Abstract
High antigen load in chronic viral infections has been associated with impairment of antigen-specific T cell responses; however, the relationship between antigen load in chronic Mycobacterium tuberculosis (Mtb) infection and functional capacity of Mtb-specific T cells in humans is not clear. We compared Mtb-specific T cell-associated cytokine production and proliferative capacity in peripheral blood from adults with progressively higher mycobacterial loads, i.e., persons with latent Mtb infection (LTBI), with smear − pulmonary tuberculosis (TB), and with smear+ TB. Patients with smear+ TB had decreased polyfunctional IFN-γ+IL-2+TNF-α+ and IL-2-producing specific CD4 T cells and increased TNF-α-single positive cells, when compared with smear − TB and LTBI. TB patients also had increased frequencies of Mtb-specific CD8 T cells, compared with LTBI. Mtb-specific CD4 and CD8 T cell proliferative capacity was profoundly impaired in individuals with smear+ TB, and correlated positively with ex vivo IFN-γ+IL-2+TNF-α+ CD4 T cells, and inversely with TNF-α single-positive CD4 T cells. During 6 months of anti-TB treatment, specific IFN-γ+IL-2+TNF-α+ CD4 and CD8 T cells increased, whereas TNF-α- and IFN-γ-single positive T cells decreased. These results suggest progressive impairment of Mtb-specific T cell responses with increasing mycobacterial load, and recovery of responses during therapy. Furthermore, these data provide a link between specific cytokine-producing subsets and functional capacity of Mtb-specific T cells, and between the presence of specific CD8 T cells ex vivo and active TB disease. Taken together, these data have potentially significant applications for diagnosis of TB and for identification of T cell correlates of TB disease progression.
Introduction
Infection with Mycobacterium tuberculosis (Mtb) can persist for the lifetime of the host. In approximately 10% of infected persons, clinical tuberculosis disease (TB) develops; approximately 9 million new cases occurred worldwide in 2009, of whom 1.5 million died (1). Clinical disease does not develop in the majority of individuals infected with Mtb, providing compelling evidence that the human immune system is capable of controlling the pathogen. However, the precise mechanisms contributing to loss of immune control and progression to active TB disease are not known.
Evidence from both animal and human models suggests an important role for both CD4 and CD8 T cells in successful immune control of Mtb infection. Mice lacking CD4 T cells show increased susceptibility to development of TB, compared with wild-type mice (2, 3). T helper 1 (Th1) cells are of particular importance, as IFN-γ-deficient mice and humans with IL-12 or IFN-γ-receptor abnormalities demonstrate increased susceptibility to mycobacterial diseases (4-6). Numerous comparisons of IFN-γ production by specific CD4 T cells in LTBI and TB disease have yielded opposing associations with infection status (7, 8); this may relate to limitations when measuring IFN-γ alone, whereas the immune response to Mtb is more complex. For example, the importance of TNF-α is suggested by observations of reactivation of latent Mtb infection (LTBI) (9), and with reduced antimycobacterial activity of CD8 T cells, when patients with autoimmune disease have received therapeutic neutralizing anti-TNF-α monoclonal antibodies (10).
Mtb-specific CD8 T cells are primed following transfer of mycobacterial antigens into the cytosol (11-13), or through cross-priming mediated by uptake of apoptotic vesicles from mycobacteria-infected macrophages by dendritic cells (DCs) (14). Recent evidence suggests progressive dysfunction of CD8 T cells in chronic Mtb infection; e.g., CD8 T cells in Mtb-infected mice gradually lose lytic potential during progression to the chronic phase of infection (15). Furthermore, CD8 T cells from individuals with pulmonary TB display decreased cytolytic activity and expression of cytotoxic molecules, compared with these cells from uninfected healthy controls (16, 17).
An overwhelming body of evidence from animal and human models of chronic viral infections indicates high levels of chronic antigen stimulation leads to functional impairment of antigen-specific T cell responses, with reduced cytokine production, cytotoxicity, and proliferative capacity (18-23). The capacity of antigen-specific T cells to produce multiple cytokines simultaneously (‘polyfunctional’ cells) has been associated with superior functional capacity (24) and has been correlated with control of human chronic viral infections such as human immunodeficiency virus (HIV) (25-27) and hepatitis C virus (HCV) (28). Moreover, polyfunctional T cells have been associated with protection against disease progression in mouse models of Leishmania major (29, 30) and Mtb (31). Polyfunctional T cells producing IFN-γ, IL-2, and TNF-α have been described in studies of Mtb-infected adults (32-35), although with differing conclusions regarding distribution of the various cytokine-producing CD4 T cell subsets in individuals with LTBI and TB.
Latent Mtb infection is characterized by the presence of Mtb-specific cellular immune responses in the absence of clinical symptoms. Pulmonary TB, the most common clinical manifestation of TB, is characterized by high levels of bacterial replication and the presence of bacilli in sputum that is directly detectable by sputum smear microscopy and/or culture. Although there is no direct quantitative measurement of bacterial load, previous clinical studies of immunocompetent adults have indicated that sputum smear grading at the time of diagnosis (negative, scanty, 1+, 2+, or 3+) correlates with sputum smear and culture conversion at two months of TB treatment, and is predictive of relapse and recurrence of TB disease (36, 37). Moreover, smear grading correlates with disease severity by chest x-ray scoring (38). The aim of this study was to determine the relationship between mycobacterial load and quantitative and qualitative measurements of Mtb-specific T cell functional capacity. We hypothesized that high mycobacterial load in individuals with high-grade smear+ TB would be associated with progressive dysfunction of Mtb-specific T cell responses, compared with lower mycobacterial load in individuals with smear − TB and LTBI.
Materials and Methods
Study population and sample collection
Participants were recruited for this study from the Cape Town region of South Africa. Participants were included when ≥ 18 years of age and seronegative for human immunodeficiency virus (HIV). Persons with LTBI and with pulmonary TB disease were enrolled. LTBI was defined by a positive response to CFP-10 and/or ESAT-6 peptides in a short-term whole blood intracellular cytokine staining (ICS) assay (see below), with no previous history of TB diagnosis or treatment. Diagnosis of pulmonary TB was based on epidemiological history, signs and symptoms, and roentgenographic findings consistent with TB; all included patients had either positive sputum smear microscopy and/or positive culture for Mtb. The number of bacilli in sputum smears was counted according to WHO guidelines (39), and two groups of TB patients were enrolled: smear − (<104 bacilli/ml sputum) and 2+ or 3+ smear-positive (>106 bacilli/ml sputum) (40). Peripheral blood was obtained in sodium heparin Vacutainer tubes (BD Biosciences), and peripheral blood mononuclear cells (PBMC) isolated by density centrifugation with Ficoll-Hypaque (Sigma-Aldrich), within 4 hours of collection. Blood from individuals with TB was obtained prior to or within 7 days of starting standard course anti-TB treatment, which was provided according to South African national health guidelines. In a subset of individuals with TB, blood was obtained again at 2 and 6 months following initiation of anti-TB treatment. All study participants gave written, informed consent for the study, which was approved by the Human Research Ethics Committee of the University of Cape Town and the Western Cape Department of Health.
Antigens
Peptides (15-mers overlapping by 10 aa) corresponding to the sequence of the Mtb antigens CFP-10 and ESAT-6 were pooled by protein, and used at 1.25 μg/ml/peptide in the whole blood intracellular cytokine staining (ICS) assay, and at 0.1 μg/ml in the 6-day PBMC proliferation assay. Tuberculin purified protein derivative (PPD; Staten Serum Institute) was used at 10 μg/ml in the whole blood ICS assay, and at 1 μg/ml in the 6-day PBMC proliferation assay. Staphylococcal enterotoxin B (SEB; Sigma-Aldrich; positive control) was used at 1 μg/ml in the whole blood ICS assay, and at 0.1 μg/ml in the 6-day PBMC proliferation assay.
Antibodies
The following fluorescently-conjugated monoclonal antibodies were used in this study: anti-CD3 Pacific Blue (UCHT1), anti-CD3 APC-H7 (SK7), anti-CD8 PerCP-Cy5.5 (SK-1), anti-IFN-γ Alexa Fluor 700 (B27), and anti-IL-2 FITC (5344.111), all from BD Biosciences, and anti-CD4 QDot605 (S3.5) (Invitrogen) and anti-TNF-α-PE-Cy7 (Mab11) (eBiosciences).
Whole blood intracellular cytokine staining (ICS) assay
One ml heparinized blood was incubated with antigen within 2 hours of blood collection, for 8 hrs at 37°C. Blood incubated with no antigen served as a negative control, and blood incubated with SEB served as a positive control. After 3 hrs, Brefeldin A (10 μg/ml; Sigma-Aldrich) was added, and the incubation continued for an additional 5 hrs. Following the 8 hr incubation, 2mM EDTA was added for 10 minutes, followed by red cell lysis and white cell fixation in FACS Lysing Solution (BD Biosciences). Stimulated, fixed white blood cells were then cryopreserved in freezing medium and stored in liquid nitrogen until use, as previously described (41). Cryopreserved, fixed white cells were later thawed and washed once in phosphate buffered saline (PBS), followed by a second wash in Perm/Wash Buffer (BD Biosciences). Cells were stained with monoclonal antibodies for 1 hr at 4°C, washed in Perm/Wash Buffer and resuspended in PBS prior to acquisition on a LSRII flow cytometer (BD Biosciences). At least 750,000 total events (median 2 × 106) were acquired for each sample.
PBMC proliferation assay
Freshly isolated PBMC were washed in PBS and labeled with 0.5 μg/ml CellTrace™ Oregon Green® 488 carboxylic acid diacetate, succinimidyl ester (OG; Invitrogen) per 1 × 107 cells. Cells were washed again in PBS and resuspended at 1 × 106/ml in complete medium (RPMI 1640 [Lonza] supplemented with 10% human male AB serum [Sigma-Aldrich], 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). OG-labeled cells were plated at 2 × 105 cells/well of a u-bottom 96-well tissue culture plate. Cells were stimulated for 6 days with CFP-10 and ESAT-6 peptide pools, or PPD. Each stimulation condition was set up in quadruplicate wells. Negative controls consisted of cells incubated with media alone; positive controls consisted of cells incubated with SEB. On day 6, cells from the quadruplicate wells were combined and washed in PBS and stained with LIVE/DEAD® Fixable Violet Dead Cell Stain (Vivid; Invitrogen). Cells were washed again in PBS, fixed with FACS Lysing Solution, and washed in Perm/Wash Buffer. Cells were stained with monoclonal antibodies for 1 hr at 4°C, washed in Perm/Wash Buffer and resuspended in PBS prior to acquisition on a BD LSRII flow cytometer. At least 55,000 total events (median 4.5 × 105) were acquired for each sample.
Flow cytometry data analysis
Multiparameter flow cytometry data were analyzed using FlowJo software (v9.0.2; Treestar). Doublet cell populations were excluded by plotting forward scatter area (FSC-A) versus forward scatter height (FSC-H). Single-stained anti-mouse Ig, κ beads (BD Biosciences) were used to calculate compensation. Combinations of cytokine-producing cells were determined using Boolean gating in FlowJo, followed by further analysis using Pestle v1.6.2 (Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and Spice v5.1 (42). Background cytokine production in the negative control of ICS assays was subtracted from each antigen-stimulated condition; background proliferation in the negative control condition of the PBMC proliferation assay was subtracted from antigen-stimulated conditions. All donors responded to SEB in the ex vivo ICS assay and in the PBMC proliferation assay. Responses in ICS and proliferation assays were considered positive if the frequency of cytokine-producing or proliferating CD4 or CD8 T cells in the stimulated sample was greater than the median plus 3 times the median absolute deviation of the negative control samples. For analysis of the ICS assay data, only individuals with positive T cell responses to Mtb antigens were included when calculating the proportion of each cytokine-producing subset contributing to the total Mtb-specific response.
Data analysis
Statistical testing was performed using GraphPad Prism v5.0a software. For analysis of whole blood ICS assay data, differences between the three participant groups was first assessed using the Kruskal-Wallis test, followed by the Dunn’s post-test. If significance was found (p<0.05), pair-wise comparisons were further analyzed using the Mann-Whitney test. The Fisher’s exact test was used to assess differences in the frequency of recognition of Mtb-specific CD8 T cell responses among the three groups. For longitudinal analysis of Mtb-specific T cell responses on anti-TB treatment, differences between time points were first assessed using the Friedman test, followed by the Dunn’s post-test. If significance was found (p<0.05), pair-wise comparisons were further analyzed using the Wilcoxon matched pairs test. Correlations were assessed using Spearman’s rank correlation.
Results
Participants
In order to assess the relationship between mycobacterial load and the quantity and quality of the Mtb-specific T cell responses, we recruited three groups of adults from the Cape Town region of South Africa, where TB is endemic: thirty healthy, asymptomatic adults with LTBI, and fifty-four individuals with pulmonary TB stratified into two groups based on acid fast bacilli (AFB) sputum smear results at the time of diagnosis. The first group had two successive 2+ or 3+ sputum smears (smear+ TB; n=38), whereas the second group had two successive AFB-negative sputum smears, while sputum cultures were positive for Mtb (smear − TB; n=16). Compared with LTBIs, TB patients were older and were more likely to be males (Table I).
Table 1.
Characteristics of study population
| Participant Group | n | AFB sputum smear grade | TB tx, days (range)2 | Age, years (range)3 | Sex (% male) |
|---|---|---|---|---|---|
| LTBI | 30 | N/A1 | N/A | 23 (18 – 50) | 33% |
| Smear− TB | 16 | Negative | 0 (0 – 7) | 45 (18 – 59)* | 77%* |
| Smear+ TB | 38 | 3+ (n=32); 2+ (n=6) | 2 (0 – 7) | 38 (19 – 60)* | 81%* |
N/A (not applicable)
Values denote median number of days on anti-TB treatment at first sample collection (range)
Values denote median (range)
P<0.05, compared with LTBI
Smear+ TB is associated with decreased polyfunctional and IL-2-producing, and increased TNF-α-producing Mtb-specific CD4 T cells, compared with smear− TB and LTBI
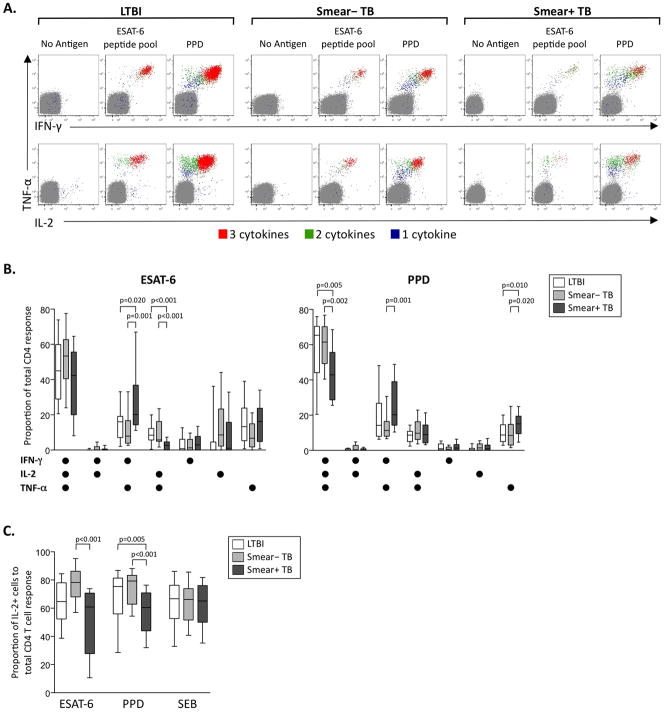
CD4 T cell responses play a key role in immune control of Mtb infection, and polyfunctional cytokine production of antigen-specific Th1 cells has been associated with control of chronic bacterial and viral infections (25, 26, 28-31). We used flow cytometry to measure the capacity of Mtb-specific CD4 T cells to produce cytokines in a short-term, whole blood ICS assay (Figure 1A, Figure S1A, Figure S2). Individuals with smear+ TB displayed decreased proportions of PPD-specific IFN-γ+IL-2+TNF-α+ and ESAT-6-specific IL-2+TNF-α+ CD4 T cells, and increased proportions of both ESAT-6- and PPD-specific IFN-γ+TNF-α+ and PPD-specific TNF-α-single positive CD4 T cells, compared with other groups (Figure 1B). These data suggested a possible selective decrease in production of IL-2 by Mtb-specific CD4 T cells in individuals with smear+ TB, and we thus determined the relative contribution of IL-2-producing cells to the total CD4 T cell response. We found decreased proportions of IL-2-producing Mtb-specific CD4 T cells in individuals with smear+ TB, compared with smear− TB and LTBI (Figure 1C). This decrease in IL-2 production was specific for Mtb, as there was no difference in IL-2 production in response to SEB stimulation. Together, these data indicate a shift in the cytokine production profiles of specific CD4 T cells with increasing mycobacterial load, characterized by progressive decreases in IL-2 and polyfunctional cytokine production capacity, co-incident with increased TNF-α production.
Figure 1. Reduced polyfunctional and IL-2-producing Mtb-specific CD4 T cells in individuals with smear+ TB, compared with smear− TB and LTBI.
Whole blood from individuals with LTBI, smear− TB and smear+ TB was stimulated for 8 hours with Mtb peptide pools, PPD, or SEB, and analyzed by flow cytometry for intracellular expression of IFN-γ, IL-2, and TNF-α in CD4 T cells. (A) Representative whole blood ICS assay data from an LTBI donor (left), a smear− TB donor (middle), and a smear+ TB donor (right). The plots shown were gated on CD3+CD4+ T cells; cells expressing all 3 cytokines (3+; red), any 2 cytokines (2+; green), or any one cytokine only (1+; blue) are shown on each plot. (B) Qualitative analysis of ESAT-6 and PPD-specific CD4 T cell responses. The contribution of each cytokine-producing subset to the total ESAT-6 or PPD-specific CD4 T cell response was assessed in donors with positive responses in the ICS assay to ESAT-6 (n=23 LTBI, n=12 smear− TB, n=23 smear+ TB) and PPD (n=30 LTBI, n=16 smear− TB, n=38 smear+ TB). (C) Contribution of IL-2-producing CD4 T cells to the total ESAT-6, PPD, and SEB-specific CD4 T cell response. The total proportion of IL-2-producing cells to the total CD4 response was determined in each donor shown in panel B. All donors had a positive CD4 response to SEB, and were thus included in the contribution of IL-2 to the total SEB response. For the data in panels B and C, differences between the three groups were assessed by the Kruskal-Wallis test, and if significance was found (p<0.05), the Mann-Whitney test was used for comparison of two groups. For box and whiskers plots, the horizontal line represents the median, the boxes the interquartile range, and the whiskers the 10th and 90th percentiles.
Increased Mtb-specific CD8 T cell responses in pulmonary TB
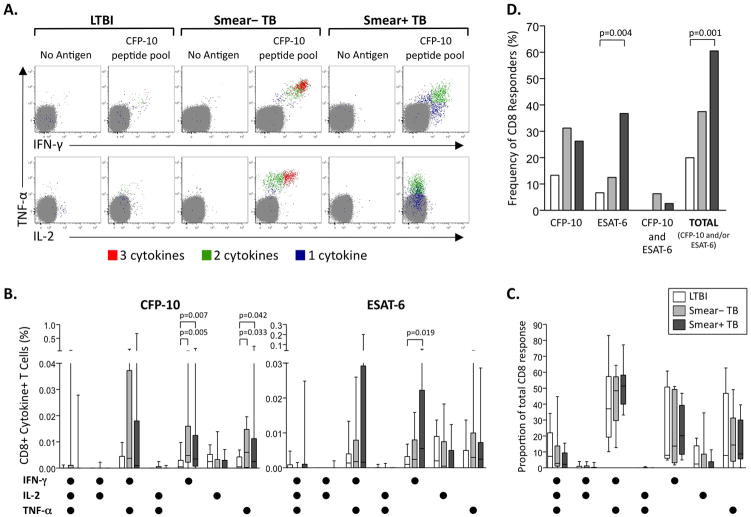
We next investigated the effect of mycobacterial load on Mtb-specific CD8 T cell responses in peripheral blood ex vivo. Individuals with TB had higher frequencies of IFN-γ and TNF-α-producing specific CD8 T cells, compared with LTBI (Figure 2A, B). In all groups, the dominant cytokine-producing subsets of Mtb-specific CD8 T cells consisted of single and double-positive IFN-γ and TNF-α-producing cells (Figure 2C).
Figure 2. Increased frequency of Mtb-specific CD8 T cell responses in individuals with pulmonary TB, compared with LTBI.
Whole blood from individuals with LTBI, smear− TB and smear+ TB was stimulated for 8 hours with Mtb peptide pools, PPD, or SEB, and analyzed by flow cytometry for intracellular expression of IFN-γ, IL-2, and TNF-α in CD8 T cells. (A) Representative whole blood ICS assay data from an LTBI donor (left), a smear− TB donor (middle), and a smear+ TB donor (right). The plots shown were gated on CD3+CD8+ T cells; cells expressing all 3 cytokines (3+; red), any 2 cytokines (2+; green), or any one cytokine only (1+; blue) are shown on each plot. (B) Comparison of the frequency of each cytokine-producing CD8 T cell subset specific for CFP-10 (left) or ESAT-6 (right) in LTBI (n=30), smear− TB (n=16), and smear+ TB donors (n=38). Background cytokine production in the negative control sample has been subtracted. Differences between the groups were assessed by Kruskal-Wallis test; if significance was found (p<0.05), the Mann-Whitney test was used for comparison between two groups. (C) Qualitative analysis of cytokine-producing CFP-10 and ESAT-6-specific CD8 T cell responses. The contribution of each cytokine-producing subset to the total CD8 T cell response was assessed in donors with positive responses to either CFP-10 or ESAT-6 (n=6 LTBI, n=6 smear− TB, n=23 smear+ TB). (D) Frequency of Mtb-specific CD8 T cell responders. The percentage of LTBI, smear− TB, and smear+ TB donors who had a positive CD8 T cell response in the ICS assay to either CFP-10 and/or ESAT-6 is shown. Differences in the frequency of recognition of these antigens were determined by the Fisher’s exact test. For all graphs, white bars represent individuals with LTBI, light grey bars smear− TB, and dark grey bars smear+ TB.
To determine if the presence of Mtb-specific CD8 T cell responses was specifically associated with pulmonary TB disease status, we compared the number of individuals in each group with positive CFP-10 and/or ESAT-6 CD8 T cell responses. Greater than 60% of individuals with smear+ TB had detectable Mtb-specific CD8 T cell responses, compared with 38% and 20% of individuals with smear− TB and LTBI, respectively. Within Mtb-specific CD8 T cell responders, individuals responded to either CFP-10 or ESAT-6, with CFP-10-specific and ESAT-6-specific CD8 T cell responses within an individual rarely observed (Figure 2D). Together, these data strongly suggest antigen-driven expansion of Mtb-specific CD8 T cell responses that are detectable ex vivo in peripheral blood of individuals with pulmonary TB, compared with LTBI.
Increase in polyfunctional Mtb-specific T cell responses following reduction in bacterial load by anti-TB treatment
The above results suggest that increasing mycobacterial load may lead to progressive quantitative and qualitative changes in Mtb-specific CD4 and CD8 T cell responses. In order to determine whether reduction of bacterial load by antibiotic treatment was associated with enhanced functional capacity of Mtb-specific T cells, we followed 13 TB patients longitudinally after initiating anti-TB treatment; all were sputum smear− by 6 months of treatment (representative data in Figures 3A and 4A).
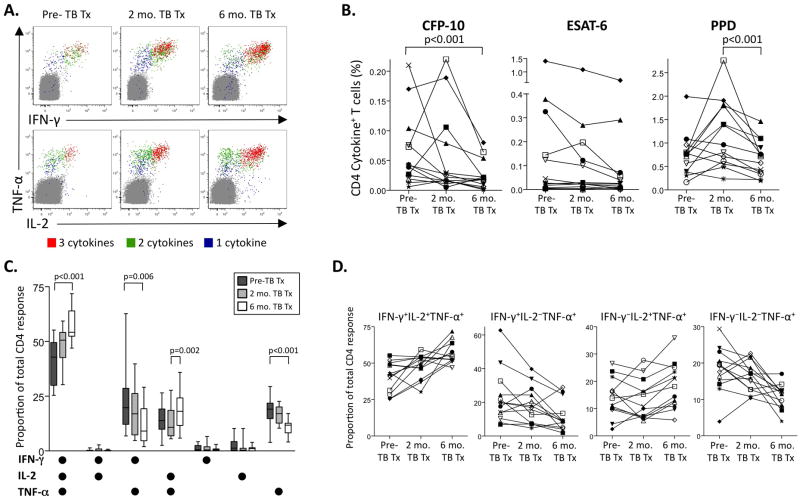
Figure 3. Polyfunctional Mtb-specific CD4 T cell responses increase following reduction in mycobacterial load by initiation of anti-TB treatment.
Whole blood ICS assays were performed on a subset of individuals with pulmonary TB (n=4 smear− TB, n=9 smear+ TB) prior to or within 7 days of anti-TB treatment (pre-TB tx), and again at 2 and 6 months following initiation of TB treatment. (A) Representative ICS data from PPD-stimulated whole blood of an individual with smear+ TB pre-treatment, and 2 and 6 months on TB treatment. Plots are gated on CD3+CD4+ T cells; cells expressing all 3 cytokines (3+; red), any 2 cytokines (2+; green), or any one cytokine only (1+; blue) are shown on each plot. The percentages shown are after subtraction of background cytokine production by CD4 T cells in the negative control at each time point. (B) Longitudinal analysis of the total frequency of IFN-γ, TNF-α, and IL-2-producing CD4 T cells following 8-hour stimulation of whole blood with CFP-10 and ESAT-6 peptide pools, and PPD. Each symbol represents a different donor, with the same symbols used for each donor in all three graphs. Differences in the total frequency of Mtb-specific CD4 T cells over the three time points were determined by the Freidman test; if significance was found (p<0.05), the Wilcoxon matched pairs test was used for comparison between two time points. (C) Contribution of each cytokine-producing subset to the total PPD-specific CD4 T cell response pre-treatment, and 2 and 6 months on TB treatment. All 13 individuals followed longitudinally maintained a positive PPD-specific response throughout treatment, and were therefore included in this analysis. Differences in the contribution of each subset between the three time points were determined by the Freidman test; if significance was found (p<0.05), the Wilcoxon matched pairs test was used for comparisons between two time points. (D) Longitudinal analysis of the proportion of cytokine-producing CD4 T cells to the total PPD-specific response. The four different cytokine-producing subsets of PPD-specific CD4 T cells that showed a significant change on TB treatment (panel C) were assessed on an individual donor level at each time point. Each symbol represents a different donor, with the same symbols used for each donor in all 4 graphs. The same symbols were used for each donor in panels B and D.
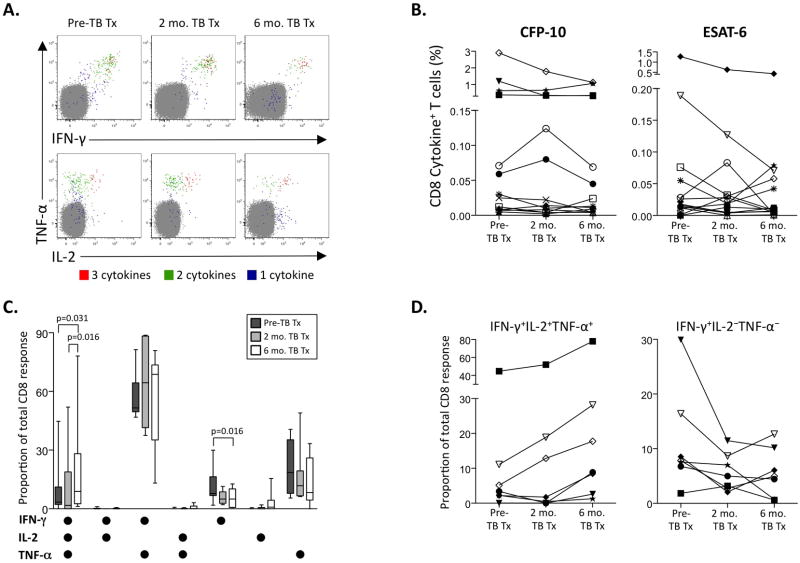
Figure 4. Polyfunctional Mtb-specific CD8 T cell responses increase following reduction in mycobacterial load by initiation of anti-TB treatment.
Whole blood ICS assays were performed on a subset of individuals with pulmonary TB as described in Figure 3. (A) Representative whole blood ICS data from ESAT-6 peptide pool-stimulated whole blood of an individual with smear+ TB pre-treatment, and 2 and 6 months on TB treatment. Plots are gated on CD3+CD8+ lymphocytes; cells expressing all 3 cytokines (3+; red), any 2 cytokines (2+; green), or any one cytokine only (1+; blue) are shown on each plot. The percentages shown are after subtraction of background cytokine production by CD8 T cells in the negative control at each time point. (B) Longitudinal analysis of the total frequency of IFN-γ, TNF-α, and IL-2-producing CD8 T cells following 8-hour stimulation of whole blood with CFP-10 and ESAT-6 peptide pools. Each symbol represents a different donor, with the same symbols used for each donor in both graphs. No differences in the total frequency of CFP-10 or ESAT-6-specific CD8 T cells over the three time points were found (Friedman test). (C) Contribution of each cytokine-producing population to the total CFP-10 and ESAT-6-specific CD8 T cell response pre-treatment, and 2 and 6 months on TB treatment. Seven individuals followed longitudinally maintained a positive CFP-10 or ESAT-6-specific CD8 T cell response at each time point, and were therefore included in this analysis. Differences in the contribution of each subset between the three time points were determined by the Freidman test; if significance was found (p<0.05), the Wilcoxon matched pairs test was used for comparisons between two time points. (D) Longitudinal analysis of the proportion of IFN-γ+TNF-α+IL-2+ and IFN-γ-single positive CD8 T cells contributing to the total CFP-10 or ESAT-6-specific response. These two cytokine-producing subsets of CFP-10 and ESAT-6-specific CD8 T cells showed a significant change on TB treatment (panel C), and were assessed on an individual donor level at each time point. Each symbol represents a different donor; the same symbols were used for each donor in panels B and D.
With the exception of CFP-10-specific CD4 T cells, the total frequency of specific CD4 T cells producing any combination of cytokines was not different after 6 months of TB treatment, compared to pre-treatment values (Figure 3B). All patients maintained a positive response to PPD at all time points, and we therefore analyzed longitudinally the proportion of each cytokine subset contributing to the total PPD response. The proportion of IFN-γ+IL-2+TNF-α+ and IL-2+TNF-α+ PPD-specific CD4 T cells increased significantly on TB treatment, coincident with a decrease in IFN-γ+TNF-α+ and TNF-α-single positive cells (Figure 3C, D). The proportion of polyfunctional PPD-specific CD4 T cells increased in all subjects following 6 months of TB treatment (Figure 3D).
We next investigated the effect of TB treatment on the total frequency of Mtb-specific CD8 T cells. Although some individuals’ frequencies declined over time, overall, there was no difference in the total frequency of cytokine-producing specific CD8 T cell responses (Figure 4B). Seven of the 13 individuals followed longitudinally maintained positive Mtb-specific CD8 T cell responses at all three time points. In these patients, the proportion of IFN-γ+IL-2+TNF-α+ CD8 T cell responses increased over time (similar to specific CD4 T cell responses), coincident with a decrease in the proportion of IFN-γ-single positive cells (Figure 4C). Taken together, these data strongly suggest the cytokine production capacity of Mtb-specific CD4 and CD8 T cell responses is associated with mycobacterial load; moreover, increased specific polyfunctional T cells coincident with reduced IFN-γ and/or TNF-α-producing cells in particular may be indicative of successful response to TB treatment.
Impaired proliferative capacity of Mtb-specific T cells in smear+ TB compared with LTBI
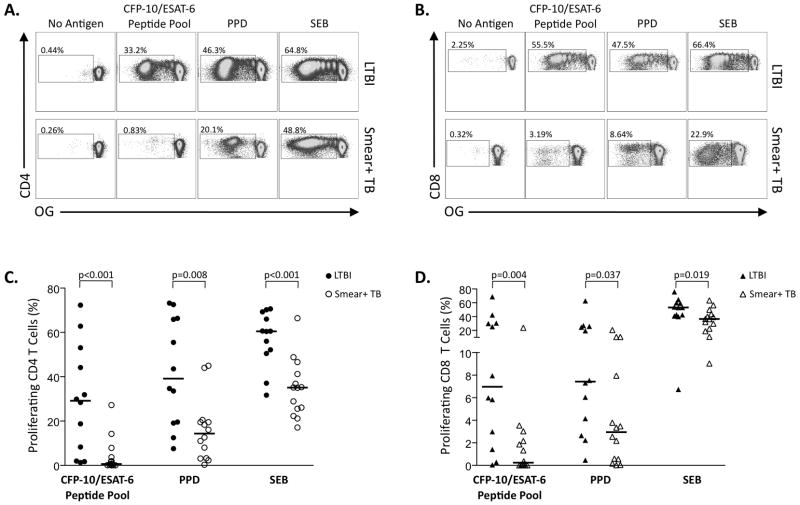
To further assess the functional capacity of Mtb-specific T cells in individuals with different mycobacterial loads, we determined these cells’ proliferative capacity in a 6-day PBMC-based assay (Figure S1B). Individuals with smear+ TB had significantly lower CD4 and CD8 T cell proliferative responses to Mtb antigens and SEB, compared with LTBI (Figure 5). Overall, these data indicate that, although cytokine-producing Mtb-specific CD4 and CD8 T cell are detectable ex vivo in individuals with smear+ TB, the proliferative capacity of Mtb-specific T cells is profoundly impaired in the context of high mycobacterial load.
Figure 5. Impaired proliferative capacity of Mtb-specific T cells in smear+ TB, compared with LTBI.
Freshly isolated PBMC from individuals with LTBI (n=12) and smear+ TB (n=14) were labeled with Oregon Green (OG) and stimulated with CFP-10 and ESAT-6 peptide pools, PPD, or SEB for 6 days. (A) Representative CD4 T cell proliferation assay data from an individual with LTBI (top row) and smear+ TB (bottom row); plots are gated on VividlowCD3+CD4+ T cells. The percentages indicate the frequency of proliferating (OGlow) cells in the gated population. (B) Representative CD8 T cell proliferation assay data from an individual with LTBI (top row) and smear+ TB (bottom row); plots are gated on VividlowCD3+CD8+ T cells. The percentages indicate the frequency of proliferating (OGlow) cells in the gated population. Summary data of CD4 and CD8 T cell proliferative capacity in individuals with LTBI (closed symbols) and smear+ TB (open symbols) are shown in (C) and (D), respectively. Background proliferation in the negative control has been subtracted. Horizontal lines represent the median; differences were assessed by the Mann-Whitney test.
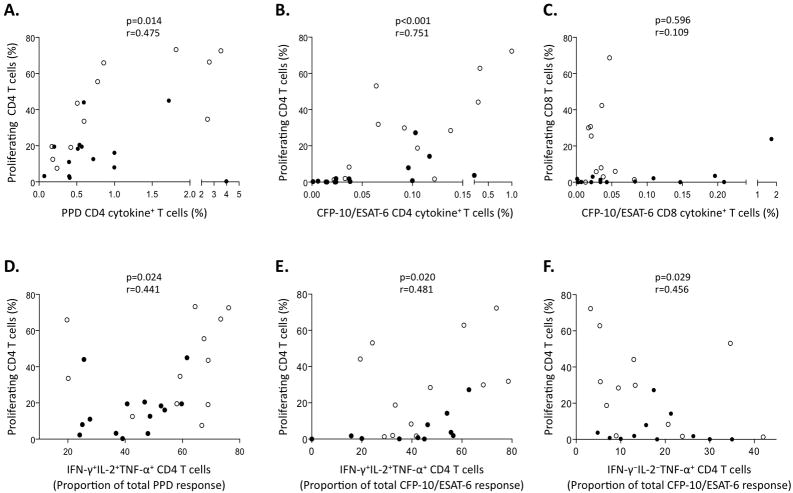
Proliferative capacity of Mtb-specific CD4 T cells correlates positively with polyfunctional IFN-γ+IL-2+TNF-α+ cells and inversely with TNF-〈-single positive cells
To establish if there is a relationship between the ex vivo cytokine profile and the functional capacity of Mtb-specific T cells, we first correlated the total frequency of cytokine-producing Mtb-specific T cells with proliferative capacity, and found a positive correlation for Mtb-specific CD4 T cell responses (Figure 6A, B). No such association was found for Mtb-specific CD8 T cells (Figure 6C), suggesting these two functions may be distinct in Mtb-specific CD8 T cells.
Figure 6. Mtb-specific CD4 T cell proliferative capacity correlates with the quantity and quality of ex vivo cytokine-producing CD4 T cells.
Correlations between the frequency of proliferating T cells with the total frequency of cytokine-producing T cells in the ex vivo whole blood ICS assay are shown for PPD-specific CD4 T cells (A), CFP-10/ESAT-6-specific CD4 T cells (B), and CFP-10/ESAT-6-specific CD8 T cells (C). Correlations between the frequency of proliferating CD4 T cells and the proportion of IFN-γ+IL-2+TNF-α+ T cells contributing to the total ex vivo ICS response are shown for PPD-specific CD4 T cells (D) and CFP-10/ESAT-6 specific CD4 T cells (E). (F) Correlation between the frequency of CFP-10/ESAT-6-specific proliferating CD4 T cells with the proportion of CFP-10/ESAT-6-specific TNF-α-single positive CD4 T cells ex vivo. Black circles represent LTBI donors and white circles represent smear+ TB donors. Correlations were assessed by the Spearman rank test.
We next correlated profiles of individual or combined cytokine production of the Mtb-specific T cells ex vivo with proliferative capacity, and found a positive correlation between the ex vivo proportion of polyfunctional IFN-γ+IL-2+TNF-α+ CD4 T cells and proliferative capacity, and an inverse correlation between the proportion of TNF-α-single positive CD4 T cells and proliferative capacity (Figure 6D–F). None of the other subsets of cytokine-producing CD4 T cells correlated with the proliferative capacity (data not shown). Together, these data indicate an association between the cytokine production capacity ex vivo and T cell functionality in Mtb infection, and indicate that specific IFN-γ+IL-2+TNF-α+ and TNF-α-single positive CD4 T cells detected in peripheral blood ex vivo are predictive of proliferative capacity.
Discussion
In this study, we recruited adults with LTBI, smear− and smear+ TB to determine the effect of mycobacterial load on the functional capacity of Mtb-specific T cell responses in peripheral blood. We found that, compared to individuals with lower mycobacterial load, high mycobacterial load in individuals with smear+ TB was associated with decreased polyfunctional and IL-2-producing cells and increased TNF-α-single positive Mtb-specific CD4 T cells, as well as increased frequencies of specific (cytokine+) CD8 T cells, and impaired proliferative capacity of both Mtb-specific CD4 and CD8 T cell responses. During therapy, polyfunctional cytokine production capacity increased in both CD4 and CD8 T cells. Moreover, polyfunctional cytokine producing capacity correlated with proliferative capacity. These data indicate the functional capacity of Mtb-specific T cells is progressively impaired with increased mycobacterial load, and recovers during therapy.
Recent studies have assessed differences in polyfunctional cytokine production profiles of Mtb-specific CD4 T cells in individuals with LTBI and with pulmonary TB (32-35), with some studies indicating increased Mtb-specific polyfunctional CD4 responses in TB patients (32, 33), and others indicating either decreased polyfunctional responses in TB patients (35), or no difference (34). Our findings of reduced polyfunctional cytokine production in individuals with smear+ TB are consistent with progressive T cell dysfunction in the context of high mycobacterial loads, as has been described in human chronic viral infections (25-28). Furthermore, the decreased contribution of IL-2-producing specific CD4 T cells in individuals with smear+ TB is consistent with previous reports of loss of IL-2-producing Mtb-specific T cells in HIV-coinfected individuals with high viral loads (43), as well as lower proportions of IL-2-single positive Mtb-specific T cells in TB patients compared with household contacts (32). Moreover, the increased contribution of TNF-α-single-positive CD4 cells in individuals with smear+ TB compared with LTBI is consistent with recent reports (32, 35), and provides further rationale for measurement of this particular subset of Mtb-specific CD4 T cells for differentiating Mtb infection versus disease (35).
Previous studies of Mtb-specific T cell polyfunctionality in humans have not differentiated mycobacterial load or disease status within individuals with pulmonary TB. By using well-defined cohorts of individuals stratified by these parameters, we were able to determine specific associations with Mtb-specific T cell functional capacity and mycobacterial load. Interestingly, we did not find differences in quantitative or qualitative measurements of Mtb-specific CD4 T cell cytokine production between LTBI and smear− TB donors, suggesting the qualitative differences observed in individuals with smear+ TB are reflective of high mycobacterial load, rather than the presence of symptomatic active TB disease alone. Possible differences in the cytokine production profiles of Mtb-specific CD4 T cells found in this study compared with recent reports (32-35) may be due to differences in antigen specificity and type, methodological differences used for detection of cytokine-producing cells, and differences in study cohort characteristics.
Secreted immunodominant Mtb antigens can be processed by cytosolic pathways for presentation by MHC class I molecules (11, 12, 44), with cytosolic entry dependent on secretion of the Mtb virulence proteins CFP-10 and ESAT-6 (13). Mtb-specific CD8 T cell responses have been detected in individuals with latent and active disease (45), and we describe here a novel association between higher frequencies of Mtb-specific CD8 responses and pulmonary TB disease in adults. IFN-γ release assays currently used for diagnosis of Mtb infection or disease do not discriminate between CD4 and CD8 T cell cytokine production (46), however our results provide rationale for measurement of Mtb-specific CD8 T cell responses in particular to further define the association between CD8 T cells and TB disease progression. Such studies may significantly aid the diagnosis of TB disease, particularly in populations such as children or immunocompromised individuals where it may be difficult to distinguish Mtb infection from disease.
Further evidence of the association between the quality of Mtb-specific T cell responses and mycobacterial load is supported by our longitudinal analysis of individuals on TB treatment, where we found increased polyfunctional cytokine production capacity along with reduced proportions of monofunctional cytokine-producing cells in both CD4 and CD8 T cell populations following successful anti-TB treatment. Our longitudinal analysis of patients on TB treatment extend initial observations by Millington et al, where simultaneous measurement of IFN-γ and IL-2 production by Mtb-specific CD4 T cells indicated an increase in IL-2-producing cells following treatment (47). By simultaneous measurement of IFN-γ, IL-2, and TNF-α, we were able to determine that the increase in IL-2-producing cells after 6 months of TB treatment is specifically within the polyfunctional IFN-γ+IL-2+TNF-α+ population for both CD4 and CD8 T cell responses. Long-term follow-up beyond 6 months of TB treatment will yield important insights regarding the longevity of polyfunctional Mtb-specific T cell responses, and their potential association with protection from either re-infection or relapse of disease.
To measure functional capacity in addition to cytokine production, we measured proliferative capacity in individuals with smear+ TB and LTBI, and found severe impairment of proliferative capacity of both CD4 and CD8 T cells in smear+ TB donors, despite detection of Mtb-specific cytokine+ T cells in these individuals ex vivo. Studies in Mtb-infected mice have indicated that cytotoxicity and cytokine production are carried out by distinct populations of CD8 T cells (48). The discordance between cytokine production ex vivo and proliferative capacity of Mtb-specific CD8 T cells further supports the notion that Mtb infection in humans primes populations of CD8 T cells with distinct functions. Furthermore, these data indicate maintenance of Mtb-specific T cell responses with robust proliferative capacity is an important correlate with successful immune control of Mtb infection. We did not find differences in the ex vivo frequency of cytokine-producing Mtb-specific T cells in the two groups of donors tested in the proliferation assay; we also did not find differences in T cell viability after 6 days in any of the stimulation conditions tested (data not shown). Unfortunately we did not have sufficient samples from smear− TB donors to compare the proliferative capacity with smear+ TB and LTBI donors. Overall these data suggest an overall deficiency in the proliferative capacity of T cells from individuals with smear+ TB, and warrant further studies to determine precise mechanisms or pathways, such as T cell exhaustion by inhibitory receptor expression (49), which may contribute to loss of proliferative capacity in individuals with smear+ TB.
The functional significance of antigen-specific polyfunctional cytokine responses has largely come from animal models or chronic human viral infections (24, 29, 30, 50-52). To better understand the relationship between ex vivo cytokine production profiles and T cell functional capacity, we determined the proliferative capacity of Mtb-specific T cells using freshly isolated PBMC from the same blood samples used for assessment of ex vivo cytokine production profiles. Polyfunctional Mtb-specific IFN-γ+IL-2+TNF-α+ CD4 T cells were the only cytokine subset that showed a positive correlation with proliferative capacity, thus providing further evidence that polyfunctional cytokine production capacity may be associated with superior functional capacity in the context of a chronic human bacterial infection. IL-2 is a T cell growth factor and important for proliferation of memory T cells following re-encounter with antigen (52, 53), and our results indicate IL-2 production capacity specifically within the context of simultaneous IFN-γ and TNF-α production is indicative of antigen-specific CD4 T cell proliferative capacity.
In contrast to polyfunctional Mtb-specific CD4 T cell responses, the proportion of ex vivo TNF-α-single positive CD4 cells, which were increased in individuals with smear+ TB, were inversely correlated with proliferative capacity. TNF-α plays an essential role in protection against TB (9, 54), and our results indicate populations of Mtb-specific CD4 T cells producing TNF-α, in the absence of IFN-γ and IL-2 co-expression, are expanded under inflammatory conditions of high mycobacterial load, and may identify a short-lived population of effector cells with limited survival and ability to expand upon re-encounter with antigen. Further studies are warranted to determine particular phenotypes of Mtb-specific T cells, such as activation, memory, and inhibitory receptors and ligands, which are associated with functional capacity in different stages of Mtb infection.
Increasing evidence indicates latent Mtb infection is not static, but rather represents a broad spectrum of individuals with varying levels of Mtb replication, with increasing mycobacterial load correlating with the development of clinical disease (55, 56). Our results suggest there are progressive changes in Mtb-specific T cell function associated with increasing mycobacterial load, with increased frequency of specific CD8 T cells in peripheral blood perhaps occurring early, followed by loss of polyfunctional and IL-2-producing CD4 T cell responses as mycobacterial load increases in individuals with smear+ TB. Recent studies of whole blood genome-wide transcriptional profiles in individuals with latent and active TB disease have provided important insights into transcriptional profiles that differentiate infection versus disease (57). Further transcriptional profiling studies of particular subsets of Mtb-specific T cells from individuals with different stages of infection and disease are warranted to further define profiles that can predict risk of development of TB disease.
In summary, we have identified qualitative and quantitative associations between Mtb-specific T cell functional capacity and mycobacterial load in the context of human Mtb infection and disease. The immune correlates of protection from TB disease progression are not well-defined, although results from this study provide rationale for future longitudinal studies of Mtb-infected individuals to determine if progressive loss of polyfunctional and/or IL-2-producing Mtb-specific CD4 T cells and increasing frequencies of Mtb-specific CD8 T cells are predictive of development of clinical TB disease. Such data would have a significant impact on rates of Mtb transmission and TB-associated morbidity and mortality worldwide by facilitating early diagnosis of TB and targeted treatment intervention in Mtb-infected populations at risk for developing TB.
Supplementary Material
Footnotes
This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIH R01 AI083156, NIH R01 AI065653, and NIH R01 AI087915), and the Wellcome Trust-funded Clinical Infectious Diseases Research Initiative (CIDRI).
References
- 1.Global Tuberculosis Control. World Health Organization; 2010. [Google Scholar]
- 2.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 3.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile JF, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli V, Heyne K, Fischer A, Holland SM, Kumararatne DS, Schreiber RD, Casanova JL. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21:370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, Zumla A. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–1937. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grotzke JE, Siler AC, Lewinsohn DA, Lewinsohn DM. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J Immunol. 2010;185:4336–4343. doi: 10.4049/jimmunol.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewinsohn DM, Grotzke JE, Heinzel AS, Zhu L, Ovendale PJ, Johnson M, Alderson MR. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J Immunol. 2006;177:437–442. doi: 10.4049/jimmunol.177.1.437. [DOI] [PubMed] [Google Scholar]
- 13.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Lazarevic V, Nolt D, Flynn JL. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J Immunol. 2005;175:1107–1117. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Klein MR, Malin AS, Sillah J, Huygen K, Andersen P, McAdam KP, Dockrell HM. Human CD8(+) T cells specific for Mycobacterium tuberculosis secreted antigens in tuberculosis patients and healthy BCG-vaccinated controls in The Gambia. Infect Immun. 2000;68:7144–7148. doi: 10.1128/iai.68.12.7144-7148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson J, Samarina A, Fink J, Rahman S, Grundstrom S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect Immun. 2007;75:5210–5222. doi: 10.1128/IAI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 21.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 23.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8(+) T-cell immune correlates against HIV. Immunol Rev. 2011;239:109–124. doi: 10.1111/j.1600-065X.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81:12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciuffreda D, Comte D, Cavassini M, Giostra E, Buhler L, Perruchoud M, Heim MH, Battegay M, Genne D, Mulhaupt B, Malinverni R, Oneta C, Bernasconi E, Monnat M, Cerny A, Chuard C, Borovicka J, Mentha G, Pascual M, Gonvers JJ, Pantaleo G, Dutoit V. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 29.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207:1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 31.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland JS, I, Adetifa M, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 33.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 34.Streitz M, Fuhrmann S, Powell F, Quassem A, Nomura L, Maecker H, Martus P, Volk HD, Kern F. Tuberculin-Specific T Cells Are Reduced in Active Pulmonary Tuberculosis Compared to LTBI or Status Post BCG Vaccination. J Infect Dis. 2011;203:378–382. doi: 10.1093/infdis/jiq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. Dominant TNF-alpha(+) Mycobacterium tuberculosis-specific CD4(+) T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hesseling AC, Walzl G, Enarson DA, Carroll NM, Duncan K, Lukey PT, Lombard C, Donald PR, Lawrence KA, Gie RP, van Helden PD, Beyers N. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis. 2010;14:560–570. [PubMed] [Google Scholar]
- 37.Gopi PG, Chandrasekaran V, Subramani R, Santha T, Thomas A, Selvakumar N, Narayanan PR. Association of conversion & cure with initial smear grading among new smear positive pulmonary tuberculosis patients treated with Category I regimen. Indian J Med Res. 2006;123:807–814. [PubMed] [Google Scholar]
- 38.Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, Wilks MJ, Waramori G, Tjitra E, Sandjaja, Kenagalem E, Pontororing GJ, Anstey NM, Kelly PM. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65:863–869. doi: 10.1136/thx.2010.136242. [DOI] [PubMed] [Google Scholar]
- 39.de Kantor IN, Kim SJ, Frieden T, Laszlo A, Luelmo F, Norval P-Y, Rieder H, Valenzuela P, Weyer K. Laboratory Services in Tuberculosis Control. World Health Organization; Geneva: 1998. [Google Scholar]
- 40.Toman K. Tuberculosis Case Finding and Chemotherapy: Questions and Answers. World Health Organization; Geneva: 1979. [Google Scholar]
- 41.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PA, Ress S, Hussey GD, Kaplan G. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Roederer M, Nozzi JL, Nason MX. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grotzke JE, Harriff MJ, Siler AC, Nolt D, Delepine J, Lewinsohn DA, Lewinsohn DM. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grotzke JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:776–788. doi: 10.1016/j.micinf.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Pai M, Dheda K, Cunningham J, Scano F, O’Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis. 2007;7:428–438. doi: 10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 47.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman P, Lalvani A. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77:4621–4630. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 52.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 53.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 55.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.