, (P < 0.001) of the values in the placebo group. Prednisolone alone reduced the mineralizing perimeter and bone formation rate to 54 and 49%, respectively, of that found in the placebo group (P < 0.03), but with OPG-Fc, these measurements were profoundly reduced or not measureable, without or with prednisolone (Fig. 2, E and F). There were no significant changes in vertebral cancellous bone area.
Fig. 2.
OPG-Fc decreased osteoid, osteoblasts, osteoclasts, and bone formation more than in mice receiving prednisolone (pred) (both male and female mice). A, Osteoid area; B, osteoid perimeter; C, osteoblast number; D, osteoclast number; E, mineralizing perimeter; F, bone formation rate (BFR) (not detectable in the OPG/pred group). *, P < 0.001; †, P < 0.03 vs. placebo; NS, not significant.
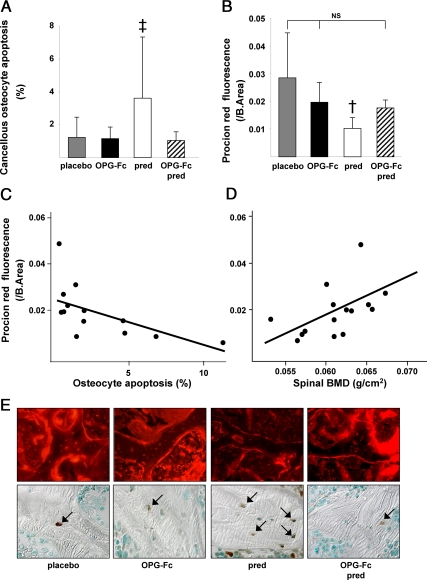
OPG-Fc prevents the increase in osteocyte apoptosis and decrease in bone interstitial fluid in mice receiving prednisolone
In both male and female mice receiving prednisolone alone, the prevalence of cancellous osteocyte apoptosis was 293% greater than in the animals receiving placebo (P < 0.05) (Fig. 3, A and E). Strikingly, OPG-Fc prevented the prednisolone-induced cancellous osteocyte apoptosis and maintained osteocyte viability at values not significantly different from the placebo group. Prednisolone also increased the prevalence of cortical osteocyte apoptosis by 335% (1.52 ± 0.67 vs. 5.01 ± 5.14%, P < 0.02), and this increase was also prevented by OPG-Fc (1.68 ± 0.92%, P < 0.05). In agreement with these findings, Procion Red fluorescence, a measurement of solute transport from the systemic circulation to the osteocyte-lacunar-canalicular network that is strongly linked to osteocyte viability (6), was reduced by 36% (P < 0.03) with prednisolone administration compared with the group receiving placebo but was preserved at levels not significantly different from placebo by OPG-Fc, without or with prednisolone (Fig. 3, B and E). Procion Red fluorescence was indirectly related to the prevalence of osteocyte apoptosis (r = −0.61; P < 0.03) (Fig. 3C) and directly related to the final spinal BMD values (r = 0.51; P < 0.05) (Fig. 3D). Changes in the prevalence of osteocyte apoptosis account for about 37% (r2) of the variance in the bone interstitial fluid measurements and about 26% of the variance in BMD.
Fig. 3.
OPG-Fc prevents the increase in osteocyte apoptosis and decrease in bone interstitial fluid in male and female mice receiving prednisolone (pred). A, Osteocyte apoptosis (n = 44); B, Procion Red fluorescence (n = 15); C, Procion Red fluorescence is inversely related to the prevalence of osteocyte apoptosis (n = 13; r = 0.61; P < 0.03); D, Procion Red fluorescence is directly related to the spinal BMD (n = 15; r = 0.51; P < 0.05); E, photomicrographs of cancellous Procion Red fluorescence (×400) and osteocyte apoptosis (in situ nick-end labeling staining viewed with Nomarski differential interference contrast microscopy, ×630) in the placebo, OPG-Fc, prednisolone, and OPG-Fc/prednisolone groups. †, P < 0.03; ‡, P < 0.05 vs. placebo; NS, not significant; B.Area, bone area.
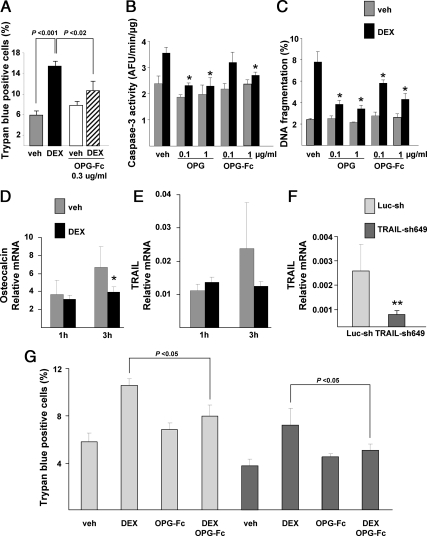
OPG-Fc and OPG prevent glucocorticoid-induced apoptosis in osteocytic cells
To explore a potential cellular mechanism underlying the in vivo findings, we used MLO-Y4 osteocytic cells to examine whether OPG-Fc or OPG directly affects osteocyte viability. Consistent with the in vivo findings, dexamethasone increased the apoptosis of MLO-Y4 cells by more than 2-fold (P < 0.001), as expressed by an increased percentage of trypan blue-positive cells. Compared with dexamethasone alone, OPG-Fc significantly blunted the proapoptotic effect of dexamethasone (P < 0.02), and there was no significant change in apoptosis with vehicle vs. dexamethasone when combined with OPG-Fc (Fig. 4A). Both OPG-Fc and OPG produced similar results when apoptosis was determined by caspase-3 activity or by DNA fragmentation (Fig. 4, B and C). As measured by caspase-3 activity, OPG was at least as, if not more, potent than OPG-Fc in the prevention of dexamethasone-induced MLO-Y4 cell apoptosis.
Fig. 4.
OPG effects on MLO-Y4 cell apoptosis and expression of TRAIL. A, OPG-Fc decreased dexamethasone (DEX)-induced apoptosis of MLO-Y4 osteocytic cells; B and C, both OPG and OPG-Fc decreased dexamethasone-induced apoptosis of MLO-Y4 cells (*, P < 0.05 vs. vehicle and DEX); D and E, dexamethasone decreased expression of osteocalcin but did not change expression of TRAIL (*, P < 0.05); F, knockdown of TRAIL with construct sh649 was effective (**, P < 0.01); G, TRAIL knockdown did not prevent OPG inhibition of dexamethasone-induced MLO-Y4 apoptosis. Luc-sh, Firefly luciferase control; TRAIL-sh649, lentivirus shRNA.
To determine whether dexamethasone induced osteocyte apoptosis in part by stimulating TRAIL expression, we measured TRAIL mRNA levels 1 and 3 h after exposure to the glucocorticoid. Dexamethasone decreased the expression of osteocalcin in MLO-Y4 cells at 3 h as expected (Fig. 4D) but did not alter TRAIL expression (Fig. 4E). Even though the glucocorticoid did not stimulate TRAIL expression, it remains possible that TRAIL was permissive for glucocorticoid-induced osteocyte apoptosis, thereby explaining the effect of the OPG-Fc. To address this possibility, we used a lentivirus-mediated shRNA to knock down TRAIL. The knockdown reduced TRAIL expression by 68% compared with the firefly luciferase Luc-sh control (Fig. 4F). Knockdown of TRAIL decreased the mean overall extent of trypan blue-positive cells by 26.8% (P < 0.03) but still did not prevent OPG-Fc from reducing dexamethasone-induced osteocyte apoptosis (P < 0.05) (Fig. 4G).
Discussion
The animal studies described here demonstrate that the administration of OPG-Fc counteracted the glucocorticoid-induced decrease in BMD and vertebral cortical thickness (Fig. 1). These findings can be explained by the ability of OPG-Fc to inhibit RANKL and thus prevent loss of vertebral BMD and cortical thickness (7, 11, 13). Unexpectedly, we found that OPG-Fc prevented the glucocorticoid-induced increase in the prevalence of osteocyte apoptosis and the decrease in solute transport from the systemic circulation to the osteocyte-lacunar-canalicular network. Decreased osteocyte apoptosis was correlated with preservation of the bone interstitial fluid and BMD (Fig. 3), suggesting that the two changes were interrelated. Hence, osteocyte viability and the hydraulic support of the bone interstitial fluid may have contributed to bone strength (6).
Studies with molecular tracers have shown that aldolase (molecular mass 158,000 Da) and transferrin (molecular mass 76,000–88,000 Da) can penetrate the canalicular network (500–600 nm diameter) within minutes to hours in anesthetized rats (19). In our 28-d experiment, the mice were freely ambulatory, permitting optimal fluid flow through the lacunar-canalicular network, and thus, canalicular penetration of OPG-Fc (100,000 Da) should have been optimal, permitting direct effects of OPG-Fc on osteocytes. The finding that both OPG-Fc and OPG reduced dexamethasone-mediated osteocyte apoptosis in MLO-Y4 cells supports the contention that the protection from glucocorticoid-induced apoptosis was, indeed, direct and that this unexpected effect was not a result of the Fc portion.
Because OPG acts as a soluble inhibitor of both RANKL and the apoptosis-inducing ligand TRAIL, it was important to investigate whether TRAIL played a role in glucocorticoid-induced osteocyte apoptosis. Although knockdown of TRAIL diminished the overall extent of MLO-Y4 apoptosis by about 27%, the antiapoptotic effect of OPG-Fc on dexamethasone-induced osteocyte viability persisted (Fig. 4). OPG protection from glucocorticoid-induced apoptosis could be indirect based on the evidence that OPG is a survival factor for endothelial cells (10, 20). Bone is unique in that nutrients and oxygen are delivered and waste is removed by a system of canaliculi that reach the elaborate network of osteocytes. Therefore, the OPG prevention of glucocorticoid-induced osteocyte apoptosis could be secondary to effects on endothelial cells resulting in maintenance of the connection between the systemic circulation and the lacunar-canalicular network and the delivery of nutrients and oxygen as well as removal of wastes (21, 22).
It is unlikely that the protection from glucocorticoid-induced apoptosis was due to the antiresorptive effect of OPG, because previous work has not established a correlation between the number of osteoclasts and prevention of glucocorticoid-induced osteocyte apoptosis. Specifically, alendronate, a bisphosphonate that incapacitates osteoclasts and decreases bone resorption, prevents glucocorticoid-induced osteocyte apoptosis (17), and IG9402, a bisphosphonate that does not induce osteoclast apoptosis, reduce bone resorption, or decrease osteoclast number, still prevents glucocorticoid-induced osteocyte apoptosis (23); mice with osteoblasts bearing the OG2-11B-HSD2 transgene, an enzyme that inactivates glucocorticoids in a prereceptor fashion, are protected from glucocorticoid-induced osteocyte apoptosis but have no change in osteoclast number (5); prevention of the glucocorticoid-induced increase in osteoclast number by deletion of the glucocorticoid receptor in osteoclasts does not prevent glucocorticoid-induced osteocyte apoptosis (24); and administration of intermittent PTH prevents glucocorticoid-induced osteocyte apoptosis but increases the number of osteoclasts (25, 26).
Prednisolone administration decreased the spinal bone density as measured by dual-energy x-ray absorptiometry and the vertebral cortical thickness as measured by μCT, but the prednisolone-induced decrease in vertebral cancellous bone by μCT or histomorphometry was not significant. However, histomorphometry of the cancellous area, BMD, and μCT measure quite different aspects of bone. The two-dimensional histomorphometric measurements of cancellous bone area were obtained from longitudinal sections of L1–L4, and the three-dimensional μCT analysis is from L5, whereas the spinal BMD analysis is a two-dimensional integral measurement of all the cortical and cancellous bone from L1–L4, a much larger sample. In addition, the coefficient of variation for repeated histomorphometric measurements of murine microarchitecture is more than 7-fold greater than that for BMD (27). The main advantages of the histomorphometric measurements lie in quantification of osteoid, bone cells, and the rate of bone formation as demonstrated by the highly significant glucocorticoid-induced decrease in osteoid, osteoblasts, osteoclasts, and bone formation rate in the mice. It is also important to appreciate that with glucocorticoid excess, bone strength is lost before bone mass.
Prednisolone administration decreased osteoblast number, osteocyte viability, and bone strength, whereas the number of osteoclasts remained similar to that found in the control mice (Table 1). OPG-Fc alone decreased the number of osteoblasts and osteoclasts, maintained osteocyte viability, and increased bone strength. When OPG-Fc and prednisolone were administered together, the number of osteoblasts and osteoclasts decreased, but osteocyte viability, BMD, cortical thickness, and bone strength were maintained. Furthermore, the amount of fluid in the osteocyte-lacunar-canalicular system was inversely related to the prevalence of osteocyte apoptosis and directly related to BMD. Fluid represents at least 25% of the wet weight of bone (28, 29) and confers to bone much of its unique strength and resilience (30). Although the ability of OPG-Fc to prevent the prednisolone-induced loss of vertebral cortical thickness and BMD evidently helped prevent the reduction in strength, based on the present results and our previous work (5, 6, 31), the findings are consistent with the contention that at least part of the preservation of strength was due to maintenance of osteocyte viability, lacunar-canalicular circulation, and the hydraulic support of bone fluid. Osteocyte apoptosis due to glucocorticoid excess is associated with loss of bone strength before the loss of bone mass that is characteristic of this condition (32). Furthermore, decreased osteocyte viability is linked to reduced fluid flow in the osteocyte-lacunar-canalicular circulation and increased microdamage (33, 34).
Table 1.
OPG-Fc and prednisolone effects on murine vertebral bone cells and strength
| Osteoblast number | Osteocyte viability | Osteoclast number | Strength | |
|---|---|---|---|---|
| Prednisolone | ↓ | ↓ | nc | ↓ |
| OPG-Fc | ↓ | nc | ↓ | ↑ |
| OPG-Fc / prednisone | ↓ | nc | ↓ | nc |
↓, Decrease; ↑, increase; nc, no change.
In conclusion, administration of OPG-Fc counteracts the glucocorticoid-induced decreases in BMD, vertebral cortical thickness, vertebral compression strength, bone interstitial fluid, and osteocyte viability. At least part of the preservation of strength by OPG-Fc may result from the ability of this protein to preserve the osteocyte-lacunar-canalicular circulation, in the setting of glucocorticoid excess.
Acknowledgments
We thank W. Webb, C. Wicker III, L. Han, S. Berryhill, T. Chambers, E. Hogan, and R. Shelton for their technical assistance. We also thank Paul J. Kostenuik (Amgen Inc., Thousand Oaks, CA) for the OPG-Fc.
This work was supported by Veterans Affairs Merit Review Grants from the Office of Research and Development Department of Veterans Affairs, the National Institutes of Health (P01-AG13918), Tobacco Settlement Funds provided by the University of Arkansas for Medical Sciences College of Medicine, and funds from Amgen.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- μCT
- microcomputed tomography
- FBS
- fetal bovine serum
- 11β-HSD2
- 11β-hydroxysteroid dehydrogenase type 2
- OPG
- osteoprotegerin
- OPG-Fc
- osteoprotegerin with the fragment crystallizable region of Ig heavy chains
- RANKL
- receptor activator for nuclear factor κB ligand
- shRNA
- short hairpin RNA
- TRAIL
- TNF-related apoptosis-inducing ligand.
References
- 1. Cushing H. 1932. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp 50:137–195 [Google Scholar]
- 2. Reid IR. 1989. Pathogenesis and treatment of steroid osteoporosis. Clin Endocrinol (Oxf) 30:83–103 [DOI] [PubMed] [Google Scholar]
- 3. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. 1998. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of the deleterious effects on bone. J Clin Invest 102:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. 2002. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest 109:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. 2004. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841 [DOI] [PubMed] [Google Scholar]
- 6. Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. 2010. Endogenous glucocorticoids decrease angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9:147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. 1999. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140:4382–4389 [DOI] [PubMed] [Google Scholar]
- 8. Zauli G, Melloni E, Capitani S, Secchiero P. 2009. Role of full-length osteoprotegerin in tumor biology. Cell Mol Life Sci 66:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flyvbjerg A. 2010. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol 6:94–101 [DOI] [PubMed] [Google Scholar]
- 10. Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. 2000. Osteoprotegerin is an αvβ3-induced, NF-κB-dependent survival factor for endothelial cells. J Biol Chem 275:20959–20962 [DOI] [PubMed] [Google Scholar]
- 11. Hofbauer LC, Zeitz U, Schoppet M, Skalicky M, Schüler C, Stolina M, Kostenuik PJ, Erben RG. 2009. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum 60:1427–1437 [DOI] [PubMed] [Google Scholar]
- 12. Kleiber M. 1961. The fire of life: an introduction to animal energetics. New York: John Wiley, Sons; 177–230 [Google Scholar]
- 13. Ominsky MS, Li X, Asuncion FJ, Barrero M, Warmington KS, Dwyer D, Stolina M, Geng Z, Grisanti M, Tan HL, Corbin T, McCabe J, Simonet WS, Ke HZ, Kostenuik PJ. 2008. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res 23:672–682 [DOI] [PubMed] [Google Scholar]
- 14. Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. 2007. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 16. Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. 2007. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res 22:1492–1501 [DOI] [PubMed] [Google Scholar]
- 17. Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. 1999. Prevention of osteocyte and osteoblasts apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. 2003. Proteasomal degradation of Runx2 shortens PTH-induced anti-apoptotic signaling in osteoblasts: a putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 278:50259–50272 [DOI] [PubMed] [Google Scholar]
- 19. Knothe Tate ML. 2003. “Whither flows the fluid in bone?” An osteocyte's perspective. J Biomech 36:1409–1424 [DOI] [PubMed] [Google Scholar]
- 20. Hofbauer LC, Schoppet M. 2001. Osteoprotegerin: a link between osteoporosis and arterial calcification? Lancet 358:257–259 [DOI] [PubMed] [Google Scholar]
- 21. Pritzker LB, Scatena M, Giachelli CM. 2004. The role of osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand in human microvascular endothelial cell survival. Mol Biol Cell 15:2834–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGonigle JS, Giachelli CM, Scatena M. 2009. Osteoprotegerin and RANKL differently regulate angiogenesis and endothelial cell function. Angiogenesis 12:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plotkin LI, Bivi N, Bellido T. 2011. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone 49:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. 2006. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest 116:2152–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. 1999. Increased bone formation by prevention of osteoblast apoptosis with PTH. J Clin Invest 104:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. 2010. Intermittent parathyroid hormone administration prevents glucocorticoid-induced osteoblast and osteocyte apoptosis, decreased bone formation, and reduced bone strength in mice. Endocrinology 151:2641–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC. 2004. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology 145:1980–1987 [DOI] [PubMed] [Google Scholar]
- 28. Lien J, Kaye M. 1978. Changes in the red cell, plasma, and inulin spaces and in the total water contents of rat femurs in cortisone induced osteoporosis. Calcif Tissue Res 25:245–248 [DOI] [PubMed] [Google Scholar]
- 29. Ishijima H, Ishizaka H, Horikoshi H, Sakurai M. 1996. Water fraction of lumbar vertebral bone marrow estimated from chemical shift misregistration on MR imaging. AJR Am J Roentgenol 167:355–358 [DOI] [PubMed] [Google Scholar]
- 30. Liebschner MA, Keller TS. 2005. Hydraulic strengthening affects the stiffness and strength of cortical bone. Ann Biomed Eng 33:26–38 [DOI] [PubMed] [Google Scholar]
- 31. Weinstein RS. 2010. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone: 46:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seeman E, Delmas PD. 2006. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261 [DOI] [PubMed] [Google Scholar]
- 33. Verborgt O, Gibson GJ, Schaffler MB. 2000. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67 [DOI] [PubMed] [Google Scholar]
- 34. Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML. 2002. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res 17:2030–2037 [DOI] [PubMed] [Google Scholar]