Abstract
There is now considerable evidence that dynorphin neurons mediate the negative feedback actions of progesterone to inhibit GnRH and LH pulse frequency, but the specific neurons have yet to be identified. In ewes, dynorphin neurons in the arcuate nucleus (ARC) and preoptic area (POA) are likely candidates based on colocalization with progesterone receptors. These studies tested the hypothesis that progesterone negative feedback occurs in either the ARC or POA by determining whether microimplants of progesterone into either site would inhibit LH pulse frequency (study 1) and whether microimplants of the progesterone receptor antagonist, RU486, would disrupt the inhibitory effects of peripheral progesterone (study 2). Both studies were done in ovariectomized (OVX) and estradiol-treated OVX ewes. In study 1, no inhibitory effects of progesterone were observed during treatment in either area. In study 2, microimplants of RU486 into the ARC disrupted the negative-feedback actions of peripheral progesterone treatments on LH pulse frequency in both OVX and OVX+estradiol ewes. In contrast, microimplants of RU486 into the POA had no effect on the ability of systemic progesterone to inhibit LH pulse frequency. We thus conclude that the ARC is one important site of progesterone-negative feedback in the ewe. These data, which are the first evidence on the neural sites in which progesterone inhibits GnRH pulse frequency in any species, are consistent with the hypothesis that ARC dynorphin neurons mediate this action of progesterone.
The ovarian cycle in mammals reflects a complex interplay between the effects of LH and FSH on the ovary and the feedback actions of estradiol (E2) and progesterone on the hypothalamo-hypophysial unit (1–3). The latter occurs at both the hypothalamus and pituitary and can be divided into negative-feedback actions that inhibit episodic GnRH and LH secretion during the follicular and luteal phases of the estrous cycle and positive-feedback actions responsible for the induction of the preovulatory GnRH/LH surge (1–3). Although both E2 and progesterone inhibit episodic GnRH/LH secretion, they do so by different mechanisms, with E2 inhibiting LH and GnRH pulse amplitude and progesterone suppressing pulse frequency (3–7). These differential actions of ovarian steroids produce changes in the pattern of pulsatile LH (1–3) and GnRH (8) throughout the estrous (2, 3) and menstrual (1, 9) cycle, with the negative feedback actions of progesterone in the luteal phase being particularly important for timing events of the ovarian cycle of mammals with prolonged luteal phases, including sheep (10) and humans (11).
Although progesterone inhibits GnRH pulse frequency via the classical progesterone receptor (PR) (12), this action most likely does not occur directly because few if any GnRH neurons contain PR (13–15). The neural sites of progesterone-negative feedback have yet to be identified in any species, but there is considerable evidence on the neurotransmitters involved. Early work in humans (16, 17) implicated endogenous opioid peptides (EOP) as important mediators of progesterone-negative feedback, and subsequent work has demonstrated that blockade of EOP action with naloxone, or similar EOP antagonists, increased LH pulse frequency during the luteal phase of monkeys (18) and sheep (19) and during pregnancy in rats (20). Similar stimulatory effects of EOP receptor blockade were observed in progesterone-treated, but not ovariectomized (OVX), animals (21, 22).
Although there is general agreement that EOP mediate progesterone-negative feedback, there is less agreement on the specific EOP involved. There is indirect evidence to support a role for β-endorphin in some species (23), but there is also considerable evidence pointing to dynorphin. In sheep, an antagonist to the receptor for dynorphin (κ-opioid receptor) increased LH pulse frequency in luteal phase ewes when implanted in the medial basal hypothalamus (MBH), whereas antagonists to the other two EOP receptors (μ, δ) did not (24). Dynorphin neurons synapse on 40% of GnRH neurons in the preoptic area (POA) and 90% of MBH GnRH cells (24), and virtually all dynorphin neurons in the ovine arcuate nucleus (ARC) and POA contain PR (25). Fewer GnRH neurons receive β-endorphin-containing inputs (24), and only a small percentage of β-endorphin neurons contain steroid receptors (26, 27). Progesterone also stimulates dynorphin synthesis and release in sheep (28) and may increase its synthesis in humans (29). There are also reports in rodents that a κ-EOP receptor antagonist increased LH pulse frequency during pregnancy (30) and that dynorphin neurons in the anteroventral periventricular area (AVPV) (31), and probably those in the ARC (32), contain PR.
Although PR are found in both rostral (POA and AVPV) and ARC dynorphin neurons, these are distinct populations. Dynorphin neurons in the ARC also contain neurokinin B (NKB) and kisspeptin (33, 34), and are thus referred to as KNDy neurons (32), whereas the POA dynorphin neurons do not contain either of these neuropeptides (32, 33). In rodents, the AVPV neurons are most likely involved in control of the LH surge (35), whereas the ARC KNDy neurons have been hypothesized to mediate steroid-negative feedback (36). In the ewe, dynorphin expression in both the ARC and POA decreases after OVX (28), so these neurons are likely candidates to mediate progesterone-negative feedback. In light of these data, this initial study on the neural sites of progesterone-negative feedback in ewes focused on the ARC and POA. Specifically it was designed to determine whether progesterone acts in either area by examining the ability of local administration of progesterone to inhibit LH pulse frequency and the PR antagonist, RU486, to block the inhibitory actions of systemic progesterone. In sheep, hypothalamic PR (37, 38) and progesterone-negative feedback (21, 22, 39) are evident in OVX animals, although both are increased by E2 treatment (37, 40). Therefore, the effects of progesterone and RU486 were examined in both OVX and E2-treated OVX (OVX+E) ewes.
Materials and Methods
Animals
Adult mixed-breed blackface ewes were maintained in an open barn and moved indoors 3–7 d before surgeries. Ewes were fed a maintenance pelleted ration once a day and had free access to water and mineral blocks. Lights were adjusted every 2 wk to mimic the duration of natural lighting. All experiments were carried out in the breeding season (October through the first half of February) because the negative-feedback actions of both E2 and progesterone are altered in the anestrous season (41). Blood samples (3–4 ml) were collected by jugular venipuncture into heparinized tubes and plasma stored at −20 C. All procedures were approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
Surgeries
All surgical procedures were carried out as previously described (42) under sterile conditions using 3% halothane supplemented with nitrous oxide as anesthesia. Ovariectomies were performed via midventral laparotomy. For implanting chronic guide cannulae, the head of the ewe was positioned in a stereotaxic apparatus, the surface of the skull was exposed, and a small portion of bone was removed just rostral to bregma to expose the surface of the brain. Radioopaque dye, Omnipaque 350 (Iohexol, Winthrop, NY) was injected into one lateral ventricle and x-ray radiography used to visualize placement of the guide cannulae. Bilateral 18-gauge cannulae were placed in the POA (2.0 mm from midline at anterior border of the optic recess, 2.0 mm dorsal to the floor of this recess) or 2 mm dorsal to the posterior ARC (1.5 mm from midline at the point at which the infundibular recess begins and 2 mm above the floor of the third ventricle). Guide cannulae were cemented into place using dental acrylic and stainless steel screws, protected with a plastic cap, and occluded using stainless steel, 22-gauge obturators. The animals were treated with dexamethasone, analgesic, and penicillin, from 1 d before surgery to 3 d afterward, as previously described (42).
Drug and hormone administration
Progesterone and E2 were administered systemically using intravaginal progesterone implants (Eazi-Breed CIDRs; InterAg, Hamilton, New Zealand) and 1 cm long SILASTIC tubing (Dow Corning Corp., Midland, MI) containing E2, as previously described (12, 40). Microimplants (42) were used for local administration of progesterone or RU486 (Sigma-Aldrich Co., St. Louis, MO). The lumen of 22-gauge tubing, cut to extend 2 mm beyond the guide tubes, was filled with crystalline progesterone or RU486 by tamping and the outside cleaned with sterile gauze. In preliminary work testing the longevity of progesterone microimplants, we observed that if tubing was filled by tamping 35 times, progesterone was depleted after 3 d of placement into the hypothalamus, but if tamped 50 times, crystalline material was still evident in the lumen of the 22-gauge tubing when it was removed 3 d after the implantation. Therefore, all microimplants were prepared by tamping at least 50 times.
Experimental protocols
General design
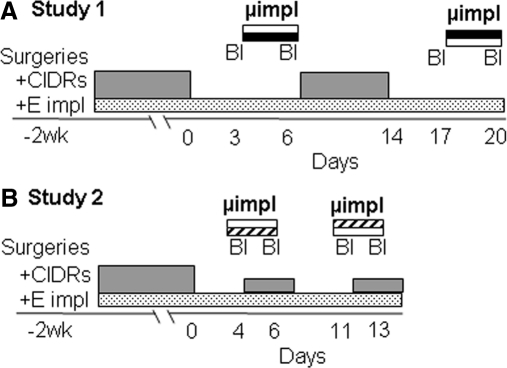
Because progesterone-negative feedback can be demonstrated for only approximately 3 wk after OVX or removal of E2 implants from E2-treated OVX (OVX+E) ewes (39), treatments were done first in OVX+E and then in OVX ewes. To minimize animal use, a cross-over design with two replicates was used so that each ewe served as its own control (Fig. 1). In the first replicate, half the animals received empty microimplants (controls) and half received microimplants containing either progesterone (study 1) or RU486 (study 2). Microimplants were then removed, the ewes were allowed to recover for a few days (with appropriate peripheral hormone treatments), and the protocol was repeated, with ewes that had been given empty microimplants in the first replicate receiving progesterone (or RU486)-containing microimplants and vice versa.
Fig. 1.
Crossover experimental designs for study 1 (A) and study 2 (B). Treatments with peripheral progesterone (CIDR) and E2 implants (starting at time of OVX and neurosurgeries) are indicated by gray and stippled bars, respectively. Frequent blood sampling (Bl) and microimplant treatments (study 1: black/open bars, progesterone/control; study 2: striped/open bars, RU486/control) are shown above peripheral implants. The treatment protocol for the first half of each study (in OVX+E ewes) is depicted; identical protocols were used for the second half of each study (OVX ewes), except that E2 implants were removed and two vaginal progesterone implants were inserted during RU486/control microimplant treatments in study 2. See text for more details.
Study 1: does progesterone act in the ARC or POA to inhibit LH pulse frequency in OVX or OVX+E ewes?
Ewes were OVX and guide tubes implanted into either the ARC (n = 7) or POA (n = 7). Two vaginal progesterone implants and one sc E2 implant were inserted at the time of OVX to maintain luteal phase concentrations of progesterone and E2 during the recovery period (Fig. 1A). After 10–14 d, the progesterone implants were removed, but E2 implants were left in place. Three days later, blood samples were collected every 10 min for 5 h, progesterone or control (empty) microimplants inserted, and samples collected again 3 d later. At the end of this sampling period, microimplants were removed and two vaginal progesterone implants inserted. Eight days later, the progesterone implants were removed and the sampling and treatment protocol repeated starting 3 d later, using a cross-over design for microimplant treatments. This portion of study 1 was completed in mid-November, and the second half done over the next 6 wk in these same animals (ending in the latter part of December). Briefly, two vaginal progesterone implants were inserted after the last blood collection from the OVX+E ewes, and both progesterone and E2 implants removed 8 d later. The cross-over microimplant treatments (Fig 1A) were then repeated with ewes receiving two progesterone and one E2 implant for the 8 d between replicates. The order of microimplant treatments were randomized for OVX+E and OVX ewes.
Study 2: does RU486 act in the ARC or POA to block the negative-feedback action of progesterone?
Ewes were OVX, guide tubes aimed at the ARC (n = 7) or POA (n = 8) implanted, and E2 implants and progesterone implants inserted as in study 1 (Fig. 1B). Ewes were allowed to recover for about 2 wk, the progesterone implants were removed, and 4 d later either RU486-filled or empty microimplants were inserted into the ARC or POA. One hour later, blood samples were collected every 12 min for 5 h. One vaginal progesterone implant was then inserted, and blood samples were collected again 2 d later. After the second sampling period, microimplants were removed. The next day, the vaginal progesterone implant was removed and 4 d later the microimplant and blood sampling protocol were repeated, using a crossover design for RU486 microimplant treatments. The same ewes were then used to examine the effects of RU486 POA microimplants in OVX ewes. Both the vaginal progesterone and E2 implants were removed 1 d after completion of the OVX+E portion, and the crossover protocol (Fig. 1B) was repeated in the absence of E2, except that two vaginal progesterone implants (instead of one) were inserted during the microimplant treatments; POA treatments lasted from mid-January through early February. The effects of the ARC RU486 microimplants in OVX ewes were tested in a new group of ewes because the treatments in OVX+E ewes were completed near the end of the breeding season (late February). After surgeries and a recovery period in September, both vaginal progesterone and E2 implants were removed from all ewes (n = 8), and the effects of RU486 or empty microimplants were examined before and during the treatment with two vaginal progesterone implants using the same crossover design for the microimplant treatments (Fig. 1B).
Tissue collection
At the end of each study, ewes were injected with two doses of heparin 10 min apart (25000 U, iv; Abraxiz Pharmaceutical Products, Schaumburg, IL) and then immediately euthanized with an overdose of sodium pentobarbitol (∼5 g), iv. The heads were removed and perfused via the carotid arteries with 6 liters of 4% paraformaldehyde in PBS (pH 7.3–7.4) with 0.1% sodium nitrite and 10 U/ml heparin. After perfusion, the brain was removed, and a block of tissue containing the POA and hypothalamus dissected and stored in paraformaldehyde overnight at 4 C. The tissue was then transferred to a solution containing 20% sucrose in phosphate buffer (pH 7.3–7.4) at 4 C until fully infiltrated. Frozen coronal sections (50 μm thick) were cut on a microtome. Every fifth section was mounted on slides, stained with cresyl violet, and examined histologically to determine microimplantation sites.
Analyses
Assays
LH concentrations were measured in duplicate 50–200 μl of plasma using reagents provided by the National Hormone and Peptide Program (Torrance, CA) as previously described (24, 42). Assay sensitivity averaged 0.07 ng/tube (NIH S24) and intra- and interassay coefficients of variation of were 5.3 and 13.8%, respectively. Progesterone concentrations were measured in duplicate 150-μl aliquots of two plasma samples from each blood collection period using a RIA that has been validated for use in sheep (24). Two assays were used for experiment 1 and two for samples from experiment 2; sensitivities and intraassay variability averaged 1.5 pg/tube and 7.3%, respectively; interassay variability was 19.3%.
Statistical analyses
LH pulses were identified using previously described criteria (4), and LH pulse frequency was analyzed by Friedman's two-way ANOVA because parametric statistics are not appropriate for this noncontinuous variable. Mean LH pulse amplitude (peak minus preceding nadir) and mean LH concentrations were calculated for each sampling period for each animal. These values were then analyzed by two-way ANOVA with repeated measures using main effects of treatment (empty vs. filled microimplants) and time (first and second sampling periods). If the complete data set was not normally distributed, but subsets of data (before and during one treatment or control) were, paired t test were used to determine whether there was a difference between the first and second sampling periods. If no data subsets were normally distributed, the Wilcoxon signed ranks test was used for statistical analyses.
Results
Study 1: does progesterone act in the ARC or POA to inhibit LH pulse frequency in OVX or OVX+E ewes?
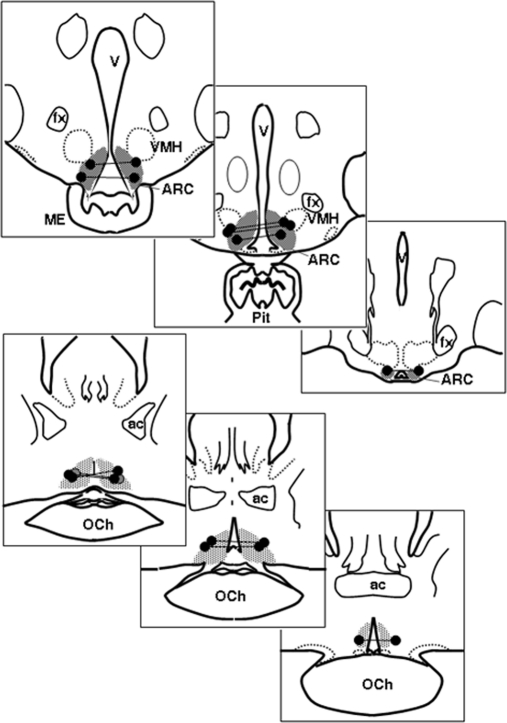
Six of the seven ewes with guide tubes aimed at the ARC (Fig. 2, top panels) had implantation sites near or within this region, whereas six of seven ewes in the POA group had sites close to the previously described (25) locations of PR-containing dynorphin neurons (Fig. 2, bottom panels). Implantation sites in the other ewes were dorsal to these target areas; data from these animals were excluded from analysis.
Fig. 2.
Sites of microimplants in study 1. The top three panels depict bilateral microimplants in the ARC; the bottom three panels are sites in the POA. Bilateral sites in the same ewe are connected by a line. The shaded area depicts distribution of PR-containing dynorphin neurons based on previous data (25). Ac, Anterior commissure; fx, fornix; ME, median eminence; OCh, optic chiasm; Pit, pituitary stalk; VMH, ventromedial hypothalamus.
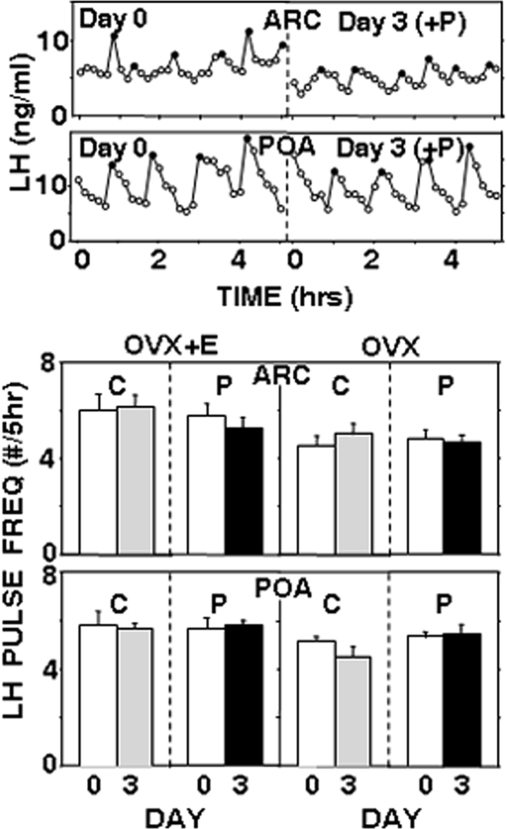
Neither empty nor progesterone-filled microimplants altered episodic LH secretion when placed in the ARC or POA (Fig. 3). In particular, there were no significant decreases in LH pulse frequency (Fig. 3) during the period of progesterone treatment in either OVX+E or OVX ewes. Similarly, no significant differences in LH pulse amplitude or mean LH concentrations were observed (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). In this and the following study, LH pulse amplitudes were 2–3 times higher in OVX ewes than in OVX+E ewes, whereas pulse frequencies were slightly lower in OVX animals. These data are consistent with previous reports on the effects of E2 on both LH pulse amplitude (4, 43) and frequency (43), but statistical comparisons of data from OVX and OVX+E ewes were not done because they were designed as independent experiments. It is worth noting that the LH pulse frequencies and amplitudes observed in the absence of any microimplants are similar to those reported in earlier studies of OVX and OVX+E ewes that had not received any neurosurgical procedures (4, 43). The lack of effect of progesterone microimplants was not due to depletion of these microimplants because crystalline progesterone was still visible in their lumen when removed from all ewes on d 3. Not surprisingly, circulating progesterone concentrations remained low during the treatment with progesterone microimplants in either the ARC (0.18 ± 0.02 ng/ml, n = 6) or POA (0.16 ± 0.01 ng/ml, n = 6).
Fig. 3.
The top two panels depict representative LH pulse patterns in OVX ewes before (d 0) and during (d 3) treatment with progesterone-containing microimplants in the ARC or POA. Solid circles depict peaks of LH pulses. The bottom two panels present mean (±sem) LH pulse frequency before (open bars) and during treatment with empty (C) (shaded bars) or progesterone-filled (solid bars) microimplants in the ARC or POA. Results from OVX+E ewes are shown on the left, and results from OVX ewes are shown on the right. There were no significant effects of progesterone. P, Progesterone.
Study 2: does RU486 act in the ARC or POA to block the negative feedback action of progesterone?
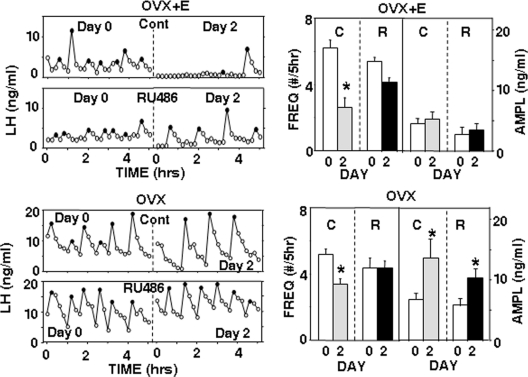
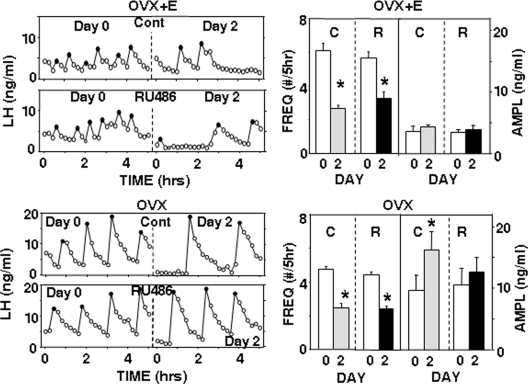
Sites of microimplantation were similar to those in study 1 (Supplemental Figs. 1 and 2), with five of seven ewes in both ARC treatment groups receiving correct placements and seven of eight correct placements in the POA group. RU486 was visible in the lumen of all microimplants at the time of removal. In OVX+E ewes, insertion of one vaginal progesterone implant increased peripheral progesterone concentrations from 0.14 ± 0.01 to 2.2 ± 0.2 ng/ml (n = 12) and suppressed LH pulse frequency by approximately 50% in control-treated OVX+E ewes (Figs. 4 and 5). This effect was blocked by RU486 microimplants in the ARC (Fig. 4) but not by RU486 microimplants in the POA (Fig. 5). With ARC microimplants, peripheral progesterone treatments also significantly inhibited mean LH concentrations (from 5.4 ± 0.7 to 2.8 ± 1.1 ng/ml; P = 0.029, paired t test) in control treatments but not when these OVX+E ewes received RU486 microimplants (before: 4.9 ± 0.8 ng/ml; during: 3.3 ± 1.1 ng/ml; P = 0.19, paired t test). In contrast, RU486 microimplants in the POA had no effect on the ability of progesterone to inhibit mean LH concentrations (P = 0.006 for time, P = 0.18 for microimplant treatment, P = 0.52 for interactions); mean LH decreased from 5.6 ± 0.7 to 3.4 ± 0.5 ng/ml and from 4.8 ± 0.5 to 3.0 ± 0.4 ng/ml during control and RU486 treatments, respectively. As illustrated in Figs. 4 and 5, there were no significant differences in LH pulse amplitudes with either ARC (controls: P = 0.30; RU486: P = 0.67, paired t tests) or POA (P = 0.46 for time; P = 0.48 for treatment, P = 0.74 for interaction) microimplants.
Fig. 4.
Effects of ARC RU486 microimplants. Left panels depict representative episodic LH secretion in OVX+E (top panels) and OVX (bottom panels) ewes receiving empty (Cont) or RU486-containing (RU486) microimplants in the ARC. LH pulse patterns before (d 0) and during (d 2) one vaginal progesterone implant are shown. Solid circles depict peaks of LH pulses. Right panels present corresponding changes in the LH pulse frequency (FREQ) and amplitude (AMPL) when ewes received empty (C) or RU486-containing (R) microimplants. Open bars depict values before and solid/shaded bars values during vaginal progesterone treatment. *, P < 0.05 vs. values before progesterone treatment.
Fig. 5.
Effects of POA RU486 microimplants. Left panels depict representative episodic LH secretion in OVX+E (top panels) and OVX (bottom panels) ewes receiving empty (Cont) or RU486-containing (RU486) microimplants in the POA. LH pulse patterns before (d 0) and during (d 2) treatment with one (OVX+E ewes) or two (OVX ewes) vaginal progesterone implants are shown. Solid circles depict peaks of LH pulses. Right panels present corresponding changes in LH pulse frequency (FREQ) and amplitude (AMPL) when ewes received empty (C) or RU486-containing (R) microimplants. Open bars depict values before and solid/shaded bars values during vaginal progesterone treatment. *, P < 0.05 vs. values before progesterone treatment.
In OVX ewes, progesterone concentrations increased from 0.16 ± 0.03 to 3.7 ± 0.31 ng/ml (n = 12) in response to the two vaginal progesterone implants, which significantly inhibited LH pulse frequency in control ewes (Figs. 4 and 5). Microimplants of RU486 in the ARC blocked this negative-feedback action of progesterone (Fig. 4), whereas those in the POA did not (Fig. 5). The effects of RU486 on LH pulse amplitude in these OVX ewes depended on the site of administration. With ARC microimplants (Fig. 4), peripheral progesterone increased the LH pulse amplitude, and this action was not significantly affected by RU486 microimplants (P = 0.023 for time, P = 0.36 for microimplant treatment, P = 0.41 for interaction). With POA treatments (Fig. 5), progesterone increased pulse amplitude in controls (P = 0.022, paired t test) but not when RU486 was microimplanted (P = 0.15, paired t test). There was no significant effect of peripheral progesterone on mean LH concentrations with either empty (before: 6.7 ± 1.0 ng/ml; during: 6.2 ± 1.0 ng/ml) or RU486 (before: 7.4 ± 2.3 ng/ml; during: 8.6 ± 1.4 ng/ml) microimplants in the ARC (P = 0.64 for time, P = 0.23 for microimplant treatment, P = 0.218 for interactions). With microimplants in the POA, progesterone inhibited mean LH during control (from 7.8 ± 1.2 to 5.7 ± 0.7 ng/ml, P = 0.028, t test) and RU486 (from 8.5 ± 1.9 to 5.9 ± 1.7 ng/ml, P = 0.016, Wilcoxon signed rank test) treatments.
Discussion
Microimplants of RU486 consistently blocked the inhibitory actions of progesterone when placed into the ARC. In contrast, RU486 had no effect on LH pulse frequency when placed in the POA in either OVX or OVX+E ewes. Thus, we propose that the ARC is one critical site for the negative-feedback action of progesterone in this species. This is the first direct evidence for the neural sites at which progesterone acts to inhibit GnRH and LH pulse frequency in any species, although this action appears to be important for timing events of the ovarian cycle in mammals with normal luteal phases (10), including women (11). In contrast, there is considerable information on the sites of progesterone action on the LH surge in rats (35, 44) and sheep (45) and on where it acts to affect sexual behavior in both these species (45–47).
In light of the effects of RU486 in the ARC and the ability of progesterone given into the third ventricle to inhibit LH pulse frequency in ewes (12), it is surprising that no inhibitory effects of progesterone microimplants were observed. There are, however, several possible explanations for this apparent discrepancy. The first possibility is that little, or no, progesterone was released from these microimplants, but this seems unlikely because of the effectiveness of similar progesterone microimplants in other studies (45) and our observation that microimplants filled with less progesterone were depleted after 3 d in situ (preliminary data). Another possibility is that RU486 was acting at glucocorticoid receptors rather than PR, but the observation that the effects of RU486 were observed only during progesterone treatments argues against it; if this drug was blocking an inhibitory action of cortisol, RU486 should have been effective both before and during progesterone treatment. A more likely possibility is that progesterone must act at multiple sites. If this is the case, local administration to one site would not be sufficient to inhibit GnRH secretion, but blockade of its action at one site would disrupt the effects of systemic progesterone. It should be noted that both sites of action could be in the ARC because this nucleus extends over several millimeters along the anterior-posterior extent of the ovine MBH and the effective spread of steroid microimplants is only approximately 1 mm (48, 49). In this regard, we have recent evidence that neurons containing orphanin-FQ, which are concentrated in the more rostral portions of the ovine ARC (50), contribute to progesterone-negative feedback in ewes (51). A final explanation for the discrepancy in the effectiveness of RU486 and progesterone is that effective concentrations of RU486 may diffuse further than those of progesterone. This possibility is supported by the evidence that progesterone is rapidly metabolized in the brain (52); because RU486 is a synthetic compound, it most likely is not a substrate for these metabolic enzymes and thus may affect a higher percentage of ARC neurons.
The lack of the effects of both progesterone and RU486 microimplants in the POA on LH pulse frequency does not support the hypothesis that PR-containing neurons in this area are important for progesterone-negative feedback. However, we cannot rule out the possibility that the RU486 and progesterone failed to reach a sufficient number of progesterone-responsive neurons in the POA because they are distributed in a fairly large volume (25). However, earlier knife-cut data also support the conclusion that neurons in this area are not critical for progesterone-negative feedback. Specifically, knife cuts between the POA and MBH had no effects on either the inhibitory actions of progesterone in OVX ewes or the ability of an EOP antagonist to increase LH pulse frequency in progesterone-treated OVX animals (53). Similar knife cuts also did not alter LH secretion during the luteal phase in ovary-intact animals (54). It is interesting to note that progesterone may act in the POA to increase LH pulse amplitude in OVX ewes because this effect was blocked by microimplants of RU486 in the POA (Fig. 5). An increase in the LH pulse amplitude has often been observed in progesterone-treated OVX ewes, but it has usually been assumed that this was caused by the decrease in GnRH and LH pulse frequency that also occurs in OVX+progesterone ewes (4). The data in this study raise the possibility that these are two independent effects of progesterone, but the physiological significance of progesterone stimulation of LH pulse amplitude is unclear because it is not always seen in OVX ewes (40) and not usually evident in the presence of E2 (Figs. 4 and 5) (40).
In light of the inability of microimplants of progesterone and RU486 near the POA dynorphin neurons (Fig. 2) to affect LH pulse frequency, this subset of progesterone-responsive dynorphin neurons is unlikely to be involved in progesterone-negative feedback. However, they could potentially play a role in either the behavioral actions of progesterone or its ability to block the preovulatory GnRH surge in sheep. Although EOP can inhibit the GnRH surge in ewes (55), it is unlikely that these dynorphin neurons mediate the inhibitory actions of progesterone for two reasons: 1) μ-receptors, not κ-receptors, mediate the EOP blockade of the surge (56), and 2) progesterone acts in the ventromedial nucleus, not the POA, to block the LH surge (45). Progesterone does act in the POA to block an estrogen-induced increase in receptivity in ewes (45), and EOP generally inhibit reproductive behavior (57). However, the behavioral effects of EOP appear to occur via δ- and μ-receptors, rather than κ-receptors, at least in rodents (57). Thus, the physiological role of the progesterone-responsive dynorphin neurons in the ovine POA remains to be determined.
Although these data point to the ARC as one critical site of progesterone-negative feedback in sheep, they do not identify the specific neurons involved. However, the most likely candidate is the KNDy neurons concentrated near the sites of the RU486 microimplants in the ARC. Virtually all of these neurons contain PR and estrogen receptor-α (25, 32), whereas only a small percentage of other subpopulations of neurons in the ARC (e.g. proopiomelanocortin, neuropeptide Y, dopaminergic) contain steroid receptors (26, 32). As previously described, there is now considerable evidence that dynorphin found in KNDy neurons mediates progesterone-negative feedback in ewes (22, 24, 25). It is interesting to note that these neurons also contain NKB and kisspeptin, both of which stimulate GnRH release in sheep (58, 59) and are necessary for GnRH secretion in humans (60–62). It is thus possible that progesterone-negative feedback occurs via inhibition of kisspeptin and/or NKB release from KNDy neurons. Kisspeptin has been implicated in the negative-feedback action of E2 in rodents (36), and E2 inhibits kisspeptin expression in sheep (63, 64). In contrast, progesterone treatment does not significantly alter Kiss1 mRNA in sheep (63, 65). The possible role of NKB in steroid-negative feedback remains to be investigated.
In conclusion, the data reported here support the hypothesis that the ARC, but not the POA, is a critical site for progesterone-negative feedback in the ewe. Taken together with earlier data on the role of dynorphin in this action of progesterone, they are consistent with the hypothesis that progesterone acts to increase dynorphin release from ARC KNDy neurons that in turn inhibits GnRH pulse frequency, although further work is needed to more directly test this hypothesis.
Acknowledgments
We thank Heather Bungard and Jennifer Lydon at the West Virginia University Food Animal Research Facility for care of animals and Paul Harton for his technical assistance in sectioning tissue. We also thank Dr. Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH.
This work was supported by National Institutes of Health Grant R01 HD39916.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular area
- E2
- estradiol
- EOP
- endogenous opioid peptides
- KNDy
- NKB, kisspeptin, and dynorphin
- MBH
- medial basal hypothalamus
- NKB
- neurokinin B
- OVX
- ovariectomized
- POA
- preoptic area
- PR
- progesterone receptor.
References
- 1. Zeleznik AJ, Pohl CR. 2006. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD. ed. Knobil and Neill's physiology of reproduction. 3rd ed Vol. 2 Amsterdam: Elsevier; 2449–2510 [Google Scholar]
- 2. Freeman ME. 2006. The neuroendocrine control of the ovarian cycle of the rat. In: Neill JD. ed. Knobil and Neill's physiology of reproduction. 3rd ed New York: Elsevier; 2327–2388 [Google Scholar]
- 3. Goodman RL, Inskeep EK. 2006. Neuroendocrine control of the ovarian cycle of the sheep. In: Neill JD. ed. Knobil and Neill's physiology of reproduction, 3rd ed Vol 2 Amsterdam: Elsevier; 2389–2447 [Google Scholar]
- 4. Goodman RL, Karsch FJ. 1980. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- 5. Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. 1984. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 58:378–383 [DOI] [PubMed] [Google Scholar]
- 6. Evans NP, Dahl GE, Glover BH, Karsch FJ. 1994. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol prior to the preovulatory surge in the ewe. Endocrinology 134:1806–1811 [DOI] [PubMed] [Google Scholar]
- 7. Karsch FJ. 1987. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol 49:356–382 [DOI] [PubMed] [Google Scholar]
- 8. Moenter SM, Caraty A, Locatelli A, Karsch FJ. 1991. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- 9. Sollenberger MJ, Carlsen EC, Johnson ML, Veldhuis JD, Evans WS. 1990. Specific physiological regulation of LH secretory events throughout the human menstrual cycle: new insights into the pulsatile mode of gonadotropin release. J Neuroendocrinol 2:845–852 [DOI] [PubMed] [Google Scholar]
- 10. Hauger RL, Karsch FJ, Foster DL. 1977. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol, and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 101:807–817 [DOI] [PubMed] [Google Scholar]
- 11. Filicori M, Flamigni C, Campaniello E, Ferrari P, Meriggiola MC, Michelacci L, Pareschi A, Valdiserri A. 1989. Evidence for a specific role of GnRH pulse frequency in the control of the human menstrual cycle. Am J Physiol 257:E930–E936 [DOI] [PubMed] [Google Scholar]
- 12. Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. 1998. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Nat Acad Sci USA 95:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fox SR, Harlan RE, Shivers BD, Pfaff DW. 1990. Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology 51:276–283 [DOI] [PubMed] [Google Scholar]
- 14. Skinner DC, Caraty A, Allingham R. 2001. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- 15. King JC, Tai DW, Hanna IK, Pfeiffer A, Haas P, Ronsheim PM, Mitchell SC, Turcotte JC, Blaustein JD. 1995. A subgroup of LHRH neurons in guinea pigs with progestin receptors is centrally positioned within the total population of LHRH neurons. Neuroendocrinology 61:265–275 [DOI] [PubMed] [Google Scholar]
- 16. Quigley ME, Yen SS. 1980. Role of endogenous opiates on LH secretion during the menstrual cycle. J Clin Endocrinol Metab 51:179–181 [DOI] [PubMed] [Google Scholar]
- 17. Reid RL, Quigley ME, Yen SS. 1983. The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab 57:1107–1110 [DOI] [PubMed] [Google Scholar]
- 18. Van Vugt DA, Lam NY, Ferin M. 1984. Reduced frequency of pulsatile luteinizing hormone secretion in the luteal phase of the rhesus monkey. Involvement of endogenous opiates. Endocrinology 115:1095–1101 [DOI] [PubMed] [Google Scholar]
- 19. Yang K, Haynes NB, Lamming GE, Brooks AN. 1988. Ovarian steroid involvement in endogenous opioid modulation of LH secretion in mature ewes during the breeding and non-breeding seasons. J Reprod Fertil 83:129–139 [DOI] [PubMed] [Google Scholar]
- 20. Devorshak-Harvey E, Bona-Gallo A, Gallo RV. 1987. Endogenous opioid peptide regulation of pulsatile luteinizing hormone secretion during pregnancy in the rat. Neuroendocrinology 46:369–378 [DOI] [PubMed] [Google Scholar]
- 21. Whisnant CS, Goodman RL. 1988. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod 39:1032–1038 [DOI] [PubMed] [Google Scholar]
- 22. Goodman RL, Gibson M, Skinner DC, Lehman MN. 2002. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl 59:41–56 [PubMed] [Google Scholar]
- 23. Wardlaw SL, Wehrenberg WB, Ferin M, Antunes JL, Frantz AG. 1982. Effects of sex steroids on β-endorphin in hypophyseal portal blood. J Clin Endocrinol Metab 55:877–881 [DOI] [PubMed] [Google Scholar]
- 24. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. 2004. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 25. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. 2002. Colocalization of progesterone receptors in the parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- 26. Lehman MN, Karsch FJ. 1993. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895 [DOI] [PubMed] [Google Scholar]
- 27. Bethea CL, Widmann AA. 1996. Immunohistochemical detection of progestin receptors in hypothalamic β-endorphin and substance P neurons of steroid-treated monkeys. Neuroendocrinology 63:132–141 [DOI] [PubMed] [Google Scholar]
- 28. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. 2005. Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin mRNA levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- 29. Rometo AM, Rance NE. 2008. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallo RV. 1990. κ-Opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol 2:685–691 [DOI] [PubMed] [Google Scholar]
- 31. Simerly RB, Young BJ, Carr AM. 1996. Co-expression of steroid hormone receptors in opioid peptide-containing neurons correlates with patterns of gene expression during the estrous cycle. Brain Res Mol Brain Res 40:275–284 [DOI] [PubMed] [Google Scholar]
- 32. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe also express dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 34. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chappell PE, Levine JE. 2000. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141:1477–1485 [DOI] [PubMed] [Google Scholar]
- 36. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scott CJ, Pereira AM, Rawson JA, Simmons DM, Rossmanith WG, Ing NH, Clarke IJ. 2000. The distribution of progesterone receptor immunoreactivity and mRNA in the preoptic area and hypothalamus of the ewe: upregulation of progesterone receptor mRNA in the mediobasal hypothalamus by oestrogen. J Neuroendocrinol 12:565–575 [DOI] [PubMed] [Google Scholar]
- 38. Dufourny L, Skinner DC. 2002. Progesterone receptor, estrogen receptor α, and the type II glucocorticoid receptor are coexpressed in the same neurons of the ovine preoptic area and arcuate nucleus: a triple immunolabeling study. Biol Reprod 67:1605–1612 [DOI] [PubMed] [Google Scholar]
- 39. Karsch FJ, Legan SJ, Hauger RL, Foster DL. 1977. Negative feedback action of progesterone on tonic luteinizing hormone secretion in the ewe: dependence on the ovaries. Endocrinology 101:800–806 [DOI] [PubMed] [Google Scholar]
- 40. Goodman RL, Bittman EL, Foster DL, Karsch FJ. 1981. The endocrine basis of the synergistic suppression of luteinizing hormone by estradiol and progesterone. Endocrinology 109:1414–1417 [DOI] [PubMed] [Google Scholar]
- 41. Goodman RL, Bittman EL, Foster DL, Karsch FJ. 1982. Alterations in the control of LH pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod 27:580–589 [DOI] [PubMed] [Google Scholar]
- 42. Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. 2001. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinising hormone via dopaminergic neurons in anoestrous ewes. J Neuroendocrinol 13:1051–1058 [DOI] [PubMed] [Google Scholar]
- 43. Karsch FJ, Foster DL, Bittman EL, Goodman RL. 1983. A role for estradiol in enhancing frequency on pulsatile LH secretion during the follicular phase of the estrous cycle of sheep. Endocrinology 113:1333–1339 [DOI] [PubMed] [Google Scholar]
- 44. Banks JA, Freeman ME. 1980. Inhibition of the daily LH release mechanism by progesterone acting in the hypothalamus. Biol Reprod 22:217–222 [DOI] [PubMed] [Google Scholar]
- 45. Blache D, Fabre-Nys C, Venier G. 1996. Inhibition of sexual behavior and the luteinizing hormone surge by intracerebral progesterone implants in the female sheep. Brain Res 741:117–122 [DOI] [PubMed] [Google Scholar]
- 46. Rubin BS, Barfield RJ. 1983. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized estrogen-primed rats. Endocrinology 113:797–804 [DOI] [PubMed] [Google Scholar]
- 47. Rubin BS, Barfield RJ. 1984. Progesterone in the ventromedial hypothalamus of ovariectomized estrogen-primed rats inhibits subsequent facilitation of estrous behavior by systemic progesterone. Brain Res 294:1–8 [DOI] [PubMed] [Google Scholar]
- 48. Blache D, Fabre-Nys CJ, Venier G. 1991. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res 546:241–249 [DOI] [PubMed] [Google Scholar]
- 49. Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. 1997. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology 138:3686–3694 [DOI] [PubMed] [Google Scholar]
- 50. Lehman MN, Coolen LM, Cheng G, Goodman RL. Orphanin FQ cells of the ovine hypothalamus express estradiol receptor-α and progesterone receptors, but not kisspeptin or tyrosine hydroxylase. Proc Annual Meeting of the Society for Study of Reproduction, 2011, Portland, OR (Abstract 603) [Google Scholar]
- 51. Nestor CC, Nesselrod GL, Valent M, Connors JM, Hileman SM, Goodman RL. Evidence that orphanin FQ is important for progesterone negative feedback in ewes. Proc Annual Meeting of Society for Study of Reproduction, 2011, Portland, OR (Abstract 604) [Google Scholar]
- 52. Micevych P, Sinchak K. 2008. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol 290:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Whisnant CS, Goodman RL. 1994. Effect of anterior hypothalamic deafferentation on the negative feedback of gonadal steroids on luteinizing hormone pulse frequency in the ewe. Domest Anim Endocrinol 11:151–159 [DOI] [PubMed] [Google Scholar]
- 54. Jackson GL, Leshin LS, Schillo KK. 1986. Effect of frontal hypothalamic deafferentation on duration of breeding season and melatonin secretion in the ewe. Biol Reprod 35:1277–1288 [DOI] [PubMed] [Google Scholar]
- 55. Currie WD, Joseph IB, Rawlings NC. 1991. Morphine, naloxone and the gonadotrophin surge in ewes. J Reprod Fertil 92:407–414 [DOI] [PubMed] [Google Scholar]
- 56. Walsh JP, Clarke IJ. 1996. Effects of central administration of highly selective opioid μ-, δ- and κ-receptor agonists on plasma luteinizing hormone (LH), prolactin, and the estrogen-induced LH surge in ovariectomized ewes. Endocrinology 137:3640–3648 [DOI] [PubMed] [Google Scholar]
- 57. Micevych P, Chaban V, Quesada A, Sinchak K. 2002. Oestrogen modulates cholecystokinin: opioid interactions in the nervous system. Pharmacol Toxicol 91:387–397 [DOI] [PubMed] [Google Scholar]
- 58. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin 3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 62. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 64. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and gonadotropin-inhibitory hormone expression and terminal connections to GnRH neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goodman RL, Rao A, Smith JT, Clarke IJ. Negative feedback control of Kiss-1 gene expression by estradiol and progesterone in the ewe. Proc First World Congress on Kisspeptin Signaling in the Brain, 2008 Cordoba, Spain p. 87 (Abstract 19) [Google Scholar]