Abstract
Mycobacterium tuberculosis is a virulent intracellular pathogen that survives in macrophages even in the presence of an intact adaptive immune response. Type I interferons (IFN) have been shown to exacerbate tuberculosis in mice and to be associated with disease progression in infected humans. Nevertheless, the mechanisms by which type I IFN regulate the host response to M. tuberculosis infection are poorly understood. In this study, we show that M. tuberculosis induces an IFN-related gene expression signature in infected primary human macrophages, which is dependent on host type I IFN signaling as well as the mycobacterial virulence factor, Region of Difference 1. We further demonstrate that type I IFN selectively limits the production of IL-1β, a critical mediator of immunity to M. tuberculosis. This regulation occurs at the level of IL1B mRNA expression, rather than caspase-1 activation or autocrine IL-1 amplification and appears to be preferentially utilized by virulent mycobacteria since avirulent M. bovis bacillus Calmette-Guerin (BCG) fails to trigger significant expression of type I IFN or release of mature IL-1β protein. The latter property is associated with decreased caspase-1-dependent IL-1β maturation in the BCG-infected macrophages. Interestingly, human monocytes in contrast to macrophages produce comparable levels of IL-1β in response to either M. tuberculosis or BCG. Together, these findings demonstrate that virulent and avirulent mycobacteria employ distinct pathways for regulating IL-1β production in human macrophages and reveal that in the case of M. tuberculosis infection the induction of type I IFN is a major mechanism utilized for this purpose.
Introduction
Mycobacterium tuberculosis is a highly virulent pathogen that infects approximately one third of the world's population and is responsible for almost 2 million deaths annually. M. tuberculosis establishes a life-long persistent infection in the majority of infected individuals despite the development of an ongoing immune response. It has been proposed that the organism does so by subverting innate defense programs in macrophages that typically eliminate invading microbes (1, 2). However, the mechanisms by which virulent mycobacteria manipulate host innate signaling pathways are not fully understood.
Studies in mice have revealed that a significant proportion of the transcriptional response to M. tuberculosis in vivo and in macrophages in vitro is MyD88 independent but type I IFN signaling dependent (3–5). The mechanisms of host-pathogen interaction that regulate M. tuberculosis-induced type I IFN have yet to be defined. However, studies on Listeria monocytogenes and Francisella tularensis infections have suggested that type I IFN induction requires the recognition of bacterial products in the host cell cytosol (6). A mycobacterial secretion system, ESX1, has been suggested to play a key role in mycobacterial virulence by facilitating the secretion of M. tuberculosis products into the host cytoplasm (7, 8). ESX1 is partially encoded by the Region of Difference 1 (RD1), a gene locus in the M. tuberculosis genome that is deleted in all strains of M. bovis bacillus Calmette-Guerin (BCG) vaccines (9). Importantly, ESX1 has been implicated in type I IFN induction as well as activation of the inflammasome, a cytosolic protein complex that cleaves pro-IL-1β into its mature bioactive form, in virulent mycobacteria-infected macrophages (4, 10–13).
The IL-1 and type I IFN signaling pathways play opposite roles in host resistance to M. tuberculosis. Mice deficient in IL-1R or IL-1β show increased susceptibility to the pathogen (14, 15), suggesting that the cytokine signaling is essential for the host control of the infection. Interestingly, a recent study has shown that a M. tuberculosis Zn2+ metalloprotease, Rv0198c (zmp1), required for full virulence of the pathogen in mice, suppresses IL-1β processing by inhibiting inflammasome activation in murine macrophages (16). This observation has led to the hypothesis that mycobacteria regulate IL-1β maturation as part of their survival strategy in macrophages (17). In contrast to IL-1β, type I IFN have been shown to promote infection by M. tuberculosis in mice. Thus, virulent clinical isolates of M. tuberculosis induce high levels of type I IFN in vivo and in most studies mice deficient in type I IFN signaling display significantly reduced bacterial loads following infection (18–20). Similarly, intranasal administration of Poly:IC, a type I IFN-inducing agent, exacerbates pulmonary TB in wild-type but not IFN-αβ receptor deficient mice (21).
The role of type I IFN in the regulation of human MTB infection is poorly understood. Nevertheless, a recent clinical study revealed that IFN-inducible genes are highly expressed in the leukocytes of active but not latent TB patients (22), strongly arguing for a contributing role of type I IFN in TB progression in humans. Previous in vitro studies have also shown that M. tuberculosis infection significantly up-regulates the expression of a number of type I IFN-inducible genes (23, 24). We report here that this induction of a type I IFN signature in human macrophages is a property of live virulent mycobacteria and is not triggered by either BCG or an RD1-deficient M. tuberculosis mutant. In addition, we report that IL-1β, also preferentially induced by virulent M. tuberculosis, is negatively regulated by type I IFN. These findings demonstrate a functional intersection between type I IFN and IL-1β signaling in M. tuberculosis-infected human macrophages.
Materials and Methods
Cell cultures and infection
Peripheral blood-derived monocytes were isolated from healthy donors by counter-flow centrifugal elutriation under protocols approved by the institutional review boards of both the National Institute of Allergy and Infectious Diseases and the Department of Transfusion Medicine of the National Institutes of Health for these studies after appropriate informed consent. Monocytes were differentiated as previously described (23), briefly, cells were cultured in antibiotic-free RPMI 1640 medium containing 10% fetal calf serum, 2mM glutamate, 50μM 2-mercaptoethanol, and 10mM HEPES buffer in 24 well plates (Corning) at a density of 1×106 cells/ml for 7 days. Recombinant human M-CSF (10 ng/ml, PeproTech) was added on days 0, 2, and 4. For differentiation of THP-1 cells, the cell line (1×106 cells / well) was stimulated with PMA (10 ng/ml) in 24 well plates for 2 days. The cells were then rested in fresh complete medium for 24 hours before infection.
For stimulation with mycobacteria, differentiated macrophages were exposed to live BCG or RD1 mutant (gift from Dr. S. Derrick, FDA) or WT M. tuberculosis H37Rv at an MOI of 5, or with irradiated M. tuberculosis at 50μg/ml. In some experiments, a mouse monoclonal antibody to human IFN-αβ receptor-2 (10 μg/ml) was included. Recombinant human IFN-β or IFN-γ were purchased from PeproTech and IL-27 were obtained from R&D Systems. All cytokines were used at a final concentration of 10 ng/ml. In some experiments, IL-27R/Fc (2μg/ml, R&D Systems) and Anakinra (30 μg/ml, Amgen) were added to the cultures.
Measurement of gene expression
Total RNA was isolated using the RNAeasy mini kit (Qiagen, Valencia, CA) and residual DNA was digested using RNAse-free DNAse (Quagen). The individual RNA samples were reversely transcribed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Unless otherwise indicated, gene expression was measured using a previously described SYBR Green-based real-time quantitative PCR (25) in which 18S mRNA was used as the housekeeping gene. The oligonucleotide primers used were: 18S: F-CACGGCCGGTACAGTGAAAC and R-CCCGTCGGCATGTATTAGCT; IL-1β: F-TTCTTCGACACATGGGATAACG and R-TGGAGAACACCACTTGTTGCT; IL-6: 5′-TAATGGGCATTCCTTCTTCT-3′ and 5′-TGTCCTAACGCTCATACTTTT-3′; TNF: F-CAGCCTCTTCTCCTTCCTGAT and R-GCCAGAGGGCTGATTAGAGA; CXCL10: F-ATTTGCTGCCTTATCTTTCTG and R-TCTCACCCTTCTTTTTCATTGTAG; IFN-β F-AGC AGT TCC AGA AGG AGG AC and R-TGA TAG ACA TTA GCC AGG AGG TT; IL-1RI: 5′-AGCTGGCTGGGTGGTTCAT-3′ and 5′-CGATTCTGGCATTTTCTCATAGTC-3′; I L-1 R I I: 5′-GGGGGAAATGATCACAGGAATGGT-3′ and 5′-CCCATGAAGGCCAGCAATACAACA-3′; IL-1RACP: 5′-CCTCTCGGGGCAACATCAAC-3′ and 5′-GACCGCCTGGGACTTTTCTTC-3′; and IL-1Ra: F-GGA AGA TGT GCC TGT CCT GT AND R-TCT CGC TCA GGT CAG TGA TG.
Analysis of expression of the twelve subtypes of IFN-a in M. tuberculosis-infected macrophages was performed using a Taqman-based PCR with either molecular beacons or locked nucleic acid probes that enhanced specificity for each subtype (V.P. Mane et al, submitted for publication). Data were analyzed with SDS Software (Version 2.3, Applied Biosystems) and Ct values were exported to a spreadsheet (Microsoft Excel, Redmond, WA) to calculate both standard curves and fold-change in IFN expression relative to the house keep gene 18S (ΔCt). Standard curves and RNA input amount were used to solve for IFN copy number per μg of RNA.
The nCounter Analysis System (NanoString Technologies) was used to screen for the expression of signature genes associated with NF-kB and IFN signaling pathways as previously described (26). Two specific probes (capture and reporter) for each gene of interest were employed. Briefly, five μl of cell lysate (2000 cells/μl in RLT buffer, Qiagen) were hybridized with customized Reporter CodeSet and Capture ProbeSet according to manufacturer's instructions for direct labeling of mRNAs of interest with molecular barcodes without the use of reverse transcription or amplification. The hybridized samples were then recovered in the NanoString Prep Station and the mRNA molecules counted with the NanoString nCounter. For analysis of expression, raw counts (+1) were transformed to log base 2 and each sample profile median-normalized to the RPS18 internal control (“housekeeping gene”). A mixed effects ANOVA model was used to calculate expression differences including the blood donor subject as a random effect, comparable to a paired t-test (JMP/Genomics software, SAS software, Cary, NC). Statistical significance was calculated using a False Discovery Rate adjustment of the raw p-value (27).
Cytokine measurement and immunoblotting
Tissue culture supernatants was screened using a commercial multiplex ELISA assay for human IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12(p70), IL-23, TNF and CXCL10 (Quansys Biosciences). Commercially available ELISA kits (R&D Systems) were also used to determine the concentrations of IL-1β in culture supernatants.
For immunoblotting, supernatants were precipitated with methanol/chloroform and the resulting protein pellet was resuspended in reducing sample buffer (Thermo Fisher).). Cells were lysed using cell lysis buffer (Cell Signaling Technology) supplemented with a protease inhibitor cocktail (Calbiochem) and PMSF (Sigma). The samples were next separated by SDS-PAGE on a 12% polyacrylamide gel and then transferred onto a PVDF membrane. The membranes were than sequentially probed with polyclonal antibodies for caspase-1 (Cell Signaling Technology) or IL-1β (R&D System or Cell Signaling Technology).
Statistics
Wilcoxon ranked sum test was used in experiments involving pairwise comparisons. P < 0.05 was considered statistically significant.
Results
Virulent M. tuberculosis induces a subset of type I IFN in human macrophages
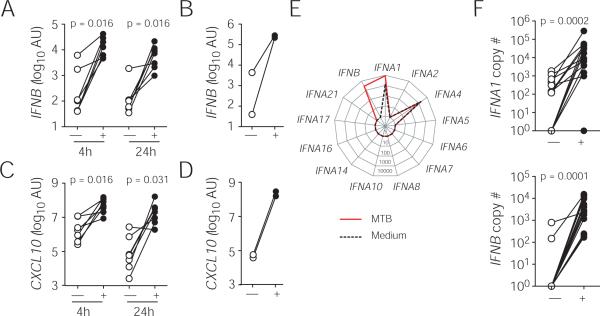
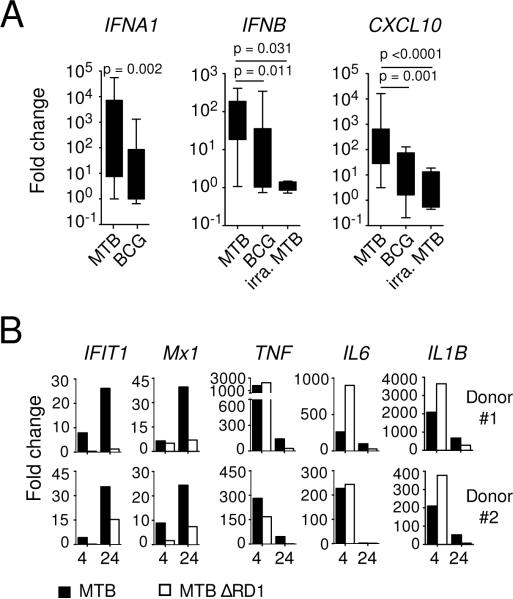
We first determined whether M. tuberculosis triggers IFNB mRNA expression in human macrophages at 4 and/or 24 hr post-infection using a SYBR™ Green-based quantitative PCR assay and found that the gene expression was significantly elevated in infected primary human macrophages (Fig. 1A) and THP-1 cells (Fig. 1B). As expected, the induction of IFNB was closely correlated with the expression of the IFN-inducible gene CXCL10 in M. tuberculosis-infected primary macrophages (Fig. 1C) and THP-1 cells (Fig. 1D). In addition to IFN-β, there are 12 closely related IFN-α sub-types in the human type I IFN family (28). To profile the sub-types of IFN-α induced by M. tuberculosis in infected macrophages, we next quantified the copy number of IFN-β and IFN-α family members using a TaqMan™ based PCR at 24 hr post-infection (Fig. 1E and F). This independent assay confirmed the induction of IFNB expression and further revealed that among all IFN-α sub-types examined, only IFNA1 is specifically up-regulated by M. tuberculosis, suggesting a restricted IFN-α sub-type usage in M. tuberculosis-infected human macrophages.
Figure 1. Induction of type I IFN in M. tuberculosis-infected human macrophages.
Expression of type I IFN and CXCL10 mRNA was analyzed in macrophages unstimulated (open symbols) or infected with M. tuberculosis (closed symbols) using a quantitative PCR. Levels of IFNB (A and B) and IFN-inducible CXCL10 (C and D) in primary human macrophages (A and C) at 4 and 24 hr post-infection or in PMA-differentiated THP-1 cells (B and D) at 24 hr post-infection were measured by a SYBR Green-based PCR. The data shown are relative fold increase of genes of interest over 18S RNA. The normalized gene expression is calculated as 2−ΔCt where ΔCt equals (treated Ct-18S Ct). (E) A representative expression profile of type I IFN in uninfected or M. tuberculosis-infected primary macrophages from a single donor and (F) summary expression data of IFNB and IFNA1 of all donors (n = 14) were determined at 24 hr using a Taqman-based quantitative PCR. The data are expressed as mRNA copy number (per μg RNA). Each paired data set represents an individual donor (A, C and F) or an independent experiment (B and D).
Regulation of M. tuberculosis-induced cytokine expression in human macrophages by type I IFN
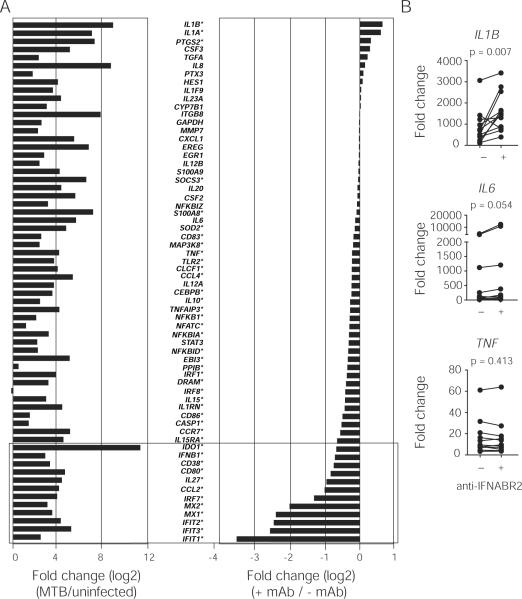
To identify innate inflammatory cytokine(s) that are regulated by type I IFN signaling, we treated M. tuberculosis-infected macrophages with a mouse monoclonal antibody specific for IFN-α/β receptor 2 (IFNABR2) thereby blocking signaling of all members of the type I IFN family. We then analyzed the effect of the signaling blockade on the expression of 64 genes that are known to be critical in mediating NF-κB- or IFN-dependent cellular responses to microbial stimulation using a novel nCounter Analysis System. We found that M. tuberculosis strongly induced both NF-κB- and IFN-related genes (Fig. 2A left panel). Importantly, the expression of IFN-inducible genes such as IFIT1, IFIT2, MX1, MX2 and IL27 was significantly reduced in the presence of the mAb to IFNABR2 (Fig. 2A, right panel), thus establishing that the induction of IFN-related genes in M. tuberculosis infected macrophages is dependent on endogenously produced type I IFN. In addition, we observed that blocking type I IFN signaling led to increased expression in a small group of genes (Fig 2A, right panel). Among these, IL1B and IL1A were the most highly up-regulated.
Figure 2. Regulation of the macrophage cytokine response to M. tuberculosis by type I IFN signaling.
(A) Primary human macrophages from five individual healthy donors were left unstimulated or infected with M. tuberculosis for 24 hr in the presence or absence of a blocking mAb to IFNABR2 and gene expression analyzed using a nCounter Gene Expression Assay. Data are expressed as the log2 ratio (“fold change”) of mRNA counts. Left panel: M. tuberculosis infected relative to uninfected. Right panel: M. tuberculosis + IFNABR2 mAb relative to M. tuberculosis alone. Known IFN-dependent genes are shown in boxed areas. * False Discovery Rate (FDR) < 0.5. (B) Increased IL1B expression in human macrophages infected with M. tuberculosis following blockade of endogenous type I IFN measured by quantitative PCR at 24 hr. The data shown are fold change over non-infected cultures. Each paired data set represents an individual donor.
To further determine whether type I IFN is a regulator of IL1B mRNA expression in M. tuberculosis infection, we extended our study to a larger cohort of healthy donors and compared the expression of IL1B in the infected macrophage cultures with or without IFNABR2 blockade. In addition to reducing the expression of the IFN-dependent CXCL10 (data not shown), blockade of type I IFN signaling significantly increased IL1B mRNA levels when compared with the cultures infected with M. tuberculosis alone (Fig. 2B). In contrast, expression of other NF-κB-dependent genes, such as IL6 and TNF, was not significantly altered by the mAb suggesting a selective regulation of IL1B expression by type I IFN.
Downregulation of M. tuberculosis-induced IL-1β by type I IFN occurs independently of alterations in IL-1R signaling, IL-27R signaling and caspase-1 activation
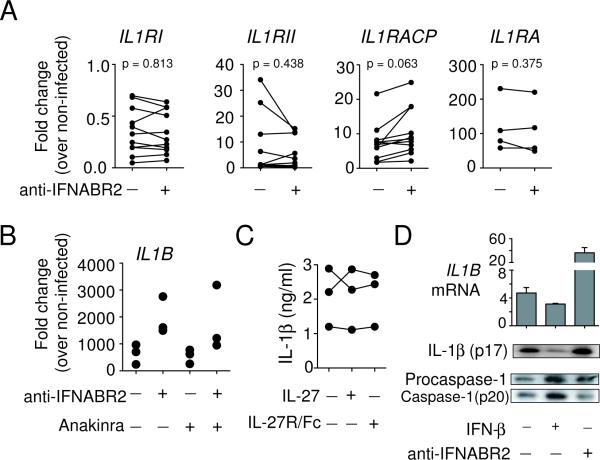
Type I IFN have been shown to modulate IL-1 receptor expression (29) and it is well established that IL-1 can autoamplify its production (30, 31) through interaction with its own receptors. Therefore, it was possible that the increase in IL1B expression in our mAb blocking experiments was due to the release of type I IFN-dependent inhibition of IL-1R signaling and subsequent autocrine stimulation of IL-1 production. To test this, we first analyzed the effect of endogenous type I IFN on the expression of the signaling receptors IL1RI and IL1RAcP, the inhibitory receptor ILRII and the receptor antagonist IL1Ra in M. tuberculosis-infected primary macrophages. We found that relative to uninfected controls, M. tuberculosis-infected macrophages displayed reduced expression of the IL1RI but increased levels of IL1RAcp, IL1RII and IL1Ra (Fig. 3A). Nevertheless, blockade of type I IFN signaling failed to significantly alter the expression of any IL-1R species.
Figure 3. Regulation of IL-1β by M. tuberculosis-induced type I IFN is independent of altered IL-1R and IL-27R signaling and caspase-1 activation.
(A) Expression of IL-1R components and IL-1Ra in the presence or absence of IFNABR2 mAb and (B) IL1B levels in the presence of Anakinra and / or IFNABR2 mAb was measured in M. tuberculosis-infected human macrophages at 24 hr using quantitative PCR. Data shown are fold change over non-infected cultures. Each symbol represents an individual donor. (C) IL-1β secretion in macrophage cultures incubated with recombinant IL-27 or IL-27R/Fc protein measured by ELISA at 24 hr after infection. Each line represents an individual donor. (D) IL1B mRNA expression, caspase-1 activation and IL-1β processing in THP-1 cells infected with M. tuberculosis alone or in combination with exogenous IFN-β or IFNABR2 mAb at 24 hr. IL1B mRNA in lysates was measured using quantitative PCR, while cleaved caspase-1 and mature IL-1β in culture supernatants were assayed by Western blotting involving sequential probing of the same membrane with antibodies against each product. The mRNA data shown are fold change over non-stimulated cultures and data shown are the mean (± SD) of 3 independent experiments with similar results.
To further exclude the possibility that endogenous type I IFN regulate IL-1-induced IL1B expression, we compared gene expression in M. tuberculosis-infected macrophages cultured with mAb to IFNABR2 or Anakinra, a recombinant IL-1Ra competing with IL-1 for IL-1RI binding, or both. Addition of Anakinra did not significantly reduce IL1B expression in macrophages infected with M. tuberculosis alone or in combination with the mAb to IFNABR2 (Fig. 3B). Together, these findings suggest that the IL-1R-dependent positive feedback loop does not appear to contribute significantly to M. tuberculosis-induced IL-1β production in human macrophages and that the augmented IL1B expression following blockade of type I IFN signaling is not due to enhanced IL-1R signaling.
Since IL-27, an IFN-inducible regulatory cytokine, has also been implicated in suppressing IL-1β production in macrophages through the regulation of IL-1 receptor expression (32), we examined its possible involvement in regulating M. tuberculosis-induced IL-1β production. We found that the addition of exogenous IL-27 or neutralization of endogenous IL-27 activity with a soluble IL-27R/Fc chimera did not significantly alter M. tuberculosis-induced IL-1β production (Fig. 3C), thus arguing against a role for this cytokine in regulating IL-1β production in infected macrophages.
In addition to mRNA induction, mature IL-1β release requires caspase-1 activation and cleavage of pro-IL-1β. To determine whether type I IFN regulate caspase-1 activation in M. tuberculosis-infected macrophages, IL1B mRNA as well as the cleavage of caspase-1 and IL-1β in culture supernatants of mycobacterial infected THP-1 cells were analyzed. Addition of exogenous type I IFN decreased the expression of IL1B mRNA, while supplementation of cultures with mAb specific for IFNABR2 increased IL1B expression (Fig. 3D). Comparable effects of type I IFN or IFNABR2 mAb were observed when the levels of mature IL-1β protein in the same cultures were analyzed by Western blotting. In contrast, the extent of caspase-1 cleavage was affected minimally by either culture condition, indicating that type I IFN play a minor role in regulating inflammasome activation and in the processing of pro-IL-1β in M. tuberculosis-infected human macrophages.
M. tuberculosis-induced IL-1β production is suppressed by type I but not type II IFN and this regulation occurs in human macrophages but not monocytes
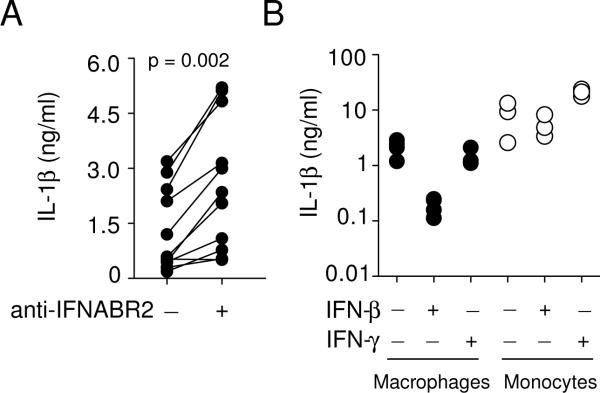
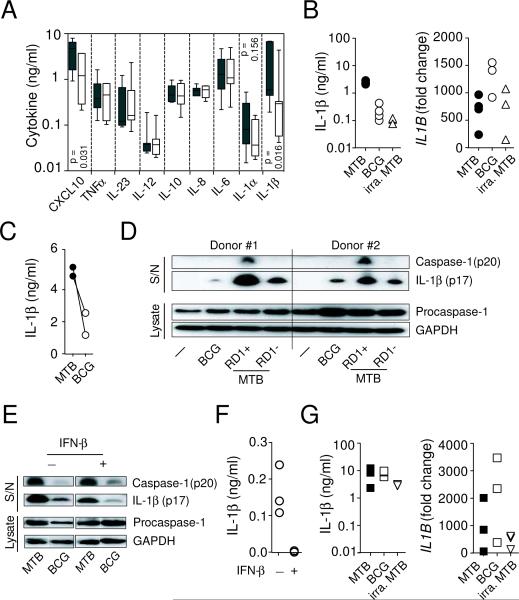
Thus far our results showed that the induction of type I IFN by M. tuberculosis negatively regulates IL1B expression. We next quantified the levels of IL-1β protein in the infected cultures and found that consistent with the IL1B mRNA expression pattern (Fig. 2B), blockade of endogenous type I IFN signaling significantly increased the amount of IL-1β protein released into the cultures (Fig. 4A). Conversely, addition of exogenous IFN-β markedly suppressed IL-1β protein secretion in M. tuberculosis-infected macrophages (Fig. 4B). Interestingly, IFN-γ, an IFN family member known to activate macrophages, had a minimal effect on M. tuberculosis induced IL-1β secretion in the same infected cultures, suggesting that type I and II IFN play distinct roles in regulating IL-1β production. Finally, we determined the effect of exogenous IFN-β and IFN-γ on the IL-1β response of human monocytes to M. tuberculosis and observed that neither IFN suppressed the production of IL-1β in the infected monocyte cultures (Fig. 4B), indicating that the IL-1β response of monocytes and macrophages to M. tuberculosis is differentially regulated by IFNs.
Figure 4. Differential effects of type I IFN on IL-1β production in M. tuberculosis-infected macrophages and monocytes.
(A) Increased IL-1β protein secretion by M. tuberculosis-infected primary human macrophages cultured with mAb to IFNABR2. Each paired data set represents an individual donor. (B) IFN-β limits M. tuberculosis-induced IL-1β secretion by macrophages but not monocytes. Primary human macrophages and monocytes from the same donors were infected with M. tuberculosis in the presence or absence of exogenous IFN-β or INF-γ. The cytokines (10 ng/ml) were added to the cultures at the beginning of the infection. The quantity of secreted IL-1β protein measured at 24 hr by ELISA. Each symbol represents an individual donor.
Virulent mycobacteria induce type I IFN expression in an RD1-dependent fashion
To determine whether the ability to induce type I IFN is a common property of all mycobacteria, we compared expression of type I IFN-related genes in macrophages exposed to virulent versus avirulent or inactivated mycobacteria. We found that BCG and irradiated M. tuberculosis stimulated significantly weaker expression of IFNA1, IFNB and CXCL10 compared to live M. tuberculosis (Fig. 5A), indicating a preferential induction of type I IFN by virulent mycobacteria in human macrophages.
Figure 5. Induction of type I IFN by virulent mycobacteria in human macrophages.
(A) Primary macrophages were exposed to live or irradiated M. tuberculosis or BCG for 24 hr and the expression of IFNA, IFNB and CXCL10 was analyzed by quantitative PCR. The data shown are fold change over non-stimulated cultures (n = 8–14). (B) Human macrophages from two individual healthy donors were infected with either WT (closed symbols) or RD1-deficient (open symbols) H37Rv M. tuberculosis and the expression of the indicated genes was analyzed using a nCounter Gene Expression Assay at 4 and 24 hr. The data shown indicate fold change over non-stimulated cultures after gene expression was normalized against RPS18.
Since the RD1 locus, which is present only in virulent M. tuberculosis, has been implicated in the induction of type I IFN in mice (4, 13), we compared IFN-related gene expression in primary human macrophages infected with an RD1-deficient mutant (ΔRD1) or wild-type (WT) M. tuberculosis. Compared to WT M. tuberculosis, the ΔRD1 mutant was significantly less effective at inducing the expression of the IFN-inducible IFIT1 and MX1 while capable of up-regulating TNF, IL6 and IL1B mRNA (Fig. 5B), indicating a requirement for the mycobacterial virulence determinant RD1 in inducing type I IFN in human macrophages.
Virulent M. tuberculosis induces higher levels of CXCL10 and IL-1β protein compared to BCG
To compare the cytokine response of human macrophages to virulent and avirulent mycobacteria at the protein level, primary human macrophages were infected with either M. tuberculosis or BCG and secreted inflammatory cytokines/chemokines quantified using a multiplex or a conventional ELISA assay. While a low level of IFN-β protein was detected in M. tuberculosis-infected macrophage cultures of some but not all donors (data not shown), large quantities of IFN-inducible CXCL10 were induced (Fig. 6A) in every donor tested. Consistent with the analysis of mRNA expression (Fig. 5A), multiplex cytokine measurements showed that M. tuberculosis-infected human macrophages secreted a significantly higher quantity of CXCL10 as well as IL-1β when compared with those infected with BCG. In contrast, the levels of other NF-κB-dependent proinflammatory cytokines were comparable. This difference in IL-1β protein secretion was subsequently confirmed using a conventional IL-1β ELISA assay showing that M. tuberculosis stimulated primary macrophages release approximately 10-fold more IL-1β protein than either BCG- or irradiated M. tuberculosis-stimulated cells (Fig. 6B, left panel). The difference was not due to a failure of avirulent mycobacteria to activate the innate system since all bacteria induced a comparable level of IL1B mRNA in the same infected macrophage cultures (Fig. 6B, right panel). Similar to primary macrophages, M. tuberculosis-infected differentiated THP-1 cells also consistently produced higher level of secreted IL-1β protein than those stimulated with BCG (Fig. 6 C).
Figure 6. Virulent but not avirulent mycobacteria stimulate IL-1β protein secretion in human macrophages.
(A and B) Expression of cytokines in mycobacterial stimulated primary macrophage cultures was analyzed at 24 hr using a multiplex cytokine assay (A, M. tuberculosis: closed symbols and BCG: open symbols. n = 7), ELISA assay (B, left panel) or quantitative PCR (B, right panel). (C) Levels of IL-1β were determined in mycobacterial infected THP-1 cell cultures by ELISA and each paired data set represents an independent experiment. (D) Expression of caspase-1 and IL-1β in infected primary human macrophage cultures was analyzed at 24 hr by Western blotting involving sequential probing of the same membrane with the corresponding antibodies. (E) M. tuberculosis and BCG-infected THP-1 cell cultures were analyzed at 24 hr post-infection by Western blotting as described in D. IFN-β (10 ng/ml) was added to some cultures at the beginning of the infection. Data shown are representative of 2 independent experiments with similar results. (F) Levels of secreted IL-1β protein in BCG-infected primary macrophage cultures with or without exogenous IFN-β were determined at 24 hr by ELISA. (G) Levels of secreted IL-1β protein (left panel) and IL1B mRNA (right panel) in mycobacterial stimulated monocyte cultures at 24 hr were measured by ELISA or quantitative PCR, respectively. Monocytes isolated from the same donors shown in B were stimulated with M. tuberculosis, BCG or irradiated M. tuberculosis. mRNA data shown are fold change over non-infected cultures. Each symbol in B, F and G represents an individual donor.
M. tuberculosis is more efficient than BCG in activating caspase-1 in infected macrophages
Given that type I IFN negatively regulate IL1B mRNA expression in M. tuberculosis infected macrophages and BCG fails to induce type I IFN expression, it was surprising that BCG-infected macrophages produce decreased rather than increased IL-1β production. To determine whether M. tuberculosis and BCG differentially regulate IL-1β production at the post-transcriptional level, we analyzed levels of cleaved caspase-1 and IL-1β in the culture supernatants as well as procaspase-1 in the cell lysates of infected primary human macrophages. We found that virulent M. tuberculosis is more efficient than avirulent BCG and RD1-deficeint M. tuberculosis in activating caspase-1 and triggering the secretion of mature IL-1β protein (Fig. 6D).
Type I IFN have been suggested to activate the inflammasome in the Francisella infection model (33). Therefore, it was possible that compared to M. tuberculosis-infected cells, the weaker caspase-1 activation observed in BCG-infected culture is due to the lower level of type I IFN expressed (Fig. 5A). However, the addition of exogenous IFN-β failed to increase caspase-1 cleavage to the level observed in M. tuberculosis-infected cells (Fig. 6E). Moreover, consistent with our earlier finding with M. tuberculosis (Fig. 4B), exogenous IFN-β reduced the quantity of BCG-induced IL-1β to undetectable levels in primary macrophage cultures (Fig. 6F), arguing that type I IFN does not regulate mycobacteria-induced IL-1β production by altering caspase-1 activation. Together, these observations suggested that the difference in IL-1β production by BCG versus M. tuberculosis infected human macrophages is due to differential caspase-1 activation rather than type I IFN induction by these bacteria.
Monocytes, in contrast to macrophages, mount equivalent IL-1β response to M. tuberculosis and BCG
We next examined the IL-1β response of human monocytes to mycobacterial infection as it has been reported previously that the production of this cytokine is differentially regulated in the two mononuclear cell populations (34, 35). In contrast to macrophages (Fig. 6B), the levels of IL1B mRNA and secreted IL-1β protein were comparable in monocyte cultures infected with M. tuberculosis, BCG or irradiated M. tuberculosis (Fig. 6G), arguing that only differentiated macrophages are capable of distinguishing virulent from avirulent mycobacteria with regard to IL-1β release. Therefore, the IL-1β response to mycobacteria is influenced both by properties of the pathogen as well as the host cell type that is infected.
Discussion
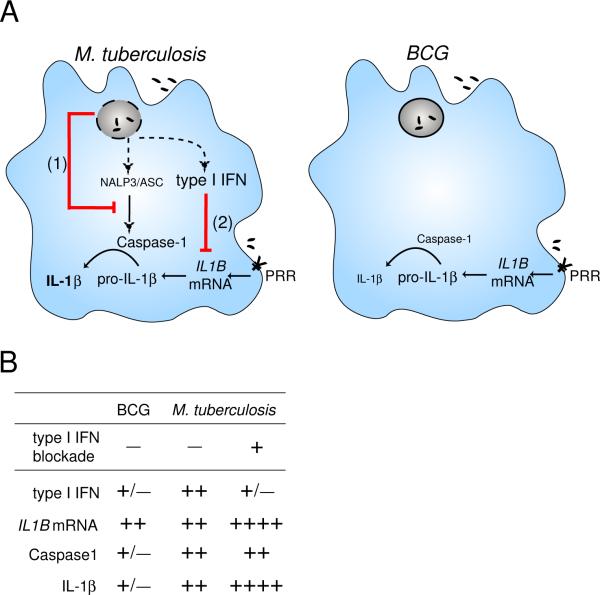
Although essential for resistance to viral infections, type I IFN have been shown to contribute to pathogenesis of intracellular bacterial infections (36, 37). In the case of M. tuberculosis, the cytokines have been shown to impair host resistance in mice (4, 18) and to be associated with TB disease progression in infected humans (22). However, the mechanisms by which type I IFN regulate the host response to M. tuberculosis infection are poorly understood in both mice and humans. In this report, we show that type I IFN are preferentially induced by virulent mycobacteria and function as regulators of IL-1β production in infected human macrophages through suppression of IL1B mRNA expression. This host cytokine-mediated, transcriptional regulation of IL-1β production is thus distinct from a previously described mechanism in murine macrophages involving direct inhibition of inflammasome activation by the mycobacterial product, zmp1 (16). Together, our findings reveal a second pathway through which M. tuberculosis can manipulate host IL-1β production indirectly by inducing type I IFN (Fig. 7). Although the molecular mechanisms required for the induction of type I IFN by M. tuberculosis have yet to be fully elucidated, it is interesting to note that live M. tuberculosis selectively induces only a subset of type I IFNs in macrophages, as shown here, and dendritic cells (24). Therefore, a detailed comparison of the M. tuberculosis induced IFN signature with that known to be triggered by viral infection may provide important clues in understanding the “probacterial” vs. anti-viral effects of this important cytokine family.
Figure 7. Schematic diagram illustrating the regulation of IL-1β in human macrophages infected with either M. tuberculosis or BCG.
(A) While M. tuberculosis and BCG trigger equivalent levels of IL1B mRNA, only M. tuberculosis can efficiently activate the inflammasome to process pro-IL-1β leading to release of mature IL-1β. In addition, M. tuberculosis is able to simultaneously limit the production of IL-1β by at least two independent pathways (indicated in red lines). The first mechanism involves the previously described direct inhibition of caspase-1 activation via zmp1 (1) while the second, revealed in the current study, entails the suppression of IL1B mRNA expression indirectly through the induction of host type I IFN secretion (2). Dotted lines indicate hypothetical pathways necessary for sensing bacterial products critical for triggering caspase-1 activation and type I IFN production. These two pathways are not effectively triggered by BCG or other RD1-deficient mycobacteria since they involve cytosolic sensors that these bacteria cannot access because they lack the ESX-1 secretion system. (B) Table summarizing the levels of key elements in the pathways described in A in infected macrophages. The effects of IFN blockade on the expression of these components in M. tuberculosis-infected macrophages are also described. PRR: pattern recognition receptor.
Our findings reveal that type I IFN selectively inhibit IL-1β mRNA induction but not caspase-1 activation or IL-1R signaling in M. tuberculosis-infected human macrophages. A similar suppressive effect of IFN-β on IL-1β gene expression has been reported in dendritic cells isolated from multiple sclerosis patients before treatment (38). Nevertheless, type I IFN has also been shown to regulate IL-1β production at the post-transcriptional level. For example, it has been reported that type I IFN positively regulate IL-1β release by enhancing inflammasome activation in Francisella tularensis-infected murine macrophages (33) although in vivo type I IFN signaling has been shown to impair host control of Francisella infection (39). Moreover, while a previous study indicated that type I IFN/Tyk2 signaling regulates LPS-induced IL-1β production at the translational level in murine macrophages (40), a more recent paper reports that type I IFN regulate LPS and alum triggered IL-1β production at the levels of both pro-IL-1β protein synthesis and inflammasome activation (41). These discordant findings have yet to be reconciled. One possibility is that the different mechanisms observed may result from variations in the strength, duration, and/or the combination of stimuli used for triggering pro-IL-1β synthesis and mature IL-1β release. Differences in the timing and duration of exogenous type I IFN exposure employed in the different studies may also have contributed to the distinct results obtained.
Interestingly, although IFN-α/β and IFN-γ share related signaling pathways, analyses performed on a subset of donors suggest that IFN-γ does not exhibit the same effects on IL-1β production by M. tuberculosis-infected human macrophages. Moreover, IFN-γ a known macrophage stimulating cytokine, is unable to antagonize the suppressive effects of endogenous type I IFN on infected human macrophages, suggesting a dominant effect of type I over type II IFN signaling in regulating the IL-1β response to M. tuberculosis in human macrophages. This distinction may be due partly to the known ability of M. tuberculosis to interfere with IFN-γ signaling in macrophages, which has been proposed as a mechanism for the pathogen's evasion of adaptive immunity (42, 43).
The functions of the type I IFN and IL-1β pathways in human TB are poorly understood. Studies in the murine model have suggested that two cytokines play opposite roles in host resistance to M. tuberculosis and the present study demonstrates that type I IFN clearly limits IL-1β production in human macrophages infected with the pathogen. Since IL-1β is host protective in murine TB, restriction of IL-1β production by type I IFN might be expected to prevent host clearance of the organism. However, due to the known pro-inflammatory effects of IL-1β on cell recruitment, it is also possible that down-regulation of its activity in some settings could be host protective through dampening excessive IL-1β-mediated tissue pathology induced by virulent mycobacteria (44) and/or even preventing the establishment of the infection by restricting macrophage recruitment to granulomas, a process that has recently been proposed to support rather than limit mycobacterial survival in vivo (45). The impact of such regulation on the establishment and maintenance of chronic M. tuberculosis infection in humans remains to be elucidated.
Regardless of the exact mechanisms involved, the balance between IL-1β and type I IFN responses may be pivotal in ensuring the survival of host as well as pathogen thereby contributing to the maintenance of persistent M. tuberculosis infection. Disturbing this balance may be detrimental for host control of the infection as evidenced by our previous finding that Poly:IC induction of type I IFN results in increased bacterial growth and host pathology in mice (21) and the observation of TB reactivation in chronic hepatitis C patients undergoing therapy with pegylated IFN-α and ribavirin (46, 47).
In agreement with previous studies with murine macrophages, avirulent mycobacteria (in this case BCG) trigger substantially less type I IFN than virulent M. tuberculosis in human macrophages. Given the negative regulatory role of type I IFN in M. tuberculosis infection, one might predict from these observations that IL-1β production would be increased in BCG-infected macrophages due to the release in type I IFN mediated suppression. Nevertheless, in both human and mouse macrophages (10–12) only low levels of mature IL-1β are triggered by avirulent mycobacteria. As indicated here in human macrophages, this decreased IL-1β production is not due to a defect in the induction of IL1B mRNA message by BCG but rather the inability of the organism to trigger caspase-1 dependent processing and secretion of mature IL-1β. A likely explanation for this caspase-1 activation defect is the different intracellular trafficking of virulent vs. avirulent mycobacteria in macrophages. Thus, virulent M. tuberculosis (which expresses the ESX-1/RD1 secretion system) has been proposed to translocate bacterial products from the phagolysosome into the cytoplasm where, as previously demonstrated in murine macrophages, they trigger host inflammasome activation and mature IL-1β protein release (10–12, 44). In contrast, avirulent BCG and other RD1-deficient mycobacteria lack this secretion system and are retained within the phagolysosomal compartment (Fig 7). Therefore, regardless of the regulation of IL1B transcription by type I IFN, avirulent mycobacteria remain incapable of stimulating the production of mature bioactive IL-1β protein. For this reason, it can be concluded that in macrophages infected with virulent vs. avirulent mycobacteria IL-1β production is governed by both overlapping and distinct mechanisms.
In addition to highlighting distinctions between M. tuberculosis and BCG, our data reveal major differences between human macrophages and monocytes in the regulation of IL-1β production following mycobacterial infection. It has been previously demonstrated that following pattern recognition receptor stimulation, human monocytes do not require a second signal for IL-1β release because caspase-1 is constitutively activated (34, 35). The latter distinction is the likely explanation of our observation that, in direct contrast to infected macrophages, M. tuberculosis and BCG infected monocytes secrete comparable levels of IL-1β. This cell-specific difference in IL-1β regulation in mononuclear phagocytes may have important in vivo implications. Thus, if mycobacterial infection is not contained locally within tissue macrophages and disseminates systemically, interaction of circulating monocytes with mycobacteria will trigger the robust production of IL-1β without the requirement for inflammasome activation thereby preventing further spread of the pathogen.
M. tuberculosis has evolved multiple strategies to counteract host defense mechanisms in infected macrophage by regulating host cytokine signaling, cell survival, antigen presentation and microbicidal activities (48, 49). In this study, we have demonstrated a cytokine regulatory loop by which M. tuberculosis can manipulate host IL-1 production through the induction of type I IFN. These findings have important implications for the understanding of M. tuberculosis virulence, persistence and pathogenesis. Since type I IFN is readily triggered by numerous viral and other pathogens, this pathway may be of particular relevance in delineating the mechanisms by which co-infections may contribute to TB progression. The extent to which type I IFN and IL-1β induction and signaling influence the outcome of M. tuberculosis infection in humans is thus an important topic for future investigation.
Acknowledgments
We thank Dr Lyudmila Lyakh (NCI) for her initial help in measuring gene expression using the nCounter Analysis System and David Kugler for his assistance with graphics. We also thank Drs Siamon Gordon and Kathryn Zoon for their invaluable discussions and Drs Dragana Jankovic and Alena Srinivasan for critical reading of the manuscript.
This research was supported by the Intramural Research Program of NIAID, NIH, Department of Health & Human Services.
Footnotes
IL-1β regulation in mycobacterial infected human macrophages
References
- 1.Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. 2003;57:641–676. doi: 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- 2.Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 3.Shi S, Blumenthal A, Hickey CM, Gandotra S, Levy D, Ehrt S. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-αβ receptor and STAT1. J Immunol. 2005;175:3318–3328. doi: 10.4049/jimmunol.175.5.3318. [DOI] [PubMed] [Google Scholar]
- 4.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 6.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 8.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 9.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurenuma T, Kawamura I, Hara H, Uchiyama R, Daim S, Dewamitta SR, Sakai S, Tsuchiya K, Nomura T, Mitsuyama M. The RD1 locus in the Mycobacterium tuberculosis genome contributes to activation of caspase-1 via induction of potassium ion efflux in infected macrophages. Infect Immun. 2009;77:3992–4001. doi: 10.1128/IAI.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 13.Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 15.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1β production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarevic V, Martinon F. Linking inflammasome activation and phagosome maturation. Cell Host Microbe. 2008;3:199–200. doi: 10.1016/j.chom.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc Natl Acad Sci U S A. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Barry C, Kaplan G. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 20.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, Nutman TB. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 24.Remoli ME, Giacomini E, Lutfalla G, Dondi E, Orefici G, Battistini A, Uze G, Pellegrini S, Coccia EM. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–374. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- 25.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-γ differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086-–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 26.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 28.Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282:20047–20051. doi: 10.1074/jbc.R700004200. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Ho HH, Lou O, Hidaka C, Ivashkiv LB. Homeostatic role of interferons conferred by inhibition of IL-1-mediated inflammation and tissue destruction. J Immunol. 2005;175:131–138. doi: 10.4049/jimmunol.175.1.131. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 31.Schindler R, Ghezzi P, Dinarello CA. IL-1 induces IL-1. IV. IFN-γ suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol. 1990;144:2216–2222. [PubMed] [Google Scholar]
- 32.Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF-α and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J Immunol. 2010;185:7047–7056. doi: 10.4049/jimmunol.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-β inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 39.Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, Ho L, Sauer JD, Iwakura Y, Metzger DW, Monack DM. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J Immunol. 2010;184:3755–3767. doi: 10.4049/jimmunol.0902065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radwan M, Stiefvater R, Grunert T, Sharif O, Miller I, Marchetti-Deschmann M, Allmaier G, Gemeiner M, Knapp S, Kovarik P, Muller M, Strobl B. Tyrosine kinase 2 controls IL-1β production at the translational level. J Immunol. 2010;185:3544–3553. doi: 10.4049/jimmunol.0904000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-γ through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 43.Kincaid EZ, Ernst JD. Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-γ without inhibiting STAT1 function. J Immunol. 2003;171:2042–2049. doi: 10.4049/jimmunol.171.4.2042. [DOI] [PubMed] [Google Scholar]
- 44.Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, Sun M, Diehl L, Brown EJ. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 2010;6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2009;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbatani S, Manfredi R, Marinacci G, Pavoni M, Cristoni L, Chiodo F. Reactivation of severe, acute pulmonary tuberculosis during treatment with pegylated interferon-α and ribavirin for chronic HCV hepatitis. Scand J Infect Dis. 2006;38:205–208. doi: 10.1080/00365540500263268. [DOI] [PubMed] [Google Scholar]
- 47.Tsai MC, Lin MC, Hung CH. Successful antiviral and antituberculosis treatment with pegylated interferon-α and ribavirin in a chronic hepatitis C patient with pulmonary tuberculosis. J Formos Med Assoc. 2009;108:746–750. doi: 10.1016/S0929-6646(09)60400-9. [DOI] [PubMed] [Google Scholar]
- 48.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296–307. doi: 10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]