Abstract
Previous research has demonstrated considerable preclinical efficacy of nicotinamide (NAM; vitamin B3) in animal models of TBI with systemic dosing at 50 and 500 mg/kg yielding improvements on sensory, motor, cognitive and histological measures. The current study aimed to utilize a more specific dosing paradigm in a clinically relevant delivery mechanism: continuously secreting subcutaneous pumps. A bilateral frontal controlled cortical impact (CCI) or sham surgery was performed and rats were treated with NAM (150 mg/kg/day) or saline (1 ml/kg) pumps 30 min after CCI, continuing until seven days post-CCI. Rats were given a loading dose of NAM (50 mg/kg) or saline (1 ml/kg) following pump implant. Rats received behavioral testing (bilateral tactile adhesive removal, locomotor placing task and Morris water maze) starting on day two post-CCI and were sacrificed at 31 days post-CCI and brains were stained to examine lesion size. NAM-treated rats had reductions in sensory, motor and cognitive behavioral deficits compared to vehicle-treated rats. Specifically, NAM-treated rats significantly improved on the bilateral tactile adhesive removal task, locomotor placing task and the reference memory paradigm of the Morris water maze. Lesion size was also significantly reduced in the NAM-treated group. The results from this study indicate that at the current dose, NAM produces beneficial effects on recovery from a bilateral frontal brain injury and that it may be a relevant compound to be explored in human studies.
Keywords: Traumatic Brain Injury, Vitamin B3, Treatment, Recovery of Function, Rat
1. Introduction
Traumatic brain injury (TBI) has been cited as a major health concern for industrialized nations. In the United States alone, the Center for Disease Control (CDC) estimates that, as of 2010, 1.7 million occur per year. Of those admitted to a hospital, 52,000 die from the injury and an additional 275,000 require hospitalization [1]. The economic burden of TBI has as much of an impact as the social cost. Estimates of the cost of the complications from TBI (such as neurological disorders), lost productivity and reduced income of those affected were in the range of $60 billion as of 2000 [2]. Despite the fact that TBI has been identified as a major economic and health issue, scientific research has yet to yield a treatment that is effective in humans.
Some studies suggest that the reasons that many treatments have not translated to humans are directly related to the method of testing, design of clinical trial or due to not targeting towards the appropriate population (i.e. aged vs. young individuals) and not necessarily due to a difference in pharmacology between species [3–5]. TBI results in a multitude of symptoms, dependent on the location of the injury, velocity of impact and other factors. Because of the varied symptoms, multiple behavioral measures combined with histological investigations are needed to accurately evaluate deficits and assess functional recovery. The causes of cellular damage from TBI are multimodal and thus a sufficient treatment needs to address a multifaceted disorder, rather than single symptoms.
While the initial impact that causes the brain injury is extremely detrimental, many of the complications of TBI as a disorder arise from the secondary cascade of effects. This cascade is where most preventive treatments are targeted, but many pharmacological interventions that are currently being explored focus on treating one specific aspect of the secondary injury cascade such as inflammation, free radical generation or excitotoxic cell death [for review see 6]. An effective treatment may need to address more than one of these processes.
Previously, our lab has shown considerable efficacy in preclinical experiments using nicotinamide (NAM; Vitamin B3). Animals treated with NAM have shown improved recovery of function on sensory, motor and cognitive measures [7–9]. This effect has been shown across multiple behavioral tests, including the bilateral tactile adhesive removal task, vibrissae-forelimb placing task, locomotor placing task and Morris water maze and in doses of both 50 mg/kg and 500 mg/kg. Anatomically, NAM treatment has also shown reductions in edema, apoptosis and activated astrocytes during the acute period after injury at the 50 mg/kg and 500 mg/kg dose [10–12]. NAM treatment has also shown differences in lesion size as early as 72 hours after injury [12].
NAM has been shown to have multiple mechanisms of action following TBI. The primary mechanism is as a precursor to nicotinamide dinucleotide (NAD+), which helps to provide cells with additional energy stores through ATP production. However, it is also a potent inhibitor of poly(ADP-ribose)polymerase- 1 (PARP-1) and the SIRT-1 receptor and contributes to free radical scavenging through NAD+-mediated actions [see 13 for a comprehensive review]. It has even been suggested to potentially be involved in inflammatory regulation of microglia and certain cytokines [14]. Each of these mechanisms work on different aspects of the secondary injury cascade, providing multimodal treatment following TBI.
Previous studies in TBI with NAM utilized either a low dose or high dose regimen (50 mg/kg or 500 mg/kg). Recent research into the pharmacology of NAM in the rat has established the half-life (t1/2) of the 50 mg/kg dose to be 2.1 h and the t1/2 of the 500mg/kg dose to be 6.9 h (Hoane et al., unpublished data). This information has allowed for more targeted dosing to attempt to reach and maintain a steady-state in TBI studies. In addition, a recent study has shown that a drug administration model using subcutaneous pumps was an effective method of administration for NAM. That study only used a dose of approximately 50 mg/kg/day delivered continuously, but still showed recovery of function on sensory and motor tasks as well as some neuronal sparing [15].
The purpose of the current study was to examine whether the effects previously seen would generalize to the frontal model of brain injury which covers a greater range of cortical function (sensory and motor, bilaterally) and to assess recovery over multiple modalities [15]. The current study included evaluation on measures of motor ability, sensory ability and cognition as well as cortical tissue sparing. In addition, it also used an increased NAM dose compared to the previous study. We expected to see improvements on sensorimotor measures, as previously demonstrated, and also hoped that the increased dose would show cognitive improvements, as these were most frequently found at the 500 mg/kg dose [8, 16].
2. Methods
2.1 Animals
Twenty-nine male Sprague-Dawley rats, approximately three months of age with an average weight of 343 g were used in this study. One rat in the treated group died of complications following injury and was not replaced. All procedures described in this study were approved in advance by the Institutional Animal Care and Use Committee and the study was conducted in laboratory facilities certified by the American Association for the Accreditation of Laboratory Animal Care. Rats were housed singly in standard cages with ad lib access to food and water on a 12 h:12 h light:dark cycle. Testing was conducted during the light cycle.
2.2 Surgery
Surgical procedures were performed according to previous studies and perfsormed under aseptic conditions [9, 12, 15]. Rats were anesthetized under combined Isofluorane (2–4%) and oxygen (0.8 L/min) and placed into a stereotaxic device. Body temperature was monitored and adjusted using a heated surgical stage (37° C). Rats received either a frontal CCI injury or sham procedures. A 6.0 mm bilateral craniotomy was made over the frontal cortex centered at AP = +1.5, ML = 0.0 using an electronic hand drill. The cortical area containing the frontal cortex and forelimb motor area was exposed without damage to the meninges or dura. A 5.0 mm diameter stainless steel impactor tip attached to an electromagnetic impactor (myneurolab.com) was used to induce the injury. The cortex was impacted at 2.75 m/s to a depth of 2.5 mm with a contact time of 0.5 s. After injury, bleeding was stopped, the incision was sutured and the rat was placed in a heated recovery chamber. Sham procedures included all of the above, with the exception of the impact.
The rats were allowed to recover motor behavior, then, 30 min after CCI, osmotic mini-pumps were implanted (s.c.) according to a previous study [15]. Briefly, under gas anesthesia an area was sterilized and an incision was made approximately 2.0 cm anterior to the rear legs on the dorsal aspect of the rat. After the incision was made, the underlying fascia and connective tissue was separated from the skin and the osmotic mini-pumps were placed within the “pocket” approximately 2.0 cm posterior to the scapulae and shifted to the right of midline. Afterwards, the incision was sutured closed. Both sham and treated animals were implanted with pumps to control for any effects of the pump surgery on behavioral tasks.
2.3 Drug Administration
Alzet osmotic mini-pumps (Model 2ML1; delivery rate of 10 μL/h) were loaded with a concentration of NAM (215 mg NAM per 1 mL of 0.9% phosphate buffered saline [PBS]; for delivery of approximately 150 mg/kg/day) or vehicle (0.9% PBS), primed and incubated in room temperature 0.9% PBS 12 h prior to surgery following procedures described in a previous study [15]. After implant, rats were given a loading dose of NAM (50 mg/kg, s.c.) or vehicle (1 ml/kg 0.9% PBS, s.c.) to boost NAM serum levels to therapeutic doses. Pumps remained in place for seven days, after which they were explanted under anesthesia. Rats were randomly assigned to four groups. Group one received CCI and was given a pump containing NAM (NAM, n = 9). Group two received CCI and was given a pump containing vehicle (Vehicle, n = 10). Groups three and four received sham procedures and were given a pump containing either vehicle or NAM (Sham-saline, n = 5; Sham-NAM, n = 5). There were no differences between the two sham groups on any behavioral measure, so they were combined into a single sham group for analysis (Sham, n = 10).
2.4 Bilateral Tactile Adhesive Removal Task
In order to test somatosensory function this test was administered on days 2, 6, 10, 14, 18, 22, 26 and 30 post-CCI following methods adapted from Komotar and colleagues [17]. There were two days of pre-testing to establish a baseline prior to injury. Small, rectangular adhesive patches (approximately 4.0 cm × 0.6 cm) were wrapped around the radial aspects of each forelimb. The rat was then returned to its home cage. The total latency (s) to removal of both adhesive patches was recorded with a stopwatch. A trial ended after both patches were completely removed or broken or after 120 s had elapsed. Rats received two trials per test day. The mean latency from the two trials was the primary dependent variable of interest. The intertrial interval (ITI) was approximately 10 min.
2.5 Locomotor Placing Task
In order to assess recovery of coordinated, locomotor limb movement, this test was administered on days 2, 6, 10, 14, 18, 22, 26 and 30 post-CCI following methods outlined in previous studies [9, 15]. Rats were pre-tested for baseline performance one day before surgery. On each test day, the rat was placed on an elevated grid floor (56.0 cm × 54.0 cm) with openings measuring 3.2 cm × 3.2 cm in size and allowed to freely explore for 180 s. A “foot-fault” occurred when a rat inaccurately placed a limb through one of these openings. The number of grid lines crossed as well as foot-faults were recorded. Rats were administered one trial per test day. The total number of foot-faults for all limbs was the primary dependent variable of interest. The following equation was used to calculate the foot faults as a function of grid lines crossed on the grid to control for movement: [(total foot-faults)/(number of grid lines crossed during the trial)].
2.6 Morris Water Maze
In order to assess deficits in cognitive function, the Morris water maze (MWM) was utilized on days 15–21 post-CCI as described in previous studies [9, 16]. There were two phases of testing: reference memory and working memory. On days 15–18, the reference memory task took place with a clear Plexiglas platform (10 cm × 10 cm) submerged in 32 cm of room temperature water (22° C) in the center of the northwest quadrant of a 1.5 m diameter circular tank. Rats were lowered into the pool facing the wall at randomized start points. A trial ended after rats reached the platform or 90 s had elapsed. Rats unable to reach the platform after 90 s were guided by hand, allowed to climb onto the platform and stay above the water for 10 s and then removed from the platform. Path data, including latencies and distances, was recorded by computer movement tracking software (SMART, San Diego Instruments). There were four trials per day with a 15 min ITI. Rats were placed under a heat lamp to dry after each trial. Latencies from the four trials were averaged to form a score for the day.
The working memory task was performed on days 19–21 following protocols described in previous studies [8, 9, 16]. The procedure was the same, except on each day, the platform was placed in the center of a randomized new quadrant of the tank (Northeast, Southwest or Southeast). There were four trials per day with a 15 min ITI. Rats were placed under a heat lamp to dry after each trial. The first trial of the day was considered an acquisition trial and was not included; the latencies from the last three trials were averaged to form a score for the day.
2.7 Serum Analysis
Under anesthesia, at seven days post-injury, subcutaneous pumps were removed and blood samples were collected from the tail vein. Blood serum was separated by microcentrifuge, snap frozen and stored at −80°. NAM levels were analyzed in serum using HPLC with UV detection at 254 nm on a Varian Pro Star 210 HPLC system according to a previously published protocol [15]. Briefly, 60 μL serum samples and the added internal standard, nicotinamide hypoxanthine dinucleotide sodium salt (de-amino NAD) were deproteinized with 1 M perchloric acid buffered with 1 M potassium phosphate (pH 7.5) and centrifuged. The supernatant was injected onto a Adsorbosil C-18, 5 μm 250 × 4.6 mm column (Grace Davidson Discovery Sciences, Deerfield, IL) and separated using a mobile phase gradient of 6% to 20% methanol over 10 min in 0.2 M ammonium phosphate (pH 5.25). The de-amino NAD eluted at 6.3 min and NAM eluted at 13.5 min. The final concentration was measured in μg/mL.
2.8 Histology
On day 31 post-CCI, rats were anesthetized with a lethal dose of sodium pentobarbital (Euthasol, Virbac Animal Health; 0.3 mL, i.p.). Once eye-blink and pedal responses disappeared, rats were transcardially perfused with ice cold 0.9% PBS, followed by 10% phosphate buffered formalin (PBF). After the brain was removed from the skull, it was post-fixed for one day in PBF. Brains were slabbed coronally and underwent paraffin processing (Tissue Tek VIP). After the brains were processed, they were sliced on a rotary microtome at 10 μm (Leica RM2125) and mounted on electrostatically charged microscope slides.
2.9 Lesion Analysis
Prior to slicing, brains were imaged dorsally through an Olympus microscope (SZX9) using an Olympus 13.5 megapixel camera (DP-70). The lesioned area was measured using imaging software (ImageTool, UTHSCSA) calibrated to measure in millimeters. The areas were compared across groups based on a previous study [18].
In order to examine the extent of the lesion, coronal sections transversing the lesion cavity from 4.75 to −1.00 from bregma were selected (4.75, 2.50, 1.25 and −0.25 from bregma), deparaffinized and stained with cresyl-violet. An Olympus microscope (BX-51) with an Olympus 13.5 megapixel camera (DP-70) was used to image sections. The area of the remaining brain tissue and the area of the lesion for each section were calculated using ImageTool according to previous studies [8, 9]. The Calvalieri method was used to calculate the volume of the overall brain area and lesion area [19]. The number of sections (4) was multiplied by the thickness (10 μm) and the area of the brain section or lesion. The subsequent volumes were then transformed into a ratio of lesion to remaining brain and compared across groups.
2.10 Data Analysis
All data were analyzed without knowledge of group assignment. The mean and standard error of the mean (SEM) was calculated for all the data. A General Linear Model (GLM) ANOVA with repeated measures was used to evaluate the effects of NAM treatment across days. The Huynh-Feldt correction was used to correct for repeated measures. Fischer’s Least Significant Difference (LSD) was used for pairwise post-hoc comparisons. One-way ANOVAs were used to evaluate histological data and were followed up with LSD post-hoc analyses. Pearson correlations were used to examine relationships between serum level, lesion size and behavioral performance. The statistical level of significance was a p-value of less than 0.05.
3. Results
3.1 Bilateral Tactile Adhesive Removal Task
The time it took for the rat to remove both adhesive patches was recorded for each day. The pre-test days before injury were averaged and analyzed with a one-way ANOVA [Group (Sham, Vehicle, NAM) x Latency]. There were no significant differences between the groups, F(2,26) = 1.22, p = 0.313.
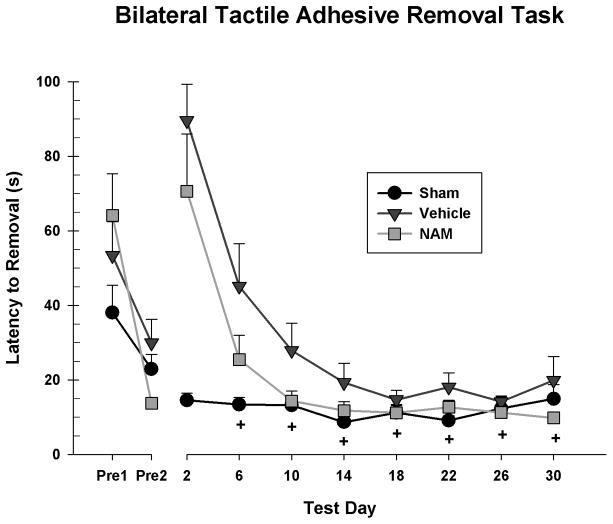
Behavioral performance post-TBI was analyzed using a 3 × 8 repeated measures ANOVA [Group (Sham, Vehicle, NAM) x Day (2, 6, 10, 14, 18, 22, 26, 30)]. There was a significant day by group interaction on the task, F(14,182) = 8.65, p < 0.001. There was a main effect of day on the latency to removal, F(7,182) = 36.41, p < 0.001, and a significant overall difference between the groups, F(2,26) = 8.17, p = 0.002. Specifically, the sham group took significantly less time to remove the patches than the vehicle group overall, LSD(18) = 18.89, p < 0.001, the NAM group took significantly less time than the vehicle group overall, LSD(17) = 10.19, p = 0.044, and there was no significant difference between the NAM and sham groups, LSD(17) = 8.7, p = 0.082 (see Figure 1).
Figure 1.
Patch removal latencies for both forelimbs on the bilateral tactile adhesive removal task. The sham group showed no effect of day, while the other groups showed large deficits which subsequently recovered over time. The NAM group took significantly less time overall than the vehicle group to remove the patches. The NAM group was not significantly different from the sham group on days 6, 10, 14, 18, 22, 26 and 30 (+ = ns).
Simple main effects were examined to interpret the interaction. There was no main effect of day for the sham group, F(7,63) = 1.28, p = 0.277, while there was for the vehicle group, F(7,63) = 13.67, p < 0.001 and NAM group, F(7,77) = 27.77, p < 0.001 demonstrating recovery from initial injury deficits. Pairwise comparisons revealed that despite an overall difference in the rate of recovery, there were no significant differences between the NAM and vehicle groups on any specific test day. The NAM group only performed significantly worse than the shams on day 2, LSD(17) = 56.01, p = 0.001.
3.2 Locomotor Placing Task
The foot-fault score was calculated for each rat for each test day. A oneway ANOVA [Group (Sham, Vehicle, NAM) x Faults] was used to analyze the pre-surgery test day. There was no difference on foot-fault scores F(2,26) = 0.27, p = 0.768.
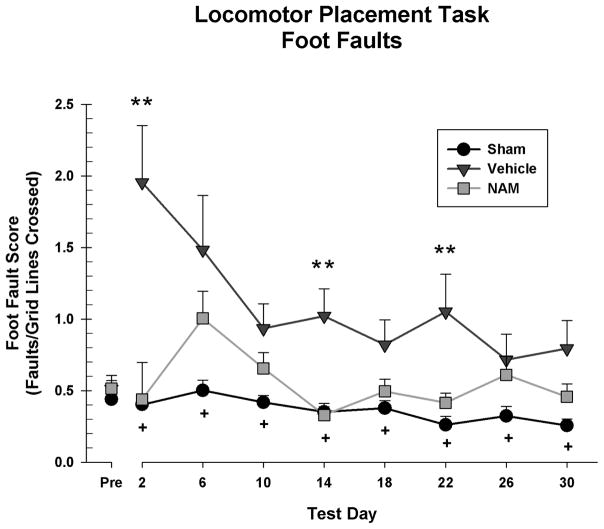
Performance post-injury was analyzed in a 3 × 8 repeated measures ANOVA [Group (Sham, Vehicle, NAM) x Day (2, 6, 10, 14, 18, 22, 26, 30)]. There was a significant day by group interaction, F(14,182) = 2.11, p = 0.008. There was also a main effect of day, F(7,182) = 4.66, p < 0.001, and a significant overall difference between groups, F(2, 26) = 11.85, p < 0.001. Specifically, the NAM group had lower overall fault scores than the vehicle group, LSD(17) = 0.21, p = 0.005, and the sham group had lower overall fault scores than the vehicle group, LSD(18) = 0.62, p < 0.001 (see Figure 2).
Figure 2.
Foot fault score on the locomotor placing task. Neither the vehicle or sham group showed an effect of day, while the NAM group did. The sham and NAM groups had significantly less overall faults than the vehicle group. The vehicle group had significantly more foot faults than the NAM group on days 2, 14, and 22 (* = p < 0.05, ** = p < 0.01). There were no significant differences between the NAM-treated and sham group on any testing day (+ = ns).
Simple main effects were examined to explore the interaction. There was a significant main effect of day for the NAM group, F(6,48) = 2.69, p = 0.025, however, there was no main effect for the vehicle group, F(6,54) = 2.25, p = 0.052 or sham group, F(6,54) = 1.40, p = 0.231, suggesting that the NAM group recovered from the initial deficits. Pairwise comparisons revealed that the NAM group had lower fault scores than the vehicle group on day 2, LSD(17) = 1.52, p = 0.002, day 14, LSD(17) = 0.69, p = 0.001 and day 22, LSD(17) = 0.64, p = 0.012. The NAM group was not significantly different than the sham group on any test day.
3.3 Morris Water Maze
The average latencies for the rats to reach the platform were analyzed for each test day. The reference memory paradigm was analyzed in a 3 × 4 repeated measures ANOVA [Group (Sham, Vehicle, NAM) x Day (15, 16, 17, 18)]. There was no day by group interaction, F(6,78) = 1.25, p = 0.289. However, there was a significant main effect of day on latency, F(3,78) = 31.04, p < 0.001 and a significant difference between the groups, F(2,26) = 20.72, p < 0.001. The sham group took significantly less time to reach the platform than the vehicle group, LSD(18) = 39.02, p < 0.001 and NAM group, LSD(17) = 23.75, p = 0.001 and the NAM group took significantly less time than the vehicle group, LSD(17) = 15.26, p = 0.022, showing that continuous NAM treatment over seven days reduced reference memory deficits in lesioned rats (see Figure 3).
Figure 3.
Latencies to locate the platform on the reference memory paradigm of the Morris water maze. Overall, the sham group had lower latencies than the vehicle and NAM groups. Overall, the NAM group had lower latencies than the vehicle group. The NAM group had significantly lower latencies than the vehicle group on days 16 and 17 (* = p < 0.05, ** = p < 0.01). The NAM group was significantly impaired compared to the sham group on all testing days.
Pairwise comparisons showed that the NAM group had significantly lower latencies than the vehicle group on day 16 LSD(17) = 23.22, p = 0.02, and on day 17, LSD(17) = 21.26, p = 0.006. The NAM group was also significantly impaired compared to the sham group on day 15, LSD(17) = 18.87, p = 0.013, day 16, LSD(17) = 22.65, p = 0.023, day 17, LSD(17) = 21.54, p = 0.005 and day 18, LSD(17) = 31.96, p = 0.003.
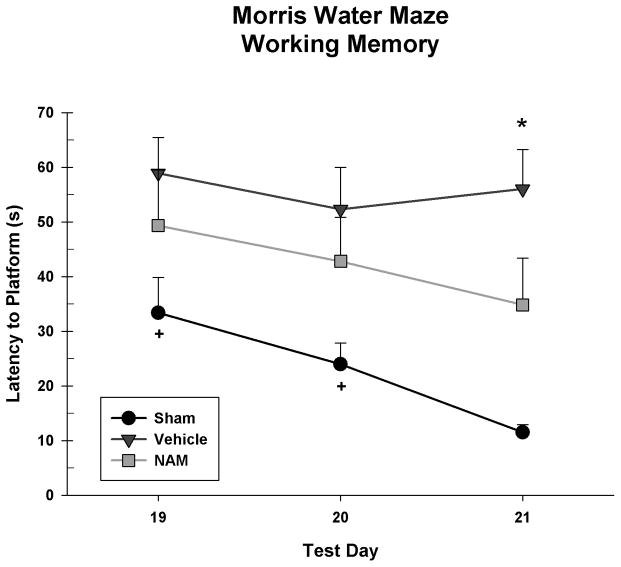
The working memory paradigm was analyzed in a 3 × 3 repeated measures ANOVA [Group (Sham, Vehicle, NAM) x Day (19, 20, 21)]. There was no significant day by group interaction, F(4,52) = 0.92, p = 0.459. However, there was a significant main effect of day, F(2,52) = 4.23, p = 0.020 and a significant difference between groups, F(2,26) = 10.02, p = 0.001. The sham group took significantly less time to reach the platform than the vehicle group, LSD(18) = 32.80, p < 0.001 and NAM group, LSD(17) = 19.37, p = 0.017. There was no significant difference between vehicle and NAM groups, LSD(17) = 13.43, p = 0.087 suggesting that continuous NAM treatment over seven days may not reduce working memory deficits in lesioned rats at the current dose (see Figure 4).
Figure 4.
Latencies to locate the platform on the working memory paradigm of the Morris water maze. Overall, the sham group took less time than the vehicle and NAM group to reach the platform. On the third day of testing (day 21) the NAM group had significantly improved latencies compared to the vehicle group (* = p < 0.05). In addition, the NAM group was not significantly different from the sham group on day 19 or 20 (+ = ns).
Pairwise comparisons showed that the NAM group only performed better than the vehicle group on day 21, LSD(17) = 21.18, p = 0.027. The NAM group was also only significantly impaired compared to the sham group on day 21, LSD(17) = 23.32, p = 0.016.
3.4 Serum Analysis
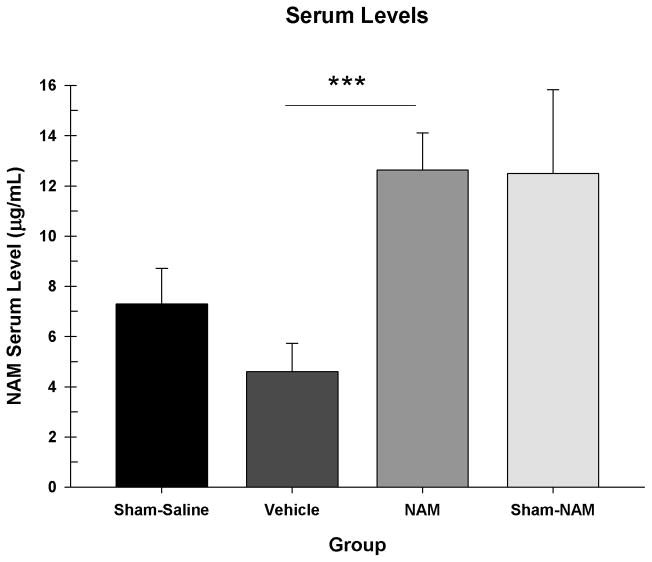
NAM levels in blood serum were analyzed in a one-way ANOVA [Group (Sham-Sal, Vehicle, NAM, Sham-NAM)]. The sham group was broken down into two groups for this analysis only since some shams received NAM treatment (see section 2.3). There was a significant difference between the groups, F(3,35) = 6.65, p = 0.001. The NAM-treated group had significantly higher NAM levels than the vehicle group, LSD(27) = 8.03, p < 0.001 but not the sham-saline group, LSD(18) = 5.33, p = 0.056 or sham-NAM group, LSD(18) = 0.13, p = 0.963. The sham-saline group did not have significantly different NAM levels than the vehicle group, LSD(17) = 2.70, p = 0.328, or sham-NAM group, LSD(8) = 5.20, p = 0.124 (see Figure 5).
Figure 5.
NAM content in serum at seven days post-CCI. The NAM-treated group had significantly higher NAM levels than the vehicle group (*** = p < 0.001), but not the sham or NAM-sham groups.
3.5 Lesion Analysis
The dorsal area of the lesion (in mm2) was compared in a one-way ANOVA [Group (Sham, Vehicle, NAM)]. There was a significant difference between the groups, F(2,26) = 54.389, p < 0.001. The sham group had smaller surface lesions than the NAM group, LSD(17) = 24.64, p < 0.001, and the vehicle group, LSD(18) = 16.57, p < 0.001. The NAM group also had significantly smaller surface lesions than the vehicle group, LSD(17) = 8.06, p = 0.003.
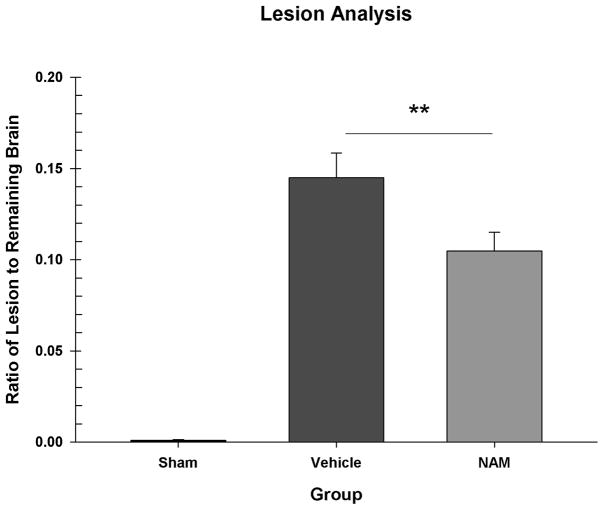
The ratio of lesion volume to total remaining brain volume was compared in a one-way ANOVA [Group (Sham, Vehicle, NAM)]. There was a significant difference between the groups, F(2,26) = 59.476, p < 0.001. The sham group had a significantly smaller ratio of lesion to brain than the vehicle group, LSD(18) =0.14, p < 0.001 and the NAM group, LSD(17) = 0.10, p < 0.001. The NAM group had a significantly smaller ratio of lesion to brain than the vehicle group, LSD(17) = 0.04, p = 0.008 (see Figure 6).
Figure 6.
Ratio of lesion volume to the total remaining brain volume. The sham group had a significantly smaller ratio than the vehicle and NAM groups. NAM administration significantly reduced the size of the injury compared to vehicle treatment (** = p < 0.01).
3.6 Lesion, Serum and Behavioral Correlations
Correlations were run to evaluate relationships between serum NAM levels and outcome measures as well as between lesion size and behavioral outcomes. In order to appropriately evaluate recovery, the sham group was not included in this analysis since the variability in the sham group would be unrelated to serum levels of NAM or to lesion sizes. Since serum was collected at day seven, testing day six was chosen as the point of comparison for the two tasks that were tested over the entirety of the study (bilateral tactile adhesive removal and locomotor placing), while the testing days of the water maze tasks were averaged to form one score for reference memory and one for working memory. There were significant correlations between serum levels and the bilateral tactile adhesive removal task, r(17) = −0.547, p = 0.015 and between lesion size and bilateral tactile adhesive removal, r(17) = 0.577, p = 0.01 as well as between lesion size and reference memory in the Morris water maze, r(17) = 0.482, p = 0.037. Table 1 shows all of the correlations between serum levels, lesion size and the behavioral outcomes.
Table 1.
Correlations between NAM serum levels, lesion size and behavioral indices of recovery.
| Serum | Lesion Size | Bilateral Tactile Adhesive Removal Task | Locomotor Placing Task | Reference Memory (MWM) | Working Memory (MWM) | |
|---|---|---|---|---|---|---|
| Serum | x |
r = −0.437 p = 0.061 |
r = −0.547* p = 0.015 |
r = 0.018 p = 0.940 |
r = −0.180 p = 0.461 |
r = −0.067 p = 0.785 |
| Lesion Size |
r = −0.437 p = 0.061 |
x |
r = 0.577** p = 0.010 |
r = 0.121 p = 0.621 |
r = 0.482* p = 0.037 |
r = 0.424 p = 0.071 |
= p < 0.05
= p < 0.01
4. Discussion
The results of this study demonstrate that NAM, administered continuously for seven days at a 150 mg/kg/day dose combined with an initial loading dose, results in multiple aspects of behavioral recovery and reduces cortical tissue loss following bilateral frontal CCI. Rats treated with NAM showed significantly fewer deficits than the vehicle-treated comparisons and in sensorimotor tasks approached sham levels of performance. The only task where NAM-treated rats did not show overall improvement was in the working memory paradigm of the Morris water maze. A primary goal of this study was to demonstrate that a more pharmacologically relevant dose of NAM, based on data on the half-life of NAM in the rat, would demonstrate recovery of function in a bilateral frontal model of injury. This hypothesis was supported across several aspects of recovery and shows that the current dose of NAM is relevant for addressing multiple deficits seen after a frontal TBI.
The bilateral tactile adhesive removal task showed that rats treated with NAM recovered to sham levels faster than the vehicle comparison group, despite considerable deficits immediately after injury, demonstrating improved sensory function. Although there was not a significant difference on any one specific day, they still recovered more quickly. In addition, with the current injury location sensory deficits would be expected to be less severe and pervasive than other motor and cognitive deficits [20]. The locomotor placing task revealed NAMtreated rats had improved locomotion and fewer foot-faults compared to vehicletreated rats overall. These results suggest a treatment effect on functional motor recovery, despite some of the variability in performance seen across days (see Figure 2).
The NAM-treated rats also learned the location of the platform better than vehicle-treated rats in the reference memory paradigm of the Morris water maze, but were not significantly different in the working memory paradigm. Though the NAM-treated rats were significantly impaired compared to the sham group, they acquired the reference memory location faster than the vehicle-treated group with lower latencies on post-CCI day 16 and 17. In the working memory paradigm, there was no overall difference between the NAM-treated and vehicletreated rats, but the NAM-treated had significantly lower latencies on day 21 of testing. The small gains made on day 21 are not sufficient to make the claim that NAM treatment improved overall performance on this task. The NAM-treated group was significantly more impaired overall compared to sham rats on working memory, but only showed specific impairments on day 21; these comparisons warrant increased investigation into behavior on this task under this dose as the frontal injury results in increased deficits in the working memory paradigm and other cognitive tasks [21]. Previously we have shown that NAM administration can reduce working memory deficits in a frontal model and it may be due to differences in injury location or administration methods that this study was unable to replicate those effects [9]. Presumably increasing serum levels of NAM to comparable levels from that study would replicate the effects seen.
In addition, the administration of NAM reduced lesion volume and while there were still significant lesions in NAM-treated rats, cortical tissue sparing occurred when compared with vehicle-treated controls. This finding matches several previous findings that demonstrated cortical sparing [7, 9, 15]. The serum analysis showed that NAM-treated rats had increases in NAM serum levels compared to the vehicle group, indicating that the pump treatment was effective for elevation of NAM levels after injury. However, the serum levels fell below the desired target (16.5μl) at seven days post-injury, suggesting that the dose may need to be increased to bring serum levels up to the full therapeutic level. Even at this slightly reduced level, NAM was shown to be effective on all behavioral measures with the exception of the working memory paradigm and also improved tissue sparing. The correlations also reveal an interesting relationship between the degree of recovery on the bilateral adhesive removal task based on serum levels (r = −0.547, p = 0.015). Despite the fact that the correlation between lesion size and serum level (r = −0.437) was non-significant (p = 0.061), it is probably the most meaningful correlation to examine as lesion size is potentially a very strong mediator of behavioral recovery. Specifically, this can be seen in the significant correlations between reduction in lesion size and improved performance on the bilateral tactile adhesive removal task (r = 0.577, p = 0.010) and the reference memory paradigm (r = 0.482, p = 0.037), as well as the nonsignificant, yet strong positive correlation on the working memory paradigm (r = 0.424, p = 0.071).
In other studies, NAM has been found to play a role in mediating the effects of experimental TBI and several potential mechanisms have been identified. The primary mechanism is through increases in available cellular energy as a precursor to NAD+. These increases in cellular energy help to reduce cellular stresses caused by ischemic conditions post-injury. It also has strong actions as a PARP-1 inhibitor, which prevents expenditure of energy on DNArepair [13, 14, 22]. Through increases in NAD+ there are reductions in oxidative stress. NAD+ has the ability to donate electrons to free radicals, thus neutralizing them and preventing oxidative damage [13]. NAM also has a complex relationship with the SIRT-1 receptor. NAM inhibits SIRT-1, which is generally beneficial for cell survival, however this inhibition may be regulatory and may be integral to its relationship with PARP-mediation [14, 23]. In addition to these actions, NAM has also been shown to activate anti-apoptotic pathways and provide reductions in apoptosis following injury [9, 12, 14]. Thus, NAM has a multi-modal mechanism following TBI and limits many different areas of the secondary injury cascade establishing explanations for its effects on recovery of function across a multitude of behaviors. It has been shown to be effective in multiple assessments of motor, including the locomotor placing, beam walk and forelimb asymmetry tasks [7, 15, 16, 24]. It has improved recovery on at least two sensory measures, the bilateral tactile adhesive removal and vibrissae-forelimb placing tasks [7–9, 15, 16, 24]. It has even been shown to improve memory and cognition following TBI, specifically on the Morris water maze [8, 9, 16].
Previous studies have established NAM treatment to be effective at alleviating behavioral deficits and providing neuroprotection in animal TBI models. The current dose (150 mg/kg/day) represents an effective dose for treatment of most of the behaviors tested in this study, which falls in line with previous work [8, 9, 15]. However, it did not improve latencies in the working memory portion of the Morris water maze. This lack of improvement may be related to the dose. Most previous studies that have shown improvement in the working memory version of the water maze have utilized a 500 mg/kg dose [8, 16, 24], while only one study found an effect at the 50 mg/kg dose [9]. The possibility of a dose-dependent effect is further supported by recent pharmacological data showing that the 150 mg/kg/day dose may not be sufficient for maintaining the steady state of NAM and as seen in the serum levels from the current study (Hoane, unpublished data). In addition, the differences in injury models may need to be considered. There is potential for dose-dependent effects that vary by injury location and these differences will need to be systematically evaluated to determine if the dose that works for bilateral frontal is optimal for unilateral parietal injuries as well. However, the findings from this study further suggest that NAM treatment generalizes well across different injury models.
Further study into the proper dosing regimen of NAM in rats is necessary to find the dose that appropriately maintains a steady state level and would potentially have the highest efficacy in treating potential cognitive deficits arising from a frontal TBI model. Despite the possible discrepancies in dosing, most behavior as well as lesion sizes showed a significant improvement compared to vehicle-treated rats. Taken together, these results show significant support for NAM as a treatment for TBI and replicate the results found in many previous studies [8, 9, 16]. Though there is still work to be done in establishing full pharmacological parameters for NAM administration in humans, it has been shown to have relevance to a number of other conditions such as animal models of ischemic stroke [22, 25] and as a TBI treatment, the compound appears to be approaching readiness for human trials.
Research Highlights.
Continuous NAM administration improved recovery of function following CCI.
Continuous NAM administration increased neuronal sparing following CCI.
Continuous administration of NAM via subcutaneous pumps is effective in increasing NAM serum levels.
Acknowledgments
The authors would like to thank Andrew Vaz for his help with behavioral data collection and scoring and Michael Emery and Kris Martens for assisting in surgical procedures. Funding for this project was provided by ARRA funds from NINDS grant NS045647-04 and NIH/NICHD grant HD061944-01.
Footnotes
Conflict of Interest
The authors have no financial relationship or commercial interests based on the outcome of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Disease Control. Injury prevention and control: Traumatic brain injury. 2010 from http://www.cdc.gov/TraumaticBrainInjury/index.html.
- 2.Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Injury Prevention. 2006;12:212–8. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas AIR, Marmarou A, Murray GD, Teasdale GM, Steyerberg EW. Prognosis and clinical design in traumatic brain injury: The IMPACT study. Journal of Neurotrauma. 2007;24:232–8. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 4.Menon DK. Unique challenges in clinical trials in traumatic brain injury. Critical Care Medicine. 2009;37:S129–S35. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 5.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. Journal of Neurotrauma. 2002;19:503–57. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathology. 2004;14:215–22. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoane MR, Pierce JL, Kaufman NA, Beare JE. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxidative Medicine and Cellular Longevity. 2008;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. Journal of Neurotrauma. 2003;20:1189–99. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- 9.Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–8. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoane MR, Gilbert DG, Holland MA, Pierce JL. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neuroscience Letters. 2006;408:35–9. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Holland MA, Tan AA, Smith DC, Hoane MR. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. Journal of Neurotrauma. 2008;25:140–52. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- 12.Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood–brain barrier breakdown following traumatic brain injury. Brain Research. 2006;1125:185–93. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxidants & Redox Signalling. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Chong ZZ, Maiese K. Cell life versus cell longevity: The mysteries surrounding the NAD+ precursor nicotinamide. Current Medicinal Chemistry. 2006;13:883–95. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goffus AM, Anderson GD, Hoane MR. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxidative Medicine and Cellular Longevity. 2010;3:145–52. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. Journal of Neurotrauma. 2006;23:1535–48. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- 17.Komotar RJ, Kim GH, Sughrue ME, Otten ML, Rynkowski MA, Kellner CP, et al. Neurologic assessment of somatosensory dysfunction following an experimental rodent model of cerebral ischemia. Nature Protocols. 2007;2:2345– 7. doi: 10.1038/nprot.2007.359. [DOI] [PubMed] [Google Scholar]
- 18.Hoane MR, Pierce JL, Holland MA, Birky ND, Dang T, Vitek MP, et al. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performances and reduces injury magnitude following cortical contusion injury. Journal of Neurotrauma. 2007;24:1108–18. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- 19.Coggeshall RE. A consideration of neural counting methods. Trends in Neuroscience. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- 20.Neafsey EJ. Motor functions of the neocortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. Cambridge: MIT Press; 1990. pp. 197–212. [Google Scholar]
- 21.Lindner MD, Plone MA, Cain CK, Frydel B, Francis JM, Emerich DF, et al. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. Journal of Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Klaidman LK, Adams JD. Medicinal chemistry of nicotinamide in the treatment of ischemia and reperfusion. Mini Reviews in Medicinal Chemistry. 2002;2:125–34. doi: 10.2174/1389557024605483. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Wang Z, Wang Q, Han J, Zhao C, Hong Y, et al. Roles of NAD+/NADH and NADP+/NADPH in cell death. Current Pharmaceutical Design. 2009;15:12–9. doi: 10.2174/138161209787185832. [DOI] [PubMed] [Google Scholar]
- 24.Quigley A, Tan AA, Hoane MR. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Research. 2009;1304:138–48. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaidman L, Morales M, Kem S, Yang J, Chang M-L, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor to NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–7. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]