Abstract
Defective clearance of apoptotic cells has been shown in systemic lupus erythematosus (SLE) and is postulated to enhance autoimmune responses by increasing access to intracellular autoantigens. Until now, research has emphasized inherited rather than acquired impairment of apoptotic cell engulfment in the pathogenesis of SLE. Here, we confirm previous results that efficient removal of apoptotic cells (efferocytosis) is bolstered in the presence of wild type mouse serum, through the C3 deposition on the apoptotic cell surface. In contrast, sera from three mouse models of SLE, MerKD, MRLlpr and NZBWF1, did not support and in fact actively inhibited apoptotic cell uptake. IgG autoantibodies were responsible for the inhibition, through the blockade of C3 recognition by macrophages. Consistent with this, IgG removal reversed the inhibitory activity within autoimmune serum and purified autoimmune IgG blocked both the detection of C3 on apoptotic cells and C3-dependent efferocytosis. Sera from SLE patients demonstrated elevated anti-C3b IgG that blocked detection of C3 on apoptotic cells, activity that was not found in healthy controls or patients with rheumatoid arthritis, nor in mice prior to the onset of autoimmunity. We propose that the suppression of apoptotic cell disposal by antibodies against deposited C3 may contribute to increasing severity and/or exacerbations in SLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multi-organ autoimmune disease that is characterized by the production of high titers of antibodies against nuclear antigens including double-stranded DNA, histones and small ribonuclear proteins (1, 2). Dying cells are thought to be the primary source of intracellular autoantigens in lupus, through the release of nucleosomes (3–5) and the display of nuclear antigens on membrane blebs (6, 7) in situations where apoptotic cell clearance is defective.
The death and disposal of aged and damaged cells is essential for the maintenance of healthy tissues. In healthy individuals, apoptotic cells are cleared with remarkable efficiency. This is in part due to alterations of the dying cell surface, which are recognized as “eat me” signals by phagocytes, which ingest apoptotic cells through a process that has been termed efferocytosis (3). Numerous serum proteins, including C1q, IgM, C-reactive protein and mannan-binding lectin deposit on apoptotic cells, initiating and amplifying the deposition of C3 and its degradation products C3b and iC3b (5) and resulting in enhanced removal via recognition by complement receptors CR3 and CR4 (4, 6, 7).
The processes that govern dying cell clearance are defective in SLE. As a result, apoptotic cells accumulate in affected tissues (8–12). The persistence of apoptotic cells is thought to result in secondary necrosis and the release of proinflammatory and proimmunogenic intracellular constituents that contribute to the pathogenic autoantibody production. Therefore, insight into the pathways that govern apoptotic cell ingestion may be critical to uncovering the mechanisms of disease progression in SLE.
Reports correlating inefficient clearance with systemic autoimmunity have evoked inherited defects in either components of the apoptotic cell recognition mechanisms, such as C1q (13), or defects in the macrophages’ ability to phagocytose apoptotic cells (14–17). However, the contribution of acquired defects in efferocytosis (those that arise as a consequence rather than the cause of disease progression) in SLE has not been fully explored.
In the present work, we investigate the hypothesis that breaks in lymphocyte tolerance may precede and be the cause of apoptotic cell clearance defects in systemic autoimmunity. We show that IgG antibodies that inhibit apoptotic cell uptake develop in three different strains of autoimmune mice. The inhibitory IgG antibodies were directed against C3b components on the apoptotic cells, yet did not alter the total amounts or composition of the bound C3. Instead, they are suggested to block the interaction between C3b bound to the apoptotic cell surface and phagocyte C3 receptors. In mice, these antibodies became apparent only as the animals developed their autoimmune state. Further analysis revealed higher titers of these antibodies in patients with SLE compared to healthy control subjects or patients with rheumatoid arthritis. Therefore, anti-C3 antibodies that develop as a consequence of deficient lymphocyte tolerance may serve to initiate and/or exacerbate defects in apoptotic cell clearance and thereby accentuate the autoimmune and/or inflammatory processes in SLE.
Materials and Methods
Mice
C57BL/6, Balb/c, ICR, MRL, MRLlpr and NZB/WF1 mice were purchased from Jackson Laboratories. MerKD mice were provided by Dr. Douglas Graham at the University of Colorado Denver Anschutz Medical Campus (Aurora, CO). C3−/ minus; mice were from Michael Carroll at Harvard (Boston, MA). MRL mice were used as a control for MRLlpr mice as they do not develop autoimmune disease until later in life. All animal studies were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at National Jewish Health approved all experimental procedures performed on the animals.
Human Cells and Serum
Whole blood was collected from healthy donors for the isolation of mononuclear cells using CPT tubes (BD) or sera in accordance with the guidelines of the IRB at National Jewish Health (Denver, CO). Deidentified serum samples from 43 SLE patients 18 patients with rheumatoid arthritis (RA), and 19 non-autoimmune controls (HC) were obtained from a research protocol approved by the IRB at University of Colorado Denver in which subjects had provided informed consent to allow their leftover samples to be used for additional research in association with linked clinical data. Deidentified serum samples from 10 additional SLE patients were obtained from the University of Colorado Clinical Rheumatology Laboratory and represent leftover samples after performance of clinical tests. Samples from lupus patients obtained from the Clinical Rheumatology Laboratory were identified based on ICD9 codes but information was not recorded that could link the samples back to the patients, thus the research involving these samples is not considered human subjects research and is exempt from the requirements of the U.S. Department of Health and Human Services under 45 CFR 46.101(b)(4). Deidentified serum samples from 16 additional healthy control (HC) subjects, also obtained from the University of Colorado Clinical Rheumatology Laboratory, were from anonymous blood bank donors used by the laboratory for quality control purposes and thus the research involving these samples is not considered human subjects research. SLE and HC sera was heat-inactivated for all experiments involving macrophage uptake so that differences in how sera were handled or changes in sera complement levels for each subject would not affect the total amount of complement in each sample. SLE sera with the greatest ability to block C3 detection and HC sera that did not block C3 detection in initial experiments were selected for further studies involving Protein G treatment.
Antibodies & Other Reagents
The FITC or HRP conjugated goat anti-mouse C3 and goat anti-human C3 antibodies were purchased from MP Biomedicals and used at 1:25 for flow cytometry and 1:1,000 for Western blots along with anti-actin (Chemicon) (1:20,000), peroxidase-conjugated rabbit anti-mouse IgG and rabbit anti-human IgG antibodies (Jackson Immunoresearch) (1:10,000 for Western blot and 1:1000 for ELISA). Control (isotype) goat IgG was from Jackson Immunoresearch and used at the same concentration as the anti-C3. Mouse serum was prepared from blood collected via cardiac puncture or tail vein. Purified human C3 protein was purchased from Quidel (San Diego, CA). Human TruStain Fc Receptor Blocking Solution was purchased from BioLegend (San Diego, CA) and used to treat HMDM at 1:20 in X-Vivo for 30 min prior to initiation of uptake assays. Mouse anti-human CD3 (BD) was used at 1 μg/106 Jurkat cells for 30 minutes prior to washing and initiation of Fc-mediated uptake assay.
Cells and Cell Culture
Murine J774 macrophages (ATCC) were cultured in DMEM (Mediatech Inc) with 10% heat-inactivated FBS (Atlanta Biological), 2 mM L-glutamine at 37°C and 10% CO2. Thioglycollate elicited peritoneal macrophages (Elicited Mø) were harvested from mice 72 hours after intraperitoneal injection of 1.5 ml sterile 4% aged thioglycollate (Sigma) and cultured similarly to J774. Bone marrow derived macrophages (BMDMø) were lifted with ice-cold PBS and plated for phagocytosis assays after seven days of culturing marrow from C57BL/6 mice on 150mm plastic dishes in DMEM with 20% Bovine Growth Serum (Hyclone), 10% L929-conditioned media, 2mM L-glutamine, 1mM sodium pyruvate and 55μM mercaptoethanol at 37°C and 7.5% CO2. Human monocyte derived macrophages (HMDMø) were cultured from buffy coats of whole blood for seven days in X-Vivo medium (Lonza) with 10% Pooled Human Sera at 37°C and 10% CO2. Human leukemia Jurkat T cell line (ATCC) was cultured in RPMI (MediaTech Inc) containing 10% heat-inactivated FBS supplemented with 2 mM L-glutamine in humidified 5% CO2 at 37°C. A primary CD4+ T cell clone isolated from NOD mice was a gift from Daniel Tonkin at National Jewish Health (Denver, CO). Murine splenocytes were prepared from C57BL/6 with a 100 μM cell strainer and were cultured similarly to Jurkats.
Induction of Apoptosis
Apoptosis of Jurkat T cells, CD4+ T cells or splenocytes (106 cells/ml in complete media) was induced by exposure to UV irradiation at 254 nm for 10 min followed by 2–3 hr of culture at 37°C. Percent apoptosis was confirmed by evaluation of nuclear morphology by light microscopy after PROTOCOL® Hema 3 staining.
Phagocytosis Assay
J774 macrophages were plated at 5 × 104 cells per 24-well two days before initiation of the assay and primary mouse macrophages at 2–3 × 105 cells per well for one day. HMDMø were cultured at 1 × 105 cells per 96-well for seven days. Macrophages were washed thoroughly with PBS and cultured for 2–3 hr in serum free media before the phagocytosis assays were performed. 0.5–1 × 106 targets, either apoptotic cells, viable cells or anti-CD3 coated Jurkats (Fc-mediated uptake), were co-cultured with effector macrophages for 1.5 hr. Macrophages were washed 4 times with ice-cold PBS, fixed and stained with PROTOCOL® Hema 3 staining. Phagocytosis (minimum 200 cells, 2–3 replicate wells per condition) was scored by visual inspection of blinded samples using light microscopy. Results are displayed as phagocytic index, which is defined as the total number of apoptotic targets ingested divided by the total number of phagocytes counted x 100.
For serum pre-treatment, apoptotic Jurkat T cells were pelleted and resuspended in DMEM alone or with 10% C57BL/6 mouse serum for 30 min at 37°C, washed with DMEM and resuspended in DMEM alone or with 10% C57BL/6 mouse serum for phagocytosis or flow cytometry. For C3 blockade, apoptotic Jurkat T cells were resuspended in DMEM plus 10% freshly prepared C57BL/6 mouse serum with anti-mouse C3 or isotype IgG before co-incubation with macrophages. For “mixed” sera samples, 2.5% autoimmune serum was added to 5% control sera just before the initiation of the phagocytosis assay. For HMDM, uptake of apoptotic Jurkats was done with 10% fresh human sera alone (Control) or mixed with 5% heat-inactivated SLE or HC sera in X-Vivo.
Flow Cytometry
Apoptotic Jurkat T cells were resuspended in DMEM containing 10% mouse serum, incubated for 30 min at 37°C, washed and stained at 0.75 × 106 cells in 0.1 ml volume on ice with the FITC-conjugated goat anti-mouse C3 or isotype antibody. 10% non-autoimmune serum + 5, 10 or 20% autoimmune mouse serum was used for mixed samples. For experiments with human sera, 15% fresh human sera in X-Vivo was used to opsonize Jurkats with C3. Cells were washed and incubated with X-Vivo (Control), 5% heat-inactivated SLE or HC sera, then washed and stained with FITC-conjugated goat anti-human C3 or isotype antibody. Cells were analyzed by flow cytometry using either the FACScan or FACSCalibur Systems running on CellQuest™ software (BD Biosciences) and plots were rendered using FlowJo software (Tree Star, Inc).
Mouse Serum Fractionation
DMEM with 6% sucrose and either MerKD or C57BL/6 sera was loaded onto a Superdex™ 200 gel filtration column and thirty-one 0.5 mL volume samples were collected. For each sample the protein concentration was determined by absorbance at 280 nM and phagocytosis assays were performed after adding C57BL/6 serum (5% final concentration) to duplicate wells for each fraction.
Depleting and purifying IgG from Sera
Protein G-Sepharose (Zymed) or Sepharose 4B (Amersham) was washed with PBS, blocked with 1% BSA, washed again and resuspended in a 50:50 bead:media (either DMEM + L-glutamine or X-Vivo) slurry. 50ul of sera and 300 μl of beads were incubated for 2 hrs while rotating at 4°C, pelleted and supernatant was collected. Beads were washed with media and mixed with the original supernatant to make 1ml total volume. All samples were filtered through a 0.45 μM filter before phagocytosis or flow cytometry assays were performed. Depletion of IgG from sera was confirmed by Western blot. IgG was eluted with 0.1 M glycine HCl (pH 2.8), neutralized with Tris (pH 9.0) and concentrated with an Amicon Ultra-4 (100,000 MWCO) filter.
Western Blots
Lysates of apoptotic cells (0.5–1×106), sera (2–20 μl) or purified human C3 (50 μg) was used for SDS-PAGE and transferred to PVD membranes. Membranes were blocked with 5% milk, probed with antibodies at concentrations listed above, washed, incubated with West Femto Maximum Sensitivity Substrate (Pierce) and visualized on film. Degradation of the C3 α-chain produces proteins of 115 kDa, 68 kDa and 43 kDA.
ELISA for C3b-specific, dsDNA-specific or Chromatin-specific IgG
200 μg of purified human C3b protein (Comptech) was coated onto ELISA plates (Falcon PVC) in PBS overnight at 4°C. The plates were blocked with 1% BSA in PBS for two hours at room temperature and washed four times with PBS + 0.05% Tween before adding 100 μl of a 1:800 dilution of human serum (SLE, RA, or HC) or 1:100 dilution of mouse sera (C57BL/6 or Mer) to each well. After two hours at room temperature, plates were washed and 100 μl peroxidase-conjugated anti-human IgG (Jackson Immunoresearch) or 100 μl peroxidase-conjugated anti-mouse IgG (ICN Pharmaceuticals, Inc) was added for one hour at room temperature. Plates were washed, and developed with 100 μL of ABTS (ThermoScientific). The optical density was read at 405nm on a microplate reader.
Anti-chromatin and anti-dsDNA Abs were detected by a sandwich ELISA as described (18) except SNF1 sera was used as a positive control and BALB/c sera as a negative control. The threshold for positive autoantibodies was set at the mean OD reading + 2 standard deviations for the C57BL/6 sera.
Statistical Analysis
Statistical analyses were performed on experiments conducted at least three times unless otherwise stated. Statistical analysis and p-value calculations were conducted using the JMP statistical program (SAS Institute). Dunnett’s tests were performed for single comparisons using mouse. Data represent the means ± S.E.M. from n independent experiments. Samples with * indicate a P-value less than 0.05 for the experimental sample compared to control. Pictures and histograms are from one representative experiment.
Results
Non-autoimmune mouse serum enhances murine macrophage-mediated apoptotic cell clearance in a C3-dependent fashion
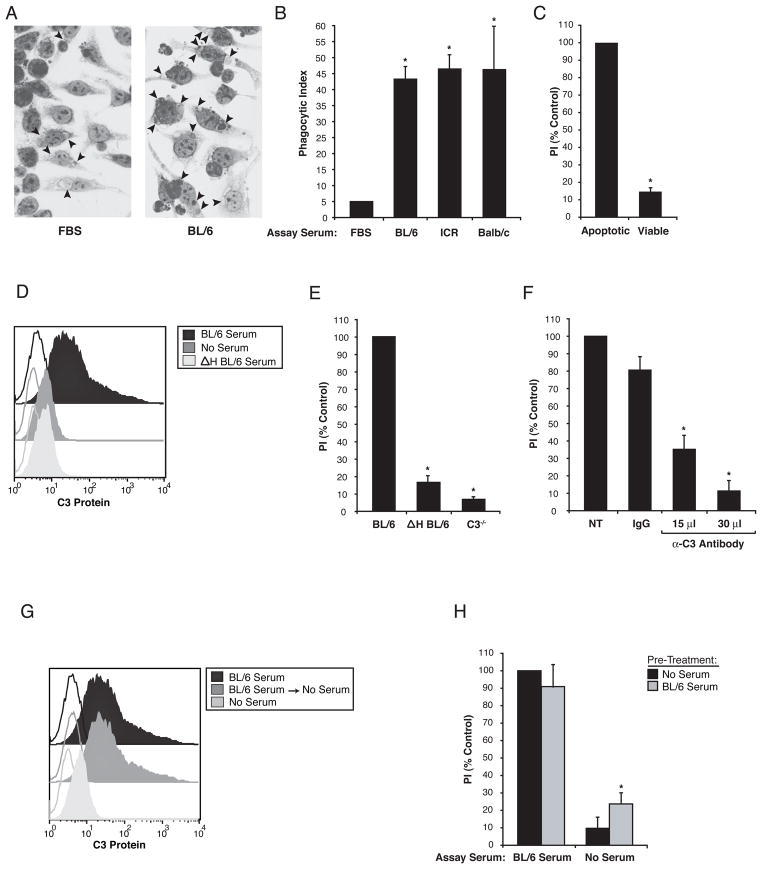
In order to examine the effect of autoimmune serum on the uptake of apoptotic cells by macrophages, we first examined the effect of non-autoimmune serum on this process. As shown in Figure 1, ingestion of apoptotic cells by murine macrophages was markedly enhanced in the presence of mouse serum compared to standard tissue culture conditions using heat-inactivated fetal bovine serum. The enhancement was equally effective with serum from three different mouse strains (Fig 1B) and did not occur with viable cells (Fig 1C). In keeping with studies using human serum reported earlier by Matsui et al (4) and Mevorach et al (6), the enhancement in this murine system was also shown to depend on complement activity and the presence of C3, i.e. it was abrogated by heat inactivation of the serum, blockade with an anti-C3 antibody or by use of serum from C3 minus;/ minus; mice (Fig 1E–F). Pretreatment of apoptotic cells with fresh mouse serum resulted in deposition of C3 on the apoptotic cell surface as demonstrated by FACS analysis (Fig 1D, G) and Western blotting (Fig 3B, D). The latter approach indicated that C3b and iC3b could be detected on the apoptotic cells. By itself, the deposition of C3 on the apoptotic cells was insufficient to mediate uptake into macrophages since the subsequent removal of the serum prevented the ingestion of C3-opsonized apoptotic cells (Fig 1H). These experiments support the notion that C3 is necessary, but not sufficient for the effect of serum in supporting uptake of apoptotic cells and that additional serum factors are required.
Figure 1. C3 deposition was necessary but not sufficient for serum enhancement of apoptotic cell engulfment.
(A) Comparison of apoptotic Jurkat T cell uptake by J774 macrophages in 10% heat-inactivated fetal bovine serum (FBS) or 10% fresh C57BL/6 mouse serum (BL/6). Arrows indicate engulfed apoptotic cells or cell fragments. (B) Quantification of engulfment assay described in (A) showed enhanced uptake using serum from BL/6, ICR or Balb/c mice compared to FBS, n=3. (C) Engulfment for apoptotic, but not viable, Jurkat T cells was seen using BL/6 serum, n=3. (D) C3 on the surface of apoptotic Jurkats was detected by FACS after treatment with BL/6 serum, but not after no serum or heat-inactivated BL/6 serum (Δ H BL/6), n=3. Compared to BL/6 serum, engulfment of apoptotic Jurkats was reduced in media containing (E) ΔH BL/6 and C3 knockout mouse serum (C3 ™/ ™), n=3. (F) Treatment of BL/6 serum with neutralizing goat anti-mouse C3 also reduced efferocytosis compared to non-treated (NT) or control antibody (IgG), n=3. (G) C3 could be detected on apoptotic Jurkats by FACS after treatment with BL/6 serum, even after a second incubation in serum free media (BL/6 Serum → No Serum), n=3. (H) Phagocytosis of Jurkats was observed when the assay was preformed in BL/6 serum but not without serum despite the presence of C3 on apoptotic Jurkats preincubated in BL/6 serum (grey bar) compared to no serum (black bar), n=3. The results in this figure are expressed as Phagocytic Index (PI), which is defined as the total number of apoptotic targets ingested divided by the total number of phagocytes counted multiplied by 100. PI is expressed as a percent of non-autoimmune serum control. Unfilled histograms are isotype labeling, filled histograms are anti-C3 labeling.
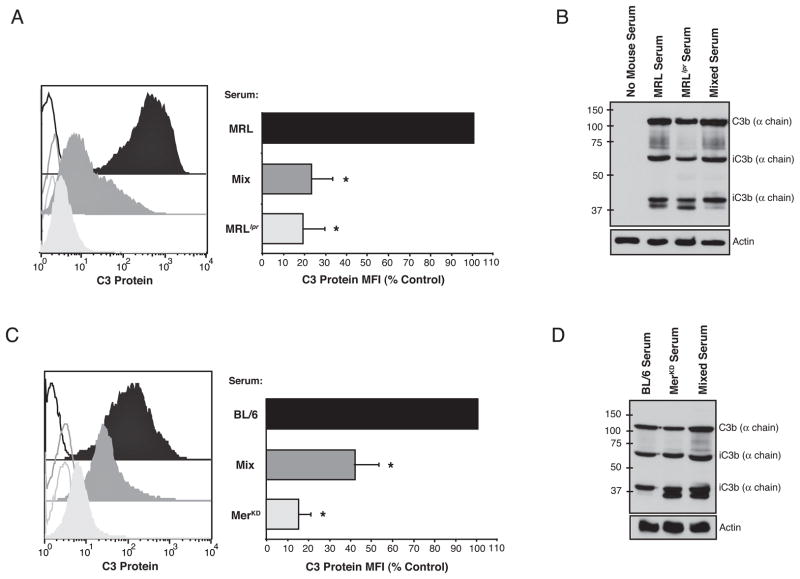
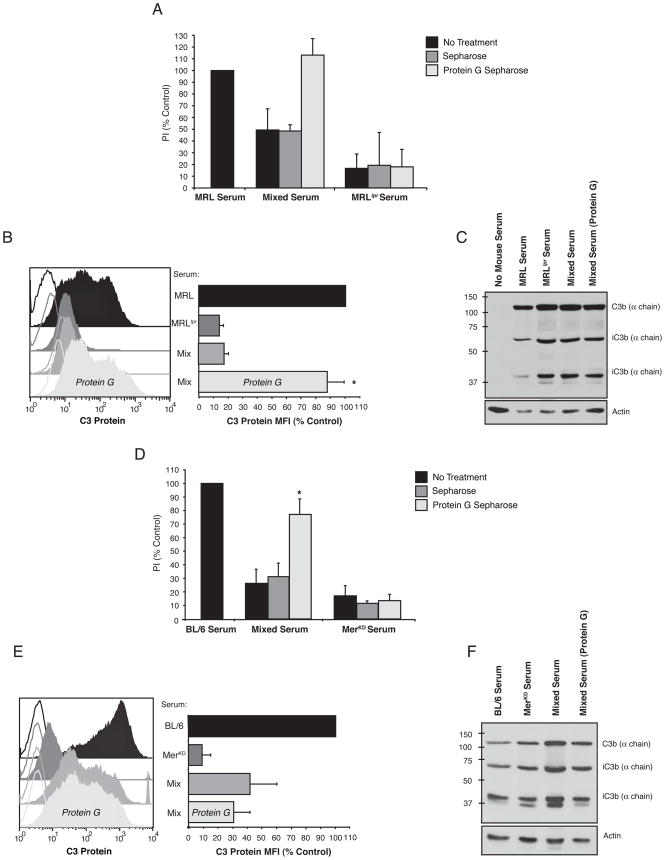
Figure 3. Sera from autoimmune mice inhibited C3 protein detection on the surface of apoptotic cells.
C3 on apoptotic Jurkats incubated in media containing non-autoimmune serum (black), non-autoimmune serum mixed with autoimmune serum (dark grey) and autoimmune serum (light grey) was detected by FACS (A,C) or Western blot (B, D). Inclusion of serum from autoimmune MRLlpr mice with pre-autoimmune MRL serum (A) blocked detection of C3 on apoptotic cells by FACS analysis (n=5) but not (B) total C3 fragment deposition determined by Western blotting of the apoptotic cell extracts (n=3). (C,D) Similar results were obtained with autoimmune MerKD serum mixed with non-autoimmune serum from C57BL/6 mice (n=3). Unfilled histograms are isotype labeling, filled histograms are anti-C3 labeling. The geometric mean fluorescence for C3 (C3 Protein MFI) is expressed as a percent of non-autoimmune serum control.
Autoimmune Serum Inhibits Apoptotic Cell Engulfment by Blocking C3 Detection
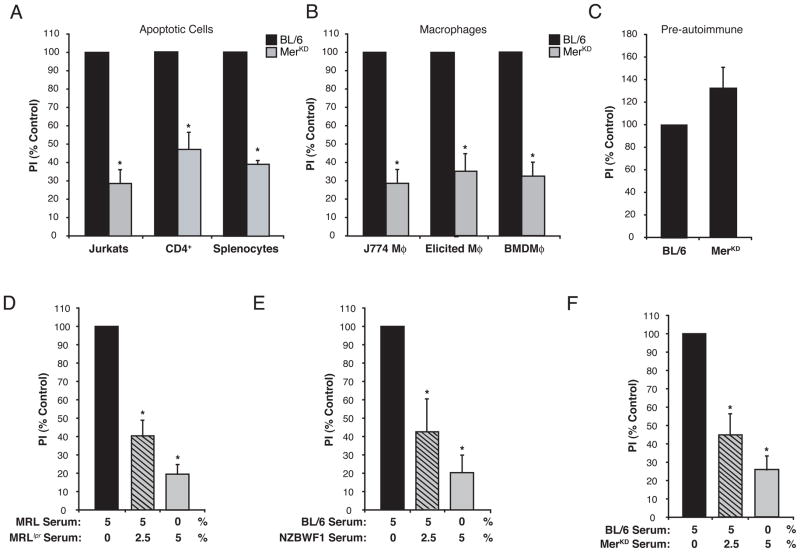
Systemic autoimmunity has previously been associated with defects in apoptotic cell clearance that were attributed to either impaired phagocyte activity or lack of serum factors to support uptake (19). Accordingly, serum from the MerKD murine model of systemic autoimmunity did not support the uptake of human or murine apoptotic targets (Fig 2A), regardless of the macrophages analyzed (primary or immortalized murine macrophages, (Fig 2B). Moreover, the defect in efferocytosis correlated with the chronological development of autoimmunity, as serum from pre-autoimmune, 6 week old MerKD mice (20) promoted similar levels of apoptotic cell uptake as that from age-matched C57BL/6 controls (Fig 2C). When sera from two other models of systemic autoimmunity were tested, neither MRLlpr nor NZB/WF1 serum proved capable of supporting efferocytosis to the levels seen in control sera (Fig 2D and E). The inability for autoimmune serum to support engulfment may be explained by either the absence of an enhancing factor or the presence of an inhibitor. To distinguish between these possibilities, efferocytosis was assessed in a mixture of non-autoimmune (C57BL/6) or pre-autoimmune (MRL) and autoimmune sera. The results demonstrated that sera from all three autoimmune strains inhibited apoptotic cell engulfment (Fig 2D–F).
Figure 2. Serum from autoimmune mice did not support, and in fact inhibited, apoptotic cell engulfment.
(A, B, C) Uptake was assessed after macrophages were incubated with apoptotic cells in media containing either C57BL/6 (black bars) or MerKD serum (grey bars). (A) Uptake of apoptotic targets, either Jurkat T cells (n=37), a mouse CD4+ T cell line (CD4+) (n=3) or mouse splenocytes (n=2), was reduced by MerKD serum compared to C57BL/6. (B) MerKD serum reduced apoptotic Jurkat engulfment by J774 macrophages (n=37), thioglycollate-elicited peritoneal macrophages (Elicited) (n=4), or bone marrow-derived macrophages (BMDM) (n=3). (C) Serum from 6 week-old Merkd mice, which have not yet developed autoimmunity, supported similar levels of efferocytosis as C57BL/6 serum, n=4. (D, E, F) J774 macrophages were co-cultured with apoptotic Jurkats in the presence of 5% non-autoimmune serum (black bar), 5% non-autoimmune serum mixed with 2.5% autoimmune serum (grey bar with black lines) or 5% autoimmune serum (grey bar). Serum from autoimmune (D) MRLlpr (n=9), (E) NZBWF1 (n=4) and (F) MerKD (n=3) did not support uptake of apoptotic Jurkats by themselves and inhibited the uptake of apoptotic Jurkats promoted by non-autoimmune (MRL or BL/6) serum. PI is expressed as a percent of non-autoimmune serum control.
To determine if autoimmune mouse sera blocked efferocytosis by interfering with C3 opsonization, levels of C3 on serum-exposed apoptotic cells were determined by both FACS analysis and Western blotting. These studies showed that C3 was nearly undetectable when cells were exposed to autoimmune serum from either MRLlpr (Fig 3A) or MerKD (Fig 3C) mice. Furthermore, C3 levels were sharply reduced when autoimmune serum was mixed with pre- or non-autoimmune serum. By contrast, C3 protein levels (and the distribution of sub-fragments) were unchanged when analyzed by Western blot (Fig 3B, D). Taken together, these results suggest that autoimmunity leads to development of an inhibitor of efferocytosis in these mice that blocks the macrophage recognition of complement C3 on the surface of apoptotic cells.
Identification of Anti-C3 Antibody as the Inhibitor Present in Autoimmune Serum
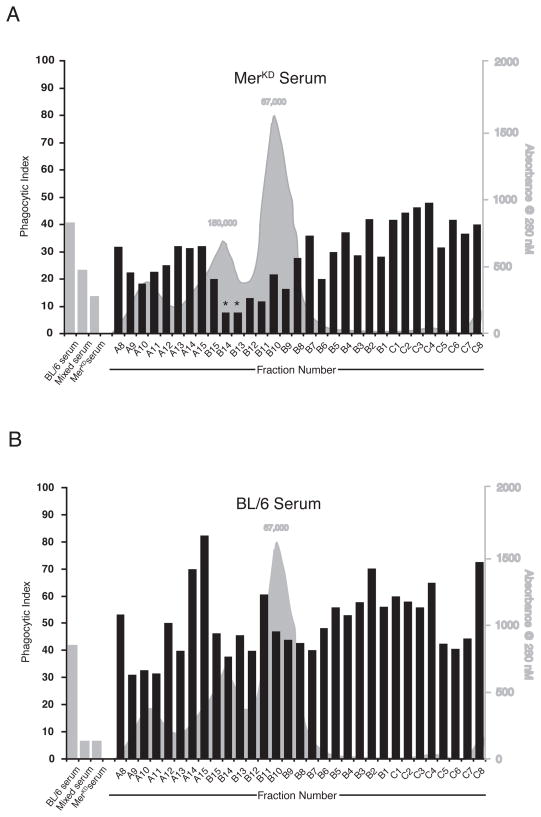
Superdex™200 gel filtration of serum was used to identify candidate molecules that inhibited the uptake of apoptotic cells by non-autoimmune sera. While several fractions from Mer sera displayed some inhibitory capacity, the strongest inhibitor found in autoimmune sera co-eluted with immunoglobulin G (IgG) (Fig 4A). The corresponding fractions from C57BL/6 serum did not inhibit engulfment (Fig 4B). Protein G removal of IgG from either MRLlpr or MerKD sera removed the inhibitor of apoptotic cell clearance (Fig 5A and D) and in the case of the MRLlpr serum, restored the ability to detect C3 on the apoptotic cells by FACS analysis (Fig 5B). In spite of the observation that protein G treatment removed the inhibitory activity present in MerKD serum, this treatment did not restore our ability to detect C3 on the surface of apoptotic cells by flow cytometry. (Fig 5E). Actual C3 deposition was unaltered by the presence of either MRLlpr or MerKD autoimmune serum (Fig 5C, F). However, protein-G-mediated removal of the inhibitory activity did not render either autoimmune sera capable of supporting high levels of apoptotic cell uptake that occurred in the presence of pre- or non-autoimmune serum (Fig 5A, D). Protein G treatment may remove enhancing factors that are required to promote optimal clearance or autoimmune serum may inherently lack these factors.
Figure 4. The inhibitory activity in autoimmune serum co-eluted with immunoglobulin.
Serum from (A) MerKD mice or (B) control C57BL/6 was fractionated on a Superdex™200 size gel filtration column. For each fraction, the relative serum protein levels (grey histogram) and apoptotic Jurkat T cell engulfment by J774 macrophages after mixing with C57BL/6 serum (black bars) are depicted. For phagocytosis assay, whole serum controls (grey bars) were 5% C57BL/6 serum, 5% C57BL/6 serum + 2.5% MerKD serum (mixed serum) and 5% MerKD serum. Analysis of the inhibitory activity in serum fractions from each strain was performed separately, and data represent the mean of samples run in duplicate from a single experiment.*Fractions in which both duplicates demonstrated inhibitory activity.
Figure 5. IgG depletion with protein G-sepharose removed the inhibitory activity present in autoimmune serum.
Analyses of MRLlpr (A–C) and MerKD (D–E) sera treated with protein G-sepharose beads to remove serum IgG. (A, D) Engulfment assays using J774 macrophages and apoptotic Jurkat T cells. Single serum samples contained 5% serum each while “Mixed Serum” samples contained 5% untreated pre- or non-autoimmune serum + 2.5% autoimmune serum. Autoimmune serum was either untreated (black bars), control sepharose bead treated (dark grey bars) or protein G-sepharose bead treated (light grey bars). Removing IgG from (A) MRLlpr serum and (C) Merkd serum using Protein G Sepharose did not restore the ability of these sera to promote uptake of apoptotic cells but did prevent these autoimmune sera from inhibiting the uptake of apoptotic cells when mixed with non-autoimmune sera, n=3. (B, E) C3 was detected by FACS on apoptotic Jurkats treated with either pre- or non-autoimmune serum (black), autoimmune serum (dark grey), pre- or non-autoimmune serum mixed with autoimmune serum (medium grey) or pre- or non-autoimmune serum mixed with protein G treated, IgG-depleted autoimmune serum (light grey). Protein G removal of IgG from (B) MRLlpr serum, but not (E) Merkd serum allowed C3 to be detected with the autoimmune sera was mixed with pre- or non -autoimmune sera, n=3. Western Blot demonstrated that C3 protein and distribution of its fragments bound to the apoptotic cells was unchanged when exposed to (C) MRL, MRLlpr or a mixture of both sera or (F) C57BL/6, Merkd or a mixture of both sera, n=3. PI is expressed as a percent of non-autoimmune serum control. Unfilled histograms are isotype labeling, filled histograms are anti-C3 labeling. The geometric mean fluorescence for C3 (C3 Protein MFI) is expressed as a percent of non-autoimmune serum control.
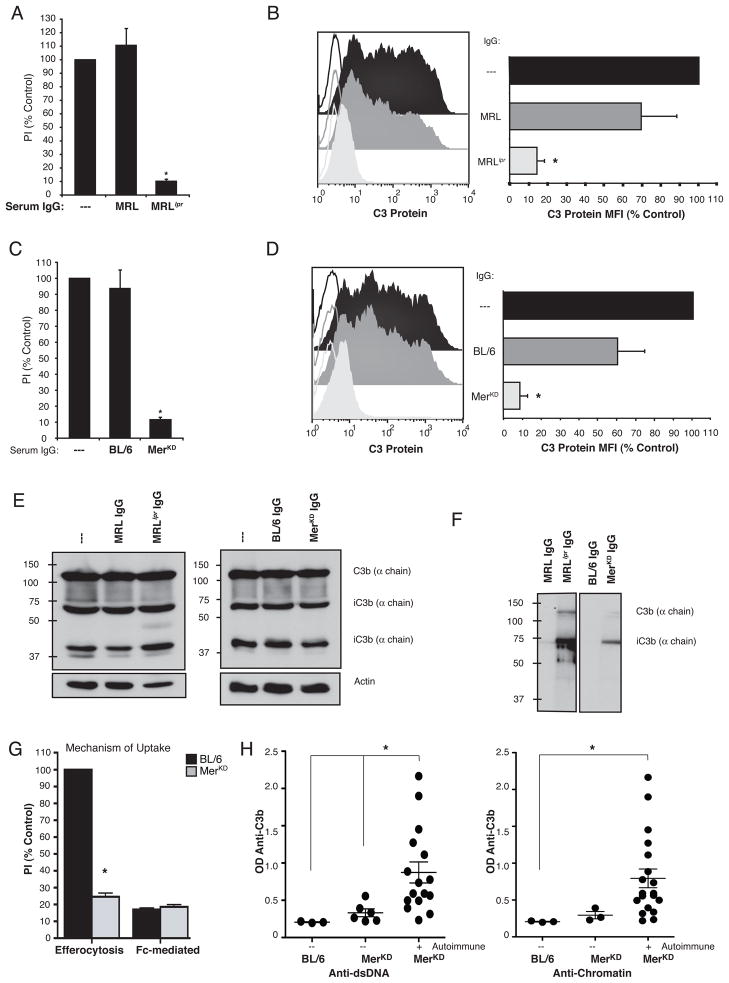
Protein G purified IgG from MRLlpr, MerKD and their control serum counterparts were examined to determine if IgG could directly block efferocytosis and C3 detection. IgG from both autoimmune strains reduced phagocytosis by 90% (Fig 6A, C) and extinguished C3 protein detection on the surface of apoptotic cells (Fig 6B, D). IgG from control sera had no effect. Western blot analysis confirmed that purified IgG purified from all sera did not alter the levels of C3 protein deposited on apoptotic cells (Fig 6E). IgG purified from MRLlpr or MerKD serum was also shown to bind directly to C3 by immunoblotting (Fig 6F). Although anti-C3 IgG bound to apoptotic cells would be predicted to facilitate FcR-mediated macrophage uptake, neither non-autoimmune C57BL/6 or autoimmune MerKD sera promoted the uptake of viable Jurkats opsonized with mouse anti-human CD3 for FcR-mediated uptake by J774, suggesting that FcR-mediated mechanisms of uptake are not utilized in this system (Figure 6G).
Figure 6. Apoptotic cell engulfment and C3 protein detection were inhibited by IgG purified from autoimmune sera.
(A, C) Engulfment of apoptotic Jurkats by J774 macrophages in pre- or non-autoimmune sera alone or mixed with IgG purified from either pre- or non-autoimmune or autoimmune sera. IgG purified from (A) MRLlpr and (C) MerKD sera inhibited the uptake of apoptotic Jurkats seen in the presence of pre- or non-autoimmune sera, n=4. (B, D) C3 was detected by FACS on apoptotic Jurkat T cells incubated with pre- or non-autoimmune serum alone (black), or pre- or non-autoimmune serum mixed with IgG purified from pre- or non-autoimmune (dark grey) or autoimmune (light grey) mouse serum. The detection of C3 was inhibited by IgG purified from (B) MRLlpr and (D) MerKD, n=3. IgG purified from non-autoimmune sera had no effect on uptake or FACS detection of C3, n=3. PI is expressed as a percent of non-autoimmune serum control. Unfilled histograms are isotype labeling, filled histograms are anti-C3 labeling. The geometric mean fluorescence for C3 (C3 Protein MFI) is expressed as a percent of non-autoimmune serum control. (E) C3 deposition was unaltered as detected by Western blot analysis of total C3 protein and distribution of its fragments bound to apoptotic cells exposed to pre- or non-autoimmune serum mixed with IgG purified from either autoimmune serum or pre- or non-autoimmune controls. (F) IgG from autoimmune MRLlpr and MerKD sera bound purified C3 and its degradation products by immunoblotting, n=3. (G) J774 macrophage phagocytosis of apoptotic Jurkats (Efferocytosis) but not anti-CD3 coated viable Jurkats (Fc-Mediated) was enhanced by C57BL/6 sera, n=3. (H–I) Sera from 23 MerKD mice were tested by ELISA for anti-C3b and markers of autoimmunity (anti-dsDNA or anti-chromatin). Each dot represents an individual animal, with the line representing the mean for the group and the whiskers representing the SEM. Elevated levels of anti-C3b were detected in the sera of MerKD mice with (H) anti-dsDNA (n=17) or (I) anti-chromatin (n=20) autoantibodies compared to the MerKD mice lacking these autoantibodies, n=6 and 3 respectively
Finally, levels of anti-C3b were measured in MerKD mice of both sexes and at varying ages and presumably stages of development of autoimmune disease. Anti-C3b levels were significantly higher in the sera of MerKD mice that had autoantibodies to anti-dsDNA compared to MerKD mice that lacked these autoantibodies (Fig 6H). A similar trend was seen in MerKD mice that had autoantibodies to anti-chromatin but the results were not statistically significant due to the much lower number of mice that lacked anti-chromatin autoantibodies in this analysis (Figure 6I). There was no correlation of anti-C3b levels with age of the mice or titers of autoantibodies (data not shown). Taken as a whole, these data suggest that IgG autoantibodies are elicited in the course of autoimmune disease that can inhibit apoptotic cell engulfment by binding to C3 on the surface of apoptotic cells and blocking its interaction with macrophage complement receptors.
C3-detection is Blocked by Immunoglobulin from the Sera of SLE patients
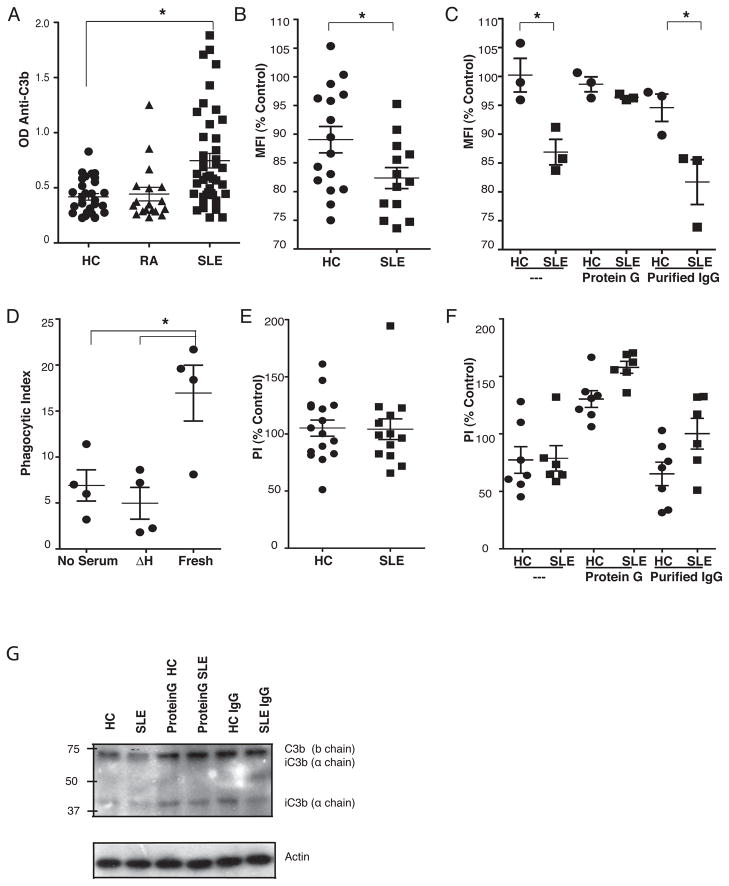
Human sera contains natural antibodies to C3 (9) as well as induced autoantibodies (immunoconglutins) that are thought to be generated in response to acute or chronic inflammation involving complement activation (10) but these antibodies have not been shown to directly interfere with macrophage uptake of C3-opsonized apoptotic cells. To test whether antibodies to C3 contribute to the clearance defects in human SLE as in murine models, the capacity of sera from SLE patients and healthy controls to block C3 accessibility and phagocytic uptake of serum-exposed apoptotic cells was evaluated. Sera from SLE patients contained significantly higher titers of anti-C3 antibodies when compared to sera from non-autoimmune controls or patients with RA (Fig 7A). Consistent with this, C3 detection on apoptotic cells incubated in fresh sera mixed with heat-inactivated SLE sera was more markedly reduced than C3 detection on apoptotic cells incubated in fresh sera mixed with heat-inactivated healthy control sera (Fig 7B). Protein G treatment of sera from the SLE patients with the highest level of inhibition of C3 detection restored the ability to detect C3 on apoptotic cells (Fig 7C) and IgG purified from these sera blocked C3 detection (Fig 7C). Deposition of C3 was not affected by SLE sera. Detection and deposition of C3 by sera from three healthy controls was not changed by these treatments (Fig 7C, G). As previously shown by Mevorach et al (6), human sera enhanced the uptake of apoptotic Jurkat cells by human monocyte-derived macrophages (HMDM) and this was blocked by heat inactivation (Figure 7D). However, the efferocytosis mediated by fresh sera was not blocked in these experiments by mixing either SLE or healthy control sera (Fig 7E). Additional experiments were performed after blockade of macrophage Fcγ receptors in order to isolate complement-mediated uptake. In these experiments, Protein G treatment of the majority of serum samples in both groups improved macrophage uptake of C3-opsonized cells and IgG purified from these sera had no effect (Fig 7F).
Figure 7. SLE sera contained IgG against C3b that blocked opsonized C3 detection.
(A) ELISA showed that the mean anti-C3b titers were higher in sera from SLE patients (n=53) compared to sera from healthy controls (HC) (n=35) or patients with rheumatoid arthritis (n =18). (B) FACS detection of C3 on apoptotic Jurkats pre-incubated with fresh human sera (Control) was reduced by mixing heat-inactivated SLE (n=16) but not heat-inactivated HC sera (n=16). (C) Removal of IgG with Protein G treatment restored the FACS detection of C3 from selected SLE sera (n=3) while the IgG purified from these SLE sera blocked C3 detection (n=3). Removal of IgG and purifed IgG from HC sera had no effect on the detection of C3 (n=3). (D) HMDM uptake of apoptotic Jurkats was enhanced by fresh, but not heat inactivated (ΔH) human sera (n=4). (E) Mixing sera from SLE patients (n=14) or healthy controls HC (n=16) with fresh human sera did not inhibit uptake of apoptotic Jurkats compared to fresh sera alone (Control). (F) Uptake of apoptotic cells with fresh human sera mixed with either SLE (n=6) or HC (n=7) was increased by protein G treatment of these sera while the purified IgG had no effect. (G) Western blots of lysates revealed no change in total C3 bound to apoptotic cells incubated in fresh sera and mixed with SLE (n=6) or HC (n=7). Dots represent each individual sera, horizonal lines are mean for the group and whiskers are the SEM. The geometric mean of C3 staining (MFI) on apoptotic Jurkats pre-incubated in 15% human sera alone was used as the control. Phagocytic index of apoptotic Jurkats by HMDM in 10% fresh human sera alone was used as the control.
Discussion
The data presented herein suggests that deficiencies in apoptotic cell engulfment need not precede the onset of systemic autoimmunity but may instead arise as a result of C3-targeted immune responses. This study describes a mechanism whereby C3-specific IgG antibodies delay efferocytosis by blocking macrophage detection of activated C3 on apoptotic cells. Consistent with previous reports, C3 opsonization enhanced efferocytosis (4, 6) and autoimmune sera did not support apoptotic cell clearance (19). The current study expanded on these observations by demonstrating that autoimmune serum can contain antibodies against opsonized C3 that inhibit apoptotic cell uptake. We propose a model where acquired defects in efferocytosis may exacerbate intrinsic, inappropriate autoimmune responses.
The exposure of phosphatidylserine on the surface of dying cells has been suggested to initiate activation of the complement system with subsequent cell surface C3 deposition that can then contribute to removal by CR3 and CR4 receptors (4, 6, 7, 12). However, as noted in the current studies, opsonization of the apoptotic cells with C3 still required additional serum factors for uptake. This raises the possibility that C3 receptors in this system serve primarily as essential tethering ligands that then need additional stimuli to optimally induce the actual ingestion (8). Since removal of the inhibitory anti-C3 IgG from autoimmune serum did not restore the ability of that serum to support efficient apoptotic cell engulfment, even when complement components (C1q or C3) were added back (data not shown), we concluded that C3 was necessary, but not sufficient for clearance and that additional pro-phagocytic components (stimuli) were also lacking in the autoimmune serum.
Consistent with the hypothesis that serum from autoimmune animals inhibited efferocytosis by blocking the C3-dependent component of the uptake, depletion of IgG from MRLlpr serum prevented its ability to inhibit the efferocytosis as well as the detection of C3 on the apoptotic cells. The situation with the MerKD system, however, appeared more complex. IgG-depletion of MerKD serum removed its ability to inhibit control serum-mediated uptake but did not restore the ability to detect C3 on the apoptotic cells with exogenous antibody. One explanation for this result is that the macrophage CR3 and CR4 may recognize different domains of C3, or with greater affinity, than those bound by the C3 detection antibody. Alternatively, additional factors may be present in the Mer serum that mask C3 detection, but do not block macrophage tethering and removal. Attempts to remove IgM autoantibodies as one potential candidate for this factor did not restore the detection (data not shown).
Nonetheless, the IgG isolated from either autoimmune mouse strain was shown to exhibit C3 reactivity and was able to inhibit efferocytosis. Thus, in contrast to the IgG purified from control sera, the IgG purified from either MRLlpr or MerKD serum inhibited both C3 detection and apoptotic cell ingestion and also demonstrated significantly greater capacities to bind purified C3 protein and its degradation products, as shown by Western blot. Notably, both autoimmune strains produced IgG that specifically bound the α chain of the iC3b protein, suggesting that C3-specific antibodies specifically block iC3b signaling through cognate receptors on macrophages.
Similar to the findings in autoimmune mice, IgG antibodies that blocked the detection of C3 on apoptotic cells were elevated in human sera from SLE patients. However, there was not a statistical decrease in efferocytosis mediated by monocyte-derived macrophages when SLE sera were mixed with fresh human serum. There are a number of explanations for the minimal effect in this in vitro mixing assay. For one, there may just not be as much anti-C3 antibody present as in the murine systems. Second, the IgG against activated C3 may block FACS detection of C3 while still allowing the recognition of C3 by HMDM complement receptors. The anti-C3 IgG might itself promote FcR-mediated clearance by the human macrophages. Finally, the presence of autoantibodies in the SLE sera that interact with antigens on the apoptotic cells other than C3 may drive removal of apoptotic cells using non-efferocytic mechanisms (9). Conversion from C3-mediated to FcR-mediated uptake of the apoptotic cells would be a pathogenic switch from an immunosuppressive and anti-inflammatory process to one that was both pro-inflammatory and potentially immunogenic.
It has been shown that the paucity of Mer expression in MerKD mice leads to a primary defect in macrophages that prevents apoptotic cell ingestion (11) that contributes to the subsequent development of autoimmunity (20). However, sera from all three murine models of systemic autoimmunity examined exhibited the inhibitory activity, independent of primary defects in macrophage-mediated efferocytosis. This suggests that autoimmunity in the MerKD mice might result from the in vivo clearance deficiency of the macrophages and be exacerbated by the development of the antibody inhibitor to efferocytosis. Our preliminary fractionation data suggested that there may also be other inhibitors present in Mer sera that we did not investigate. In the other autoimmune strains, as well as human patients, additional and/or alternative uptake defects may also contribute to the decreased apoptotic cell clearance that has been hypothesized as the source of autoantigens for the autoimmune response.
It is intriguing to note that autoantibodies termed immunoconglutinins, and later shown to react against “fixed” C3 have been noted since the 1930s (21), and in fact represented a subject of investigation for one of us in his PhD project (22). Elevated levels of immunoconglutins, with varying immunoglobulin isotypes, have been reported in the sera from patients with chronic infection or autoimmune diseases, including SLE and rheumatoid arthritis where they increased during exacerbations (10). Whether in man these exhibit variable effects in apoptotic cell clearance depending on isotype (i.e. ability to react with Fc receptors) or fine specificity against the C3 antigen is not clear, but the possible effect described herein on blocking C3 recognition could be a contributing factor in the progression and/or exacerbations seen in systemic autoimmune diseases.
Acknowledgments
J.M. Thurman is a stockholder in and consultant for Taligen Therapeutics, Inc.
Footnotes
This work was funded by National Institutes of Health grants GM61031 and HL81151. Additional support for J.M. Thurman through DK076690, S.A. Boackle through AI070304, and D.L. Bratton through A1058228 and HL4303.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501–537. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 4.Matsui H, Tsuji S, Nishimura H, Nagasawa S. Activation of the alternative pathway of complement by apoptotic Jurkat cells. FEBS Lett. 1994;351:419–422. doi: 10.1016/0014-5793(94)00897-3. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CA, Elkon KB. Role of complement and other innate immune mechanisms in the removal of apoptotic cells. Curr Dir Autoimmun. 2006;9:120–142. doi: 10.1159/000090776. [DOI] [PubMed] [Google Scholar]
- 6.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269–272. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz HU, Jelezarova E. Complement amplification revisited. Mol Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Ekdahl KN, Sjoholm A, Nilsson UR, Sturfelt G. Detection and characterization of immunoconglutinins in patients with systemic lupus erythematosus (SLE): serial analysis in relation to disease course. Clin Exp Immunol. 1992;90:251–255. doi: 10.1111/j.1365-2249.1992.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 12.Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–955. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 14.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, Voll RE, Kalden JR, Herrmann M. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M. SLE--a disease of clearance deficiency? Rheumatology (Oxford) 2005;44:1101–1107. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 18.Giles BM, Tchepeleva SN, Kachinski JJ, Ruff K, Croker BP, Morel L, Boackle SA. Augmentation of NZB autoimmune phenotypes by the Sle1c murine lupus susceptibility interval. J Immunol. 2007;178:4667–4675. doi: 10.4049/jimmunol.178.7.4667. [DOI] [PubMed] [Google Scholar]
- 19.Licht R, Jacobs CW, Tax WJ, Berden JH. An assay for the quantitative measurement of in vitro phagocytosis of early apoptotic thymocytes by murine resident peritoneal macrophages. J Immunol Methods. 1999;223:237–248. doi: 10.1016/s0022-1759(98)00212-9. [DOI] [PubMed] [Google Scholar]
- 20.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombs AM, Coombs RR. The conglutination phenomenon. IX. The production of immuno-conglutinin in rabbits. J Hyg (Lond) 1953;51:509–531. doi: 10.1017/s0022172400036792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henson PM. Immunoconglutinins of different immunoglobulin classes demonstrated by the antiglobulin reaction. Immunology. 1968;14:697–705. [PMC free article] [PubMed] [Google Scholar]