Abstract
Known for years as professional antigen presenting cells (APC), dendritic cells (DC) are also endowed with tumoricidal activity. This dual role of DC as killers and messengers may have important implications for tumor immunotherapy. However, the tumoricidal activity of DC has mainly been investigated in animal models. Cancer cells inhibit antitumor immune responses using numerous mechanisms including the induction of immunosuppressive/tolerogenic DC which have lost their ability to present antigens in an immunogenic manner. We herein evaluate the possibility of generating tumor killer DC from patients with advanced stage cancers. We demonstrate that human monocyte-derived DC are endowed with significant cytotoxic activity against tumor cells following activation with LPS. The mechanism of DC-mediated tumor cell killing primarily involves peroxynitrites. This observed cytotoxic activity is restricted to immature DC. Additionally, after killing, these cytotoxic DC are able to activate tumor Ag specific T cells. These observations may open important new perspectives for the use of autologous cytotoxic DC in cancer immunotherapy strategies.
Introduction
Dendritic cells play a central role in the initiation and regulation of innate and adaptive immune responses. As such, they represent strategic elements of cancer vaccination approaches. In response to pro-inflammatory signals, DC generated from patients' monocytes differentiate into activated cells which release cytokines and up-regulate MHC class I, class II and co-stimulatory molecules. When appropriately loaded with tumor antigens, DC are capable of presenting antigenic peptides and of activating tumor specific T lymphocytes leading to specific antitumor immune responses (1). However to date, only limited clinical responses have been obtained in trials evaluating the efficacy of DC-based tumor vaccines (2–7).
The possibility of improving the efficacy of DC-based cancer vaccines by triggering their direct tumor killing activity has not been evaluated. Several subsets of killer DC (KDC) have been described in mice (8–14), rats (15–22) and humans (23–49). However, few data concerning the killing ability of human DC generated from cancer patients are available (26, 44, 49). In most human studies, KDC were obtained by in vitro differentiation of monocytes from healthy donors in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL-4) (23–24, 27, 31–32, 45–46) followed by exposure to diverse inflammatory signals such as IFN-β (35), IFN-γ, LPS (27, 33, 46), Imiquimod, Poly I:C (48), OK432 (a penicillin-inactivated of Streptococcus pyogenes) (30) or CD40L (33). The mode(s) of induction (LPS, CpG, Interferons, CD40L) and the effector mechanism(s) (Fas-L, TNF-family members underlying the killing activity of these cells remain a subject of controversy (26, 29, 34–35, 38, 44, 47–48). In addition, the ability of these KDC to capture dead tumor cells and to induce specific T cell activation has not been fully elucidated. In terms of clinical applications, triggering of the cytotoxic activity of autologous DC from cancer patients may further enhance their therapeutic potential by fostering the release of tumor antigens immediately available for capture and presentation to specific T cells. Clear evidence has been provided that cancers can inhibit the anti-tumor immune response by numerous mechanisms including the induction of tolerogenic DC. However no information regarding the possible negative regulation of KDC cytotoxic activity by cancer cells is currently available. To address this question, we herein investigated the possibility of generating human DC endowed with tumor-killing activity from cancer patients.
Our results indicate that following activation with LPS, human monocyte-derived DC generated from advanced stage cancer patients are strongly cytotoxic against tumor cells. These human KDC (hKDC) were capable of triggering necrotic death of a wide variety of tumor cell lines through direct cell contact. The killing mechanism involves peroxynitrite release. Interestingly, immature but not mature DC exhibit cytotoxic activity against cancer cells. Importantly, after killing of tumor cells, these cytotoxic DC were able to activate tumor antigen specific T lymphocytes.
Materials and methods
Cell lines
The following human cancer cell lines were purchased from the American Tissue and Cell Collection (ATCC, Manassas, USA): the cervix carcinoma cell line HeLa, the breast adenocarcinoma cell line MCF7 and the human colorectal cancer cell lines HT29, SW480, HCT116. The T1 melanoma cell line and the LT12 CTL clone specific for the Melan-A25–36 peptide were kindly provided by Dr. Chouaib (INSERM U753, Villejuif, France). The human embryonnary cells and the A7R5 rat vascular smooth muscle cells were kindly given by Dr. Lagrost (INSERM U866, Dijon, France). HT29 expressing the green fluorescent protein (HT29-GFP) was obtained by retroviral transfection (9). The percentage of GFP expressing cells was measured by flow cytometry.
Patients
Ten patients with different confirmed cancers were enrolled in the clinical study before treatment after giving their written informed consent. Lymphoma (n= 2), pulmonary (n=4) or colon cancer (n=4) patients at stage IV incurable cancer were enrolled. None of the patient had received chemotherapy or any other immunosuppressive treatment during the previous 3 months.
Reagents
LPS, Flagellin, Poly I:C, Gardiquimod and crystal violet were purchased from Sigma-Aldrich (Saint Louis, USA). 5,10,15,20-Tetrakis(4-sulfonatophenyl)prophyrinato iron (III), Chloride (FeTPPS) was obtained from Calbiochem (San Diego, USA). IFN-γ and TNF-α were purchased from Peprotech (Rocky Hill, USA), CpG from Invivogen (Carlsbad, USA) and Pam3Cys-SK4 from EMC (Tubingen, Germany). IL-6 and IL-1β were purchased from R&D systems (Minneapolis, USA) and prostaglandin E2 (PGE2) from Sigma Aldrich.
Antibodies and flow cytometry analysis
The following human antibodies (Ab) were purchased from BD Biosciences (San Jose, USA): APC-CD1a, FITC-CD11c, FITC-CD40, FITC-CD80, FITC-CD83, FITC-CD86, FITC-HLADR, PE-CD14, PE-CD163, FITC-CD3, FITC-CD56. For flow cytometry analysis, cells (5×105) were washed in PBS with 0.5% BSA and 0.1% sodium azide, incubated with the appropriate Ab or isotype controls for 1 h, and then were washed and analyzed by flow cytometry using an LSRII (BD Biosciences).
Generation of DC
Human PBMC were isolated from the blood of cancer patients or from buffy coats of healthy donors (EFS, Besançon, France) by Ficoll-Percoll density gradient centrifugation. Monocytes were purified from human PBMC using CD14 microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) according to the manufacturer's recommendations. CD14 purity was determined using flow cytometry analysis, after staining with a PE-conjugated anti-CD14 Ab (BD Biosciences) and was routinely found to be >95%. Immature DC were obtained by incubating monocytes (5×105cells/ml) in RPMI 1640 (BioWhittaker, Basel, Switzerland) supplemented with 10% FBS and recombinant human GM-CSF (100 ng/ml) and IL-4 (20ng/ml) (both from PromoCell GmbH Heidelberg, Germany) for 5 days. Day 5 DC exhibited a phenotype (low expression of co-stimulatory molecules such as CD40, CD80, CD83, CD86 and HLA-DR) and function (high capability of endocytosis but low IL-12 production and limited ability to induce T cell proliferation) consistent with that of immature DC. DC maturation was triggered with LPS (100 ng/ml) or IFN-γ (1000 UI/ml) or a cocktail of activators: TNF (20 ng/ml) + poly I:C (50 μg/ml) or IL-1β (25 ng/ml) + IL-6 (10 ng/ml) + TNF-α (50 ng/ml) + PGE2 (1 μg/ml) at day 5 for 48 h. Moreover DC cultures were not contaminated with conventional cytotoxic cells such as macrophages, T lymphocytes or NK cells (supplemental fig. 1).
Cytokine assay
The concentration of IL-12 in cell culture supernatants was determined using ELISA kits according to the manufacturer's procedures (Diaclone, Besancon, France and Gen Probe, San Diego, USA).
Transwell assays
The tumor cells were plated (106 cells/well) in 24-well plates. Untreated or LPS-activated DC (5×106) were deposited in the inner chamber of a transwell membrane (0.45 μm pore size) (Dutscher, Brumath, France). Cytotoxic assays were performed after 48 h.
Cytotoxicity assay
DC and target tumor cell were co-cultured for 48 h. The number of residual adherent cells was then evaluated by crystal violet staining as previously described (50). Data are presented as the percentage of relative absorbance calculated from the formula Atest/Acontrol, where Atest is the absorbance of tumor cells cultured with DC in the different conditions, and Acontrol is the absorbance of tumor cells cultured alone.
Measurement of reactive oxygen and nitrogen species production by human dendritic cells
The production of reactive oxygen and nitrogen species (RONS) by cultured human dendritic cells was evaluated with electron spin resonance (ESR) spectroscopy using 1-hydroxy-3-methoxycarbonyl −2,2,5,5-tetramethylpyrrolidine (CMH, Noxygen, Germany) as the spin probe. CMH is a cell membrane-permeable hydroxylamine which can be oxidized by superoxide anions, peroxynitrites and transition metals into a paramagnetic nitroxide CP•, that forms a characteristic triplet EPR signal with a half-life of several hours (51–53). Briefly, activated and non-activated human dendritic cells (1×106) were incubated for 30 min at 37°C in a 5% CO2 atmosphere with 10−4 mol/l CMH in 200 μl pH 7.4 ESR Krebs-Hepes buffer containing 5 10−6 mol/l diethyldithiocarbamate and 2.5 10−5 mol/l desferrioxamine as transition metal chelators, as described elsewhere (53). After incubation, the cells and medium were immediately frozen in liquid nitrogen in polyethylene tubes, and kept in liquid nitrogen until measurement. ESR spectra were recorded at 100°K using a Bruker EMX-100 spectrometer (Bruker, Wissembourg, France). ESR spectra were obtained using the following instrument settings: center field 3330.2 G; sweep width 100 G, microwave frequency, 9.378 GHz; microwave power, 20 mW; modulation amplitude, 5 G; conversion time, 40.96 ms; time constant 327.68 ms; receiver gain, 1×106. The amplitude of center field anisotropic signal was measured (arbitrary units) in order to determine CP• nitroxide formation in activated or non-activated human dentritic cells as well as in buffer alone.
Annexin V/propidium iodide
The percentage of apoptotic cells and necrotic cells was determined by using an FITC-conjugated Annexin V Apoptosis Detection Kit I according to the manufacturer's recommendations (BD Biosciences).
T lymphocyte proliferation
T lymphocytes were obtained from buffy coats by using a Pan-T cell isolation kit (Miltenyi Biotec, Bergish Gladbach, Germany). T cells were then stained using Cell Trace Violet cell proliferation kit according to the manufacturer's procedure (Cell Trace™, Carlsbad, CA). Labeled cells (1 × 105 cells/well) were co-cultured with DC (1 × 104) and cell division was detected by Flow Cytometry and analyzed by ModFit software after 5 days as indicated by the manufacturer (Cell Trace™).
Statistical analyses
Unless specified, all experiments were reproduced at least 3 times and performed on samples from at least three different patients or healthy donors. Paired t tests were used to compare the different groups. When more than 2 groups were compared at the same time, we used repeated measures ANOVA tests. Results were considered statistically significant for p<0.05. Analysis was performed with GraphPad Prism®.
Results
LPS induces human monocyte-derived dendritic cell cytotoxic activity
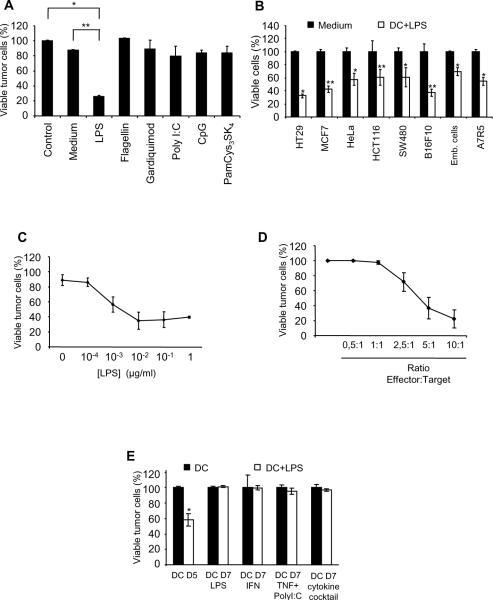
We have previously reported that in rats or mice, DC killing activity can be triggered by the TLR4 ligand, LPS (9, 19). To evaluate whether similar activation conditions may induce the cytotoxic potential of ex vivo generated human monocyte-derived DC, cells were exposed on day 5 to the indicated TLR ligands. Our results indicate that LPS was the only TLR agonist capable of triggering DC killing activity (Fig. 1A). LPS-activated day 5 DC were able to kill several human tumor cell lines (HT29, MCF7, HeLa, HCT 116, SW480) and some non-malignant cells (Fig. 1B). Importantly, the viability of total T lymphocytes was minimally affected after culture with LPS-activated day 5 DC (supplemental fig. 1B). Cells obtained after 3 days of culture (early in the differentiation process toward DC) did not exhibit significant tumoricidal activity after LPS activation (supplemental fig. 1C). Interestingly, even low concentrations of LPS were sufficient to trigger the cytotoxic function of DC with a minimum optimal concentration between 0.01 and 0.1 μg/ml (Fig. 1C). A direct cytotoxic effect of LPS on tumor cells was excluded as the survival of tumor cells was not affected by exposure to the TLR4 agonist for extensive periods of time (supplemental fig. 2A). Moreover, the pre-treatment of tumor cells with LPS did not sensitize them to DC-mediated cytotoxicity (supplemental fig. 2B). In addition, the results depicted in Fig. 1D indicate that the elimination of tumor cells was dependent on the effector DC : target tumor cell ratio.
Figure 1. Killing activity of monocyte-derived DC from healthy donors.
A: HT29 tumor cells were cultured alone (Control) or with day 5 monocyte-derived DC (DC:tumor cell ratio = 5:1) with or without (Medium) the indicated TLR ligands (TLR4L: LPS (100ng/ml); TLR5L: flagellin (100ng/ml); TLR7L: gardiquimod (1μg/ml); TLR3L: polyI:C (10μg/ml); TLR9L: CpG (1μg/ml) and TLR-1,2,6L: PamCys3SK4, (1μg/ml). DC killing was evaluated after 48 h as reported (9, 19). *, ** Significant difference when compared with tumor cells cultured in the absence of DC (Control) or in the presence of non-activated DC (Medium) *, p<0.05; **, p<0.005. B: Day 5 DC were added to the indicated cancer cell lines at a DC:tumor cell ratio = 5:1 for 48 h, in the absence (DC) or presence of LPS (100 ng/ml) (DC+LPS). Tumor cell viability was determined. *, **, Significant difference when compared with tumor cells cultured in DC in absence of LPS *, (p<0.05); **, p<0.005. C: HT29 tumor cells were cultured for 48 h with day 5 monocyte-derived DC, at a DC: tumor cell ratio= 5: 1, in the presence of LPS at the indicated concentrations. Tumor cell survival was determined as described in material and methods. D: Day 5 monocyte-derived DC were cultured for 48 h with HT29 tumor cells in the presence of LPS at the indicated effector (DC):target (HT29 tumor cells) ratios and killing of tumor cells was evaluated. E: HT29 tumor cells were cultured for 48 h with day 5 immature monocyte-derived DC (DC D5) or with day 7 activated DC (DC D7), in the presence or absence of LPS. Day 7 mature DC were obtained after exposure to different maturation stimuli such as LPS (D7 LPS), IFN-γ (D7 IFN), TNF-α + polyI:C (D7 TNF+PolyI:C) and IL-1β+IL-6+TNF-α+PGE2 (DC D7 cytokine cocktail). Tumor cell viability was then assessed. A–E: data are representative of the results obtained with monocyte-derived DC from 12 different healthy donors.
Maturation state of cytotoxic DC remains a subject of controversy. To answer this question, we investigated the cytotoxic potential of day 5 DC and day 7 DC which had been matured from day 5 to day 7 with different reagents such as LPS, IFN-γ, TNF-α+PolyI:C, or IL-1β+IL-6+TNF-α+PGE2 (supplemental fig. 3A). These day 7 DC were activated with LPS and cultured for 48 h with tumor cells, then survival of the tumor cells was assessed. The data presented in Fig. 1E indicate that LPS failed to trigger the killing activity of day 7 mature DC whatever the maturation cocktail used to induce DC maturation (Fig. 1E). Only immature day 5 DC were capable of triggering tumor cell death after activation with LPS (Fig. 1E). We therefore focused our investigation on the killing properties of LPS-activated day 5 monocyte-derived DC (hereafter referred to as hKDC) from healthy donors and from cancer patients.
hKDC generated from cancer patient blood monocytes exhibit cytotoxic activity against tumor cells
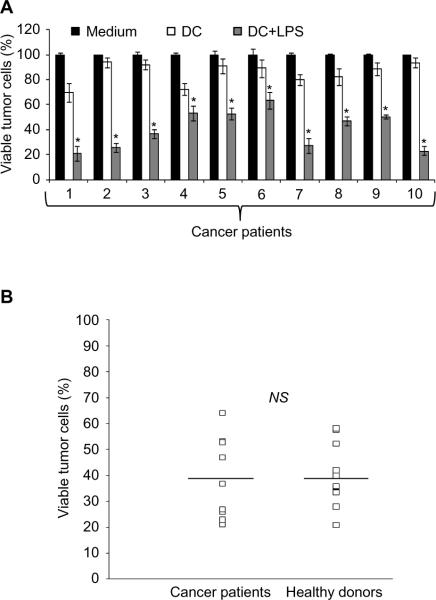
The inhibition of DC by tumors and their maintenance at a non-functional stage are two of the numerous mechanisms used by cancer cells to escape the antitumor response. These `tolerogenic' DC, typically characterized by an immature or semi-mature phenotype, are capable of anergizing effector T lymphocytes and/or driving the differentiation of immunosuppressive FoxP3+ Treg (54–55). It was therefore fundamental and clinically relevant to determine whether the tumoricidal activity of DC generated from cancer patients was impaired. Monocyte-derived DC generated from 10 patients with stage IV cancers (lung and colon cancers or lymphomas) were characterized by the same immature phenotype as DC from healthy volunteers (supplemental fig. 3Aa, 3Ab). Upon LPS stimulation DC generated from cancer patients or healthy donors exhibited a similar mature phenotype and functional properties (supplemental fig. 3Ac–f and supplemental fig. 3B) and significant cytotoxic activity against tumor cells (40 to 80%) when activated with LPS (Fig. 2A). These results therefore indicate that the tumor killing ability of DC generated from individuals with stage IV cancer is similar to that in DC from healthy volunteers (Fig. 2B).
Figure 2. Monocyte-derived DC generated from cancer patients can be differentiated into potent hKDC.
A: HT29 tumor cells were cultured alone (Medium) or with monocyte-derived DC generated from 10 cancer patients (1–10) (DC:tumor cell ratio = 5:1) in the presence (DC+LPS) or absence of LPS (DC). Tumor cell viability was determined after 48 h *, Significant difference when compared with tumor cells cultured in absence of DC (Medium) or with non-activated DC (DC) (p<0.05). B: The cytotoxic activity of monocyte-derived DC generated from 10 cancer patients was compared to the killing potential of monocyte-derived DC generated from 12 healthy donors. The values represent the percentage of viable HT29 tumor cells after 48 h of co-culture with LPS-activated, monocyte-derived DC from healthy donors or from cancer patients.
hKDC cytotoxic activity depends on peroxynitrite
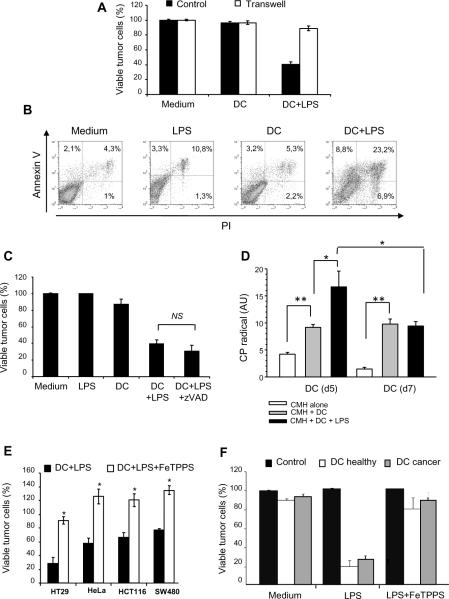
We next investigated the mechanism underlying hKDC cytotoxicity. We first determined that the tumoricidal activity of hKDC was dependent on direct cell-cell contact as separation from their targets by a microporous membrane prevented killing of cancer cells (Fig. 3A). Death receptor ligands such as TRAIL or FasL have been reported to play a role in the cytototoxic activity of DC (26, 32, 35–36, 38, 43–46, 48–49). We therefore evaluated whether the tumor killing activity of hKDC was dependent on similar processes. Our results indicate that hKDC were capable of eliminating tumor cell lines which are resistant to TNF, TRAIL or FasL (not shown). Consistent with these results, LPS activated day 5 DC did not secrete TRAIL or Fas-L (not shown). In line with this result, Annexin V and propidium iodide staining indicated that hKDC induced the necrosis of tumor cells as evidenced by early plasma membrane disruption (Fig. 3B). Consistent with these results, zVAD-fmk, a broad spectrum caspase inhibitor, failed at inhibiting hKDC-induced tumor cell death thus excluding a conventional apoptotic-related process (Fig. 3C).
Figure 3. Human monocyte-derived DC killing activity depends on peroxynitrites.
A: HT29 cells were cultured alone (Medium) or with monocyte-derived DC from healthy donors, in the presence (DC+LPS) or not of LPS (DC). The cells were cultured together (Control) or separated by a transwell insert (Transwell). Tumor cell killing was determined after 48 h. B: HT29 tumor cells were cultured alone (Medium), with LPS (LPS), with day 5 monocyte-derived DC from healthy donors with (DC+LPS) or without LPS (DC). After 48 h, the cells were stained with CD1a Ab and with annexine V-FITC/propidium iodide (PI). The percentage of PI−/Annexine-V+ (apoptotic) or PI+/Annexine-V+ (necrotic) tumor cells was determined after gating on CD1a negative HT29 tumor cells. C: HT29 tumor cells were cultured alone (Medium), with LPS (100 ng/ml) (LPS), with day 5 monocyte-derived DC (DC), with DC in the presence of LPS (DC+LPS) or DC in the presence of LPS + z-VAD-fmk (40 μM) (ratio DC:tumor cell = 5:1). Tumor cell survival was determined after 48 h. D: Production of reactive oxygen species by non activated or LPS-activated human monocyte-derived DC was determined by resonance electron spin. CMH was used as the spin probe. Day 5 monocyte-derived DC from healthy donors were cultured with (DC+LPS) or without LPS (DC) for 6 h. Activated and non-activated human DC (2×106) were harvested and incubated with 1 mmol/l CMH. The amplitude of center field anisotropic signal was quantified in arbitrary units in order to determine CP• nitroxide formation in LPS-activated or non-activated human dendritic cells at day5 and day7. This experiment was done 3 times with DCs from 3 different healthy donors and from 3 cancer patients.*, p<0.05; **, p<0.005. E: HT29 tumor cells were cultured alone (Control) or with day 5 monocyte-derived DC from healthy donors (DC): without LPS (Medium), with LPS (LPS) or with LPS and FeTPPS (50μM) (LPS+FeTPPS). Tumor cell viability was determined after 48 h. F: Different human tumor cell lines (HT29, HeLa, HCT116, SW480) were co-cultured with LPS-activated human monocyte-derived DC from healthy volunteers or from cancer patients in the presence (DC+LPS+FeTPPS) or absence (DC+LPS) of FeTPPS (50μM) (DC:tumor cell ratio = 5:1). Tumor cell viability was determined after 48 h. Similar results were obtained with DC generated from 6 healthy donors and 4 cancer patients. *, Significant difference when compared with tumor cells cultured with activated DC in the absence of FeTPPS (p<0.05).
We and others have previously reported that nitric oxide (NO) or peroxynitrites represent critical effector molecules required for the tumoricidal activity of both rat and mouse DC (9, 12, 19, 56). To define whether these cytotoxic products may also play a role in tumor cell killing by hKDC, we analyzed ROS production by these killer cells using electron spin resonance (ESR). ESR is one of the few techniques which allows the direct measurement of free radical species. However most of biological reactive oxygen and nitrogen species (RONS) of interest are too short-lived to be measured directly with ESR spectroscopy. Hydroxylamine spin-probes such as CMH can be oxidized by superoxide anion or peroxynitrites, giving rise to a more stable long-lived ESR-detectable CP• nitroxide. This allows the detection of these unstable species in cell cultures or isolated organs (57–58). Our results indicate that both immature (day 5) and mature (day 7) DCs oxidize CMH into CP• radical, in a time dependent manner. However, after 6 hours of stimulation with LPS, immature day 5 DC produced a significantly increased RONS quantity, a characteristic that was lost in mature day 7 DC (Fig. 3D). Moreover, a peroxynitrite inhibitor, FeTPPS, significantly abrogated the cytotoxic activity of hKDC against several human tumor cells lines (Fig. 3E). We also confirmed that the cytotoxic activity of monocyte-derived DC generated from cancer patients was inhibited by FeTPPS (Fig. 3F). These results, therefore, strongly suggest that hKDC generated either from healthy donors or from cancer patients kill tumor cells by a mechanism involving peroxynitrite production.
hKDC are capable of capturing tumor cell debris and of activating tumor specific T cells
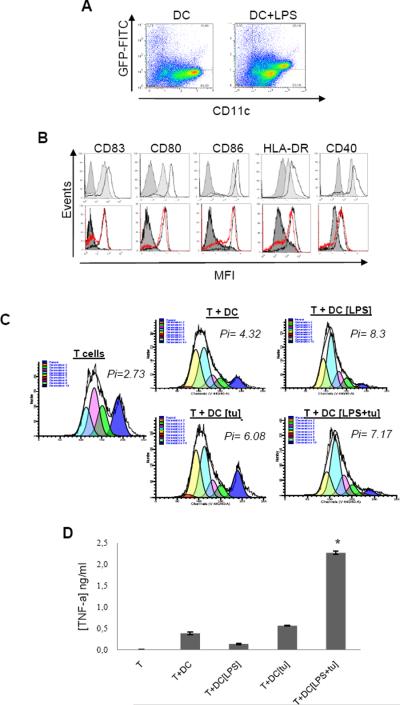
Whether hKDC that had killed cancer cells are also capable of presenting tumor antigens and activating specific lymphocytes has remained controversial. A prerequisite for tumor antigen presentation to T cells is their uptake and processing by DC. Using GFP-transfected HT29 tumor cells, we demonstrated that hKDC were able to engulf tumor fragments from the tumor cells they had killed (Fig. 4A). In addition, after tumor cell killing in presence of LPS, hKDC (generated from healthy donors or cancer patients) exhibited a phenotype consistent with that of mature DC (Fig. 4B). Following killing and the uptake of tumor cell fragments, these hKDC retained their functional properties as they were capable of inducing the proliferation of autologous T lymphocytes (Fig. 4C). Moreover, using the human melanoma cell line T1, we demonstrated that after killing cancer cells, KDCs were capable of inducing the activation of the tumor antigen (Melan-A25–36) specific human CD8+ T cell clone (Fig. 4D). These results thus indicate that hKDC generated ex vivo from healthy volunteers or cancer patients that had killed tumor cells are efficient at presenting tumor Ag from the cancer cells they have killed.
Figure 4. hKDC exhibit the phenotypical and functional characteristics of mature activated APC after killing of tumor cells.
A: GFP transfected HT29 tumor cells were cultured with day 5 monocyte-derived DC (DC) or with LPS (DC+LPS) for 72 h. The cells were then stained with CD1a Ab and analysed by flow cytometry for GFP expression. Representative data of three experiments performed with DC from healthy donors. B: Upper histograms: Phenotype of day 5 monocyte-derived DC (dark gray) compared with mature DC (light gray) or hKDC from healthy patient (black line); Lower histograms: Phenotype of day 5 monocyte-derived DC (full gray) compared with hKDC from healthy patient (black line) or from cancer patients (red line). hKDC were collected from a 48 h-culture with HT29 tumor cells and LPS. Cells were stained with the indicated Ab and analysed by flow cytometry. C: Autologous T lymphocyte proliferation was assessed using Cell Trace Violet cell proliferation kit as described in material and methods. Cell Trace Violet labelled purified T cells were co-cultured with day 5 immature DC without activator (T+DC), with LPS (100 ng/ml) (T+DC+LPS), with HT29 tumor cells (T+DC+[tu]) or with HT29 tumor cells in the presence of LPS (T+DC [LPS+tu]) (DC:T ratio = 1:10). Non-activated T cells were used as a negative control and T cells stimulated with anti-CD3/anti-CD28 T cell expansion beads were used as a positive control (T cells). The T cell proliferation index (Pi) was defined after 5 days of T cell/DC co-cultures using ModFit software. 4B, 4C: Representative results from 3 experiments performed with DC from 3 different healthy donors and from 3 different cancer patients. D: Immature day 5 DC were cultured with T1 melanoma cell line in the presence or absence of LPS (100 ng/ml). After 48 h DC were harvested from these co-cultures and plated with anti-T1 CD8+ T cells (CTL clone specific for Melan-A25–36 peptide) for an additional 4 h (DC:Tcell ratio = 5:1). TNF-α production by CTL was measured using a TNF-α ELISA kit.
Discussion
We have previously reported that KDC can be generated from rat (19) and mouse bone marrow (9). In the current report we provide evidence that human peripheral blood monocytes from both healthy individuals and more clinically relevant, from patients with advanced cancer, can also be driven to differentiate into potent killer dendritic cells. The killing mechanism mediated by these hKDC involves the production of peroxynitrites. After killing of cancer cells, hKDC are capable of capturing necrotic tumor cells and of differentiating into activated and fully functional APC that produce IL-12, express high levels of HLA-DR and co-stimulatory molecules and induce T cell proliferation. Moreover, these ex vivo generated hKDC are capable of activating tumor antigen specific CTL, which strongly advocate for their therapeutic use in cancer patients. To facilitate the clinical application of hKDC in clinic, we also demonstrated that KDC could be generated in serum free medium (supplemental fig. 4).
The cytotoxic activity of human immature peripheral blood monocyte-derived DC has already been reported (27, 35, 37, 45–46) but no or only a low killing activity at an effector:target ratio of 5:1 was observed. In addition, in these studies, LPS was used at high concentration, ranging from 5 μg/ml (27, 43) to 10 μg/ml (37). In contrast to these high concentrations, we now report that LPS induces hKDC killing activity even at doses as low as 10 ng/ml. The mechanisms underlying KDC-mediated cytotoxic function has been related to death receptors and their ligands (25–27, 29, 32–36, 42, 44, 46–47). Our results indicate that hKDC induce tumor cell necrosis by a process that is independent of the above mentioned mechanisms of cytotoxicity. By using the electron spin resonance technique and specific inhibitors, we identified peroxynitrites as the primary molecule responsible for KDC tumoricidal function. The inhibition of KDC killing activity by the peroxynitrite catalyst, FeTPPS, (59–63) confirmed the primary role of these molecules. It is important to underline, that although the mechanism of cytotoxicity used by these hKDC is not specific to tumor cells and may affect normal cell survival, we confirmed that no significant killing of T lymphocytes was detectable in presence of hKDC. This can be explained by the fact that T lymphocyte killing might be a minor phenomenon compared to the high proliferation rate of T cells induced by these DC. Another explanation may stem from the fact that the high levels of proinflammatory cytokines produced by hKDC can support T cell survival by activating anti-apoptotic mechanisms that render T lymphocytes resistant to DC-mediated cytotoxicity. In support of this hypothesis, recent studies have reported on cytokine driven up-regulation of Bcl2 or related anti-apoptotic molecules in subsets of effector and memory T cells, allowing them to survive (64–66).
Whether KDC can be generated from cancer patients has not been previously elucidated. This is a relevant question insofar as the tumor microenvironment is able to suppress many aspects of the anti-cancer immune response in order to escape from recognition by the immune system(67–68). One of the mechanisms of tumor-induced immunosuppression consists in inhibiting the ability of DC precursors to differentiate into functional cells (5, 68–69). We therefore reasoned that the tumor killing activity of DC generated from cancer patient monocytes may be impaired. Our results indicate that KDC generated from all the cancer patients evaluated in this study were able to kill tumor cells, indicating that this cytotoxic potential was not inhibited by the tumor environment. Moreover, whatever the type of cancer, hematological or solid tumors, hKDC from cancer patients were as cytotoxic as those from healthy donors. This is a clinically important finding which opens new perspective for the use of autologous hKDC in cancer patients. The use of hKDC could circumvent extensive ex vivo manipulations during DC vaccines protocols, such as tumor antigen preparation and loading, and DC activation. Autologous DC from cancer patients may be differentiated into hKDC and activated ex vivo and then used to kill autologous tumor cells and to acquire and process released tumor Ag in vitro before being re-injected into the patient. These novel findings may be relevant for the design of new improved DC-based vaccines.
Supplementary Material
Acknowledgements
We thank A. Morizot, P. Bastable, A. Legrand and A. Bouchot (SERCOBIO) for their technicalassistance.
Grant Support: This work was supported by grants from La Ligue contre le cancer (BB) and le Conseil Régional de Bourgogne (BB), the Universitary Hospital of Dijon (BB), the NIH grant R01 CA104926 (E.K. and N.L.), the AZ Cancer Center Support Grant CA023074 (EK and NL), the Tee Up for Tots and the PANDA Funds (E.K. and N.L.).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 6.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 7.Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010;2:57–68. doi: 10.2217/imt.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 9.Fraszczak J, Trad M, Janikashvili N, Cathelin D, Lakomy D, Granci V, Morizot A, Audia S, Micheau O, Lagrost L, et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J Immunol. 184:1876–1884. doi: 10.4049/jimmunol.0900831. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Tatsumi T, Pizzoferrato E, Vujanovic N, Storkus WJ. Nitric oxide sensitizes tumor cells to dendritic cell-mediated apoptosis, uptake, and cross-presentation. Cancer research. 2005;65:8461–8470. doi: 10.1158/0008-5472.CAN-05-0654. [DOI] [PubMed] [Google Scholar]
- 11.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 13.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, Alber SM, Watkins SC, Okada H, Storkus WJ. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer research. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 15.Alli R, Savithri B, Das S, Varalakshmi C, Rangaraj N, Khar A. Involvement of NKR-P2/NKG2D in DC-mediated killing of tumor targets: indicative of a common, innate, target-recognition paradigm? European journal of immunology. 2004;34:1119–1126. doi: 10.1002/eji.200324793. [DOI] [PubMed] [Google Scholar]
- 16.Chauvin C, Josien R. Dendritic cells as killers: mechanistic aspects and potential roles. J Immunol. 2008;181:11–16. doi: 10.4049/jimmunol.181.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Chauvin C, Philippeau JM, Hemont C, Hubert FX, Wittrant Y, Lamoureux F, Trinite B, Heymann D, Redini F, Josien R. Killer dendritic cells link innate and adaptive immunity against established osteosarcoma in rats. Cancer research. 2008;68:9433–9440. doi: 10.1158/0008-5472.CAN-08-0104. [DOI] [PubMed] [Google Scholar]
- 18.Josien R, Heslan M, Soulillou JP, Cuturi MC. Rat spleen dendritic cells express natural killer cell receptor protein 1 (NKR-P1) and have cytotoxic activity to select targets via a Ca2+-dependent mechanism. The Journal of experimental medicine. 1997;186:467–472. doi: 10.1084/jem.186.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolas A, Cathelin D, Larmonier N, Fraszczak J, Puig PE, Bouchot A, Bateman A, Solary E, Bonnotte B. Dendritic cells trigger tumor cell death by a nitric oxide-dependent mechanism. J Immunol. 2007;179:812–818. doi: 10.4049/jimmunol.179.2.812. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava RM, Varalakshmi C, Khar A. Cross-linking a mAb to NKRP2/NKG2D on dendritic cells induces their activation and maturation leading to enhanced anti-tumor immune response. Int Immunol. 2007;19:591–607. doi: 10.1093/intimm/dxm024. [DOI] [PubMed] [Google Scholar]
- 21.Trinite B, Chauvin C, Peche H, Voisine C, Heslan M, Josien R. Immature CD4− CD103+ rat dendritic cells induce rapid caspase-independent apoptosis-like cell death in various tumor and nontumor cells and phagocytose their victims. J Immunol. 2005;175:2408–2417. doi: 10.4049/jimmunol.175.4.2408. [DOI] [PubMed] [Google Scholar]
- 22.Trinite B, Voisine C, Yagita H, Josien R. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202–4208. doi: 10.4049/jimmunol.165.8.4202. [DOI] [PubMed] [Google Scholar]
- 23.Ayres FM, Narita M, Takahashi M, Alldawi L, Liu A, Osman Y, Abe T, Yano T, Sakaue M, Toba K, et al. A comparative study of the JAM test and 51Cr-release assay to assess the cytotoxicity of dendritic cells on hematopoietic tumor cells. Immunological investigations. 2003;32:219–227. doi: 10.1081/imm-120025102. [DOI] [PubMed] [Google Scholar]
- 24.Ayres FM, Narita M, Takahashi M, Yano T, Liu A, Toba K, Furukawa T, Aizawa Y. Human dendritic cells mediate anti-tumor activity against hematopoietic tumor cells without direct contact and Fas/FasL killing pathway. Oncology reports. 2004;11:1017–1023. [PubMed] [Google Scholar]
- 25.Beaulieu S, Lafontaine M, Richer M, Courchesne I, Cohen EA, Bergeron D. Characterization of the cytotoxic factor(s) released from thymic dendritic cells upon human immunodeficiency virus type 1 infection. Virology. 1998;241:285–297. doi: 10.1006/viro.1997.8977. [DOI] [PubMed] [Google Scholar]
- 26.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 27.Chapoval AI, Tamada K, Chen L. In vitro growth inhibition of a broad spectrum of tumor cell lines by activated human dendritic cells. Blood. 2000;95:2346–2351. [PubMed] [Google Scholar]
- 28.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) The Journal of experimental medicine. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill KS, Errington F, Steele LP, Merrick A, Morgan R, Selby PJ, Georgopoulos NT, O'Donnell DM, Melcher AA. OK432-activated human dendritic cells kill tumor cells via CD40/CD40 ligand interactions. J Immunol. 2008;181:3108–3115. doi: 10.4049/jimmunol.181.5.3108. [DOI] [PubMed] [Google Scholar]
- 31.Hubert P, Giannini SL, Vanderplasschen A, Franzen-Detrooz E, Jacobs N, Boniver J, Delvenne P. Dendritic cells induce the death of human papillomavirus-transformed keratinocytes. Faseb J. 2001;15:2521–2523. doi: 10.1096/fj.00-0872fje. [DOI] [PubMed] [Google Scholar]
- 32.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 33.Joo HG, Fleming TP, Tanaka Y, Dunn TJ, Linehan DC, Goedegebuure PS, Eberlein TJ. Human dendritic cells induce tumor-specific apoptosis by soluble factors. International journal of cancer. 2002;102:20–28. doi: 10.1002/ijc.10656. [DOI] [PubMed] [Google Scholar]
- 34.Lichtner M, Maranon C, Vidalain PO, Azocar O, Hanau D, Lebon P, Burgard M, Rouzioux C, Vullo V, Yagita H, et al. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res Hum Retroviruses. 2004;20:175–182. doi: 10.1089/088922204773004897. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–5415. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 36.Lu G, Janjic BM, Janjic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha(1)beta(2), Fas ligand, and TNF-related apoptosis-inducing ligand. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 37.Manna PP, Mohanakumar T. Human dendritic cell mediated cytotoxicity against breast carcinoma cells in vitro. Journal of leukocyte biology. 2002;72:312–320. [PubMed] [Google Scholar]
- 38.Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 39.Schakel K, Kannagi R, Kniep B, Goto Y, Mitsuoka C, Zwirner J, Soruri A, von Kietzell M, Rieber E. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 40.Schakel K, Mayer E, Federle C, Schmitz M, Riethmuller G, Rieber EP. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. European journal of immunology. 1998;28:4084–4093. doi: 10.1002/(SICI)1521-4141(199812)28:12<4084::AID-IMMU4084>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz M, Zhao S, Deuse Y, Schakel K, Wehner R, Wohner H, Holig K, Wienforth F, Kiessling A, Bornhauser M, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174:4127–4134. doi: 10.4049/jimmunol.174.7.4127. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz M, Zhao S, Schakel K, Bornhauser M, Ockert D, Rieber EP. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood. 2002;100:1502–1504. [PubMed] [Google Scholar]
- 43.Shi J, Ikeda K, Fujii N, Kondo E, Shinagawa K, Ishimaru F, Kaneda K, Tanimoto M, Li X, Pu Q. Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer science. 2005;96:127–133. doi: 10.1111/j.1349-7006.2005.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. The Journal of experimental medicine. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderheyde N, Aksoy E, Amraoui Z, Vandenabeele P, Goldman M, Willems F. Tumoricidal activity of monocyte-derived dendritic cells: evidence for a caspase-8-dependent, Fas-associated death domain-independent mechanism. J Immunol. 2001;167:3565–3569. doi: 10.4049/jimmunol.167.7.3565. [DOI] [PubMed] [Google Scholar]
- 46.Vanderheyde N, Vandenabeele P, Goldman M, Willems F. Distinct mechanisms are involved in tumoristatic and tumoricidal activities of monocyte-derived dendritic cells. Immunol Lett. 2004;91:99–101. doi: 10.1016/j.imlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Vidalain PO, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidalain PO, Azocar O, Yagita H, Rabourdin-Combe C, Servet-Delprat C. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167:3765–3772. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 49.Yang R, Xu D, Zhang A, Gruber A. Immature dendritic cells kill ovarian carcinoma cells by a FAS/FASL pathway, enabling them to sensitize tumor-specific CTLs. International journal of cancer. 2001;94:407–413. doi: 10.1002/ijc.1484. [DOI] [PubMed] [Google Scholar]
- 50.Bonnotte B, Favre N, Reveneau S, Micheau O, Droin N, Garrido C, Fontana A, Chauffert B, Solary E, Martin F. Cancer cell sensitization to fas-mediated apoptosis by sodium butyrate. Cell death and differentiation. 1998;5:480–487. doi: 10.1038/sj.cdd.4400371. [DOI] [PubMed] [Google Scholar]
- 51.Dikalov S, Skatchkov M, Fink B, Bassenge E. Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide. 1997;1:423–431. doi: 10.1006/niox.1997.0139. [DOI] [PubMed] [Google Scholar]
- 52.Wyche KE, Wang SS, Griendling KK, Dikalov SI, Austin H, Rao S, Fink B, Harrison DG, Zafari AM. C242T CYBA polymorphism of the NADPH oxidase is associated with reduced respiratory burst in human neutrophils. Hypertension. 2004;43:1246–1251. doi: 10.1161/01.HYP.0000126579.50711.62. [DOI] [PubMed] [Google Scholar]
- 53.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 54.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 55.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimamura H, Cumberland R, Hiroishi K, Watkins SC, Lotze MT, Baar J. Murine dendritic cell-induced tumor apoptosis is partially mediated by nitric oxide. J Immunother. 2002;25:226–234. doi: 10.1097/00002371-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Delemasure S, Sicard P, Lauzier B, Moreau D, Vergely C, Rochette L. Acute administration of epirubicin induces myocardial depression in isolated rat heart and production of radical species evaluated by electron spin resonance spectroscopy. J Cardiovasc Pharmacol. 2007;50:647–653. doi: 10.1097/FJC.0b013e31815571f7. [DOI] [PubMed] [Google Scholar]
- 59.Bolton C, Scott GS, Smith T, Flower RJ. The acute and chronic phases of chronic relapsing experimental autoimmune encephalomyelitis (CR EAE) are ameliorated by the peroxynitrite decomposition catalyst, 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinatoiron (III) chloride, (FeTPPS) European journal of pharmacology. 2008;601:88–93. doi: 10.1016/j.ejphar.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Duplain H, Sartori C, Dessen P, Jayet PY, Schwab M, Bloch J, Nicod P, Scherrer U. Stimulation of peroxynitrite catalysis improves insulin sensitivity in high fat diet-fed mice. The Journal of physiology. 2008;586:4011–4016. doi: 10.1113/jphysiol.2008.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, Neviere R. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. Journal of the American College of Cardiology. 2004;43:2348–2358. doi: 10.1016/j.jacc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 62.Lauzier B, Sicard P, Bouchot O, Delemasure S, Moreau D, Vergely C, Rochette L. A peroxynitrite decomposition catalyst: FeTPPS confers cardioprotection during reperfusion after cardioplegic arrest in a working isolated rat heart model. Fundamental & clinical pharmacology. 2007;21:173–180. doi: 10.1111/j.1472-8206.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 63.Sharma SS, Dhar A, Kaundal RK. FeTPPS protects against global cerebral ischemic-reperfusion injury in gerbils. Pharmacol Res. 2007;55:335–342. doi: 10.1016/j.phrs.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, Hildeman DA. Bcl-2 Allows Effector and Memory CD8+ T Cells To Tolerate Higher Expression of Bim. J Immunol. 2011;186:5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 67.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature reviews. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 68.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annual review of immunology. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonnotte B, Crittenden M, Larmonier N, Gough M, Vile RG. MIP-3alpha transfection into a rodent tumor cell line increases intratumoral dendritic cell infiltration but enhances (facilitates) tumor growth and decreases immunogenicity. J Immunol. 2004;173:4929–4935. doi: 10.4049/jimmunol.173.8.4929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.