Abstract
Background
HIV testing is still stigmatized among many high-risk groups in China while routine syphilis testing has been widely accepted at STI clinics. This project used the platform of a rapid syphilis screening test to expand HIV test uptake. The objective of this study was to use multilevel modeling to analyze determinants of syphilis and HIV testing uptake at STI clinics in China.
Methods
2061 STI patients at six clinics in Guangdong Province were offered free rapid syphilis and free rapid HIV testing. Test uptake was defined by patient receipt of results and a multilevel model was used to analyze predictors of uptake.
Results
This was the first syphilis or HIV test for the large majority (1388, 77.7%) of participants. Syphilis test uptake and HIV test uptake were high (1681, 81.6%, syphilis test uptake; 1673, 81.2% HIV test uptake). HIV test uptake was significantly concordant with syphilis test uptake (τb = 0.89, p < 0.001). The most parsimonious model of HIV test refusal included the following variables: being married, having a previous HIV test, being unaccompanied, and participating in the last two months of the study.
Conclusions
STI-clinic based screening for syphilis and HIV represents an excellent opportunity for scaling up integrated services, especially in South China where syphilis and sexually transmitted HIV cases are both rapidly increasing. Effective integration of HIV testing into routine clinical practice requires an understanding not only of individual test uptake but also of the broader social context of HIV testing.
INTRODUCTION
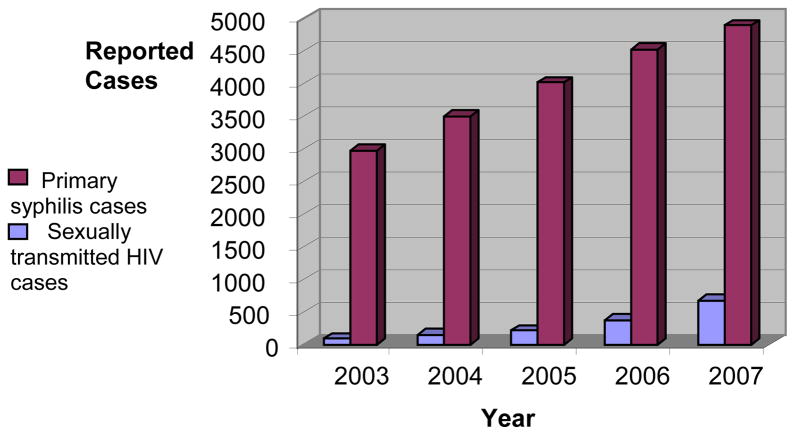
South China, especially Guangdong Province, has witnessed an alarming increase in both reported syphilis infections1,2 and sexually transmitted HIV infections (Figure 1).3,4 Syphilis increases the risk of HIV acquisition and transmission and patients with either STI frequently attend the same STI clinics.5 Although the syphilis epidemic and sexually transmitted HIV epidemic have common high risk groups and testing sites in South China,6 widespread syphilis testing has been successful while HIV testing has had poor uptake.7,8 An estimated 71% of those with HIV do not know their serostatus in China9 and high levels of test refusal have been noted at STI clinics.10 HIV voluntary counseling and testing (VCT) sites in China have had problems attracting high-risk groups.7 Addressing these challenges, China issued draft provider-initiated testing and counseling (PITC) guidelines and established provider-initiated HIV testing programs in four provinces.7
FIGURE 1.
Reported primary stage syphilis cases (purple) and heterosexually transmitted HIV cases (blue) in Guangdong Province.1,4
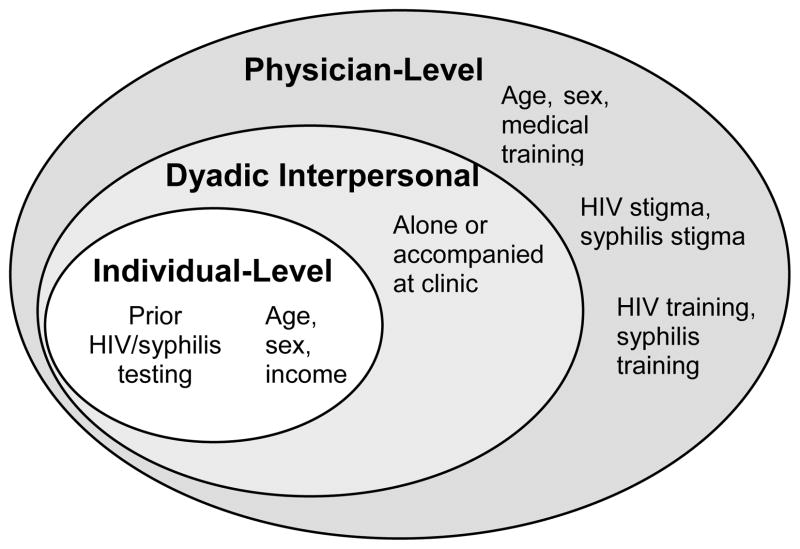
However, transforming a single HIV test into a comprehensive HIV screening program is no small task, complicated by several factors that limit routine HIV screening, particularly among the groups at higher risk of sexually transmitted HIV infection. Integration of HIV testing into routine clinical care has proven useful for expanding PITC in a number of clinical settings.11 Normalizing HIV testing within the framework of existing routine screening tests may help decrease barriers to HIV testing at several levels (Figure 2). At the individual level, high risk individuals may be more willing to accept an HIV test if it is offered in tandem with syphilis testing as part of a routine sexual health screening experience. At the interpersonal/dyadic level, peer effects of those who accompany individuals to be tested could influence integrated test uptake. Finally, physician-level determinants related to linked syphilis/HIV training and stigma might affect integrated syphilis/HIV testing programs.
FIGURE 2.
Socio-ecological model of various levels that may influence integrated HIV/syphilis test uptake.
The highly related epidemiology, biology, and social context of syphilis and HIV infection suggest integrated testing programs in China. In addition, there have been very few analyses of HIV testing at STI clinics in China despite the expansion of these twin epidemics. The objective of this study was to analyze determinants of syphilis and HIV test uptake at STI clinics in China in order to establish a model for integrated syphilis/HIV screening.
PROGRAM DESCRIPTION
Project Setting
Guangdong Province, South China, was chosen for this study because of recent increases in reported sexually transmitted HIV infection4 and syphilis.1 In 2007 the total burden of syphilis in China was 17.2 syphilis cases per 100,000 population with Guangdong Province reporting 31.6 cases per 100,000 population.12 Nearly half of Guangdong Province’s primary syphilis cases are clustered in the more developed Pearl River Delta Region,13 a more developed region that has many counties reporting higher primary syphilis case rates.14 Like other provinces in China, Guangdong has a network of public STI clinics that reaches down to the township level and provides syphilis testing, HIV testing, and related clinical STI services according to national guidelines.1
Study Participants
The Plum Blossom Project was launched in September 2009 as part of a Guangdong province-wide effort to expand syphilis and HIV testing at public STI clinics. A collaboration of both local government and international agencies, the Plum Blossom Project ensured that free rapid syphilis and free rapid HIV testing were widely available at STI clinics. The Pearl River Delta region of Guangdong has a disproportionate burden of sexually transmitted HIV infection4 and syphilis.15 Three municipalities in the Pearl River Delta were chosen to implement this research based on laboratory capacity, availability of trained research assistants, and space to conduct the interview. Two public STI clinics in each of the three cities were selected using a probability proportional-to-size sampling method. Administrative data were used to estimate the number of public STI patients evaluated per day at each site. The total number of study participants at each site ranged from 336 to 628 over the course of the five month study. Each site had 4–14 STI physicians who evaluated patients.
Survey Development
The first research phase was a formative qualitative study to test survey items and determine local syphilis/HIV testing capacity, referral patterns, and the availability of free syphilis/HIV treatment and care services. All of these essential characteristics were present at local Guangdong public STI clinics, and more detailed results of the initial feasibility study are described elsewhere.16 All items from the physician survey were field tested among local Guangdong Province STI physicians, and a series of one-on-one interviews and focus groups helped refine the survey format.
The STI physician survey consisted of three domains – sociodemographic and training background information, clinical scenarios and vignettes, and stigma assessment. All physicians who completed the survey had a medical license and formal medical training according to national guidelines.17 Sociodemographic and training items were adapted from other published measures18 while stigma items were validated among Chinese physicians in a previous study.19 All physicians were given the survey prior to Plum Blossom Project training and survey completeness was checked by research assistants.
The STI patient survey consisted of ten items, including marital status, previous HIV testing, monthly income, age, whether or not they were accompanied or alone, education completed, date of survey, duration of local residency, occupation, and sex. STI patient survey items were previously validated in the China Family Health and Life Survey.20 All surveys were self-administered and checked by a research assistant for completeness. In addition, research assistants filled out a short section linking each patient to their respective physician and asking the patient about the following: whether the physician offered syphilis/HIV testing, whether the participant was willing to be tested for syphilis/HIV, whether the participant accepted syphilis/HIV testing, whether the participant returned for results, and the result of the rapid syphilis or rapid HIV test. The primary outcome of this study was test uptake defined by receipt of test results.
Study Procedure
From September 2009 until January 2010, potential study subjects were identified by physicians, nurses, laboratory staff, and research assistants at selected STI clinics. All STI patients older than 17 years old were eligible for participation, regardless of their interest in receiving syphilis or HIV testing. Patients who refused both HIV and syphilis testing had a ten-item survey completed by their physician; patients who accepted either HIV testing, syphilis testing, or both were asked to fill out the longer form. Participation in the survey was voluntary and no incentives were given to physicians or patients to participate in the study. Those who agreed to participate in the study were given STI/HIV counseling after providing verbal informed consent. All patients received appropriate medical care for STI/HIV according to national guidelines. Venous samples were tested for HIV by ELISA (Acon, Hangzhou, China) and syphilis by an immunochromatographic rapid treponemal test (Wantai, Beijing, China). Both the rapid syphilis test and rapid HIV test were approved by China’s State Food and Drug Administration and then underwent further local field evaluation. Test results for both of the rapid tests were available within 30 minutes. STI patients were told to wait for the results of their tests. Post-test counseling for syphilis/HIV testing was implemented according to standard STI clinic procedures as each of these clinics already diagnosed both syphilis and HIV infection.
All participants provided informed consent and this research protocol was approved by the Medical Ethics Committee of Chinese Academy of Medical Sciences Institute of Dermatology (Nanjing, China), the University of North Carolina Public Health and Nursing IRB (Chapel Hill, USA), and the Partners Committee on Human Subjects Research (Boston, USA).
Statistical Analysis
The main outcomes in this analysis were HIV and syphilis test uptake as defined by patient receipt of test results, both analyzed as dichotomous outcomes. Test uptake was defined as patient receipt of results since this is the most relevant outcome for operationalizing widespread HIV screening. Since only ten survey items were available from both those who accepted and refused HIV testing, only these variables were analyzed in the multi-level models. Data were double entered into a database and SPSS 17.0 (Chicago, IL) was used to perform all the non-modeling statistical analyses. Kendall tau-b tests 21 were used to estimate the probability of syphilis/HIV test uptake concordance.
Given our known clustering in outcomes and the potential for interactions between individual-level and physician-level predictors of HIV and syphilis test outcome, we used multilevel modeling to analyze the primary outcome.22,23 Since patients were allowed to enroll only once in the study, each patient was assigned to exactly one physician and the data set was naturally hierarchically organized. A two-level model binomial logit model incorporated patient (Level 1) and physician (Level 2) variables. Two dyadic (marital status, accompaniment) were treated as Level 1 variables since more detailed information about those who accompanied participants to the clinic was not available (Supplemental Digital Content 1). While city and clinic-level factors are potentially important determinants of HIV test uptake, we had an insufficient number of cities (n = 3) and clinics (n = 6) to create a Level 3 variable. Instead, city was treated as a potential Level 2 covariate of interest. The binomial logit two-level model was of the form:
This is a binomial logistic multilevel model with random intercepts, and the binary response yij equals 1 if the individual i who saw physician j was tested for HIV. The variable Xij is a set of independent variables measured at the individual level. The intercept consists of a fixed component β0 and a physician-specific component, the random effect u0j. The assumptions of homoskedasticity and independent residuals were empirically analyzed (Supplemental Digital Content 2). Patient-physician interactions related to age (less than 10 years age difference compared to more than 10 years age difference) and sex (concordant versus discordant) were also assessed. The HIV test uptake model was constructed using MLwiN 2.20 (Bristol, UK), a software program used in multilevel modeling (http://www.cmm.bristol.ac.uk/MLwiN/), and Markov Chain Monte Carlo estimators.24,25 Missing values accounted for less than 5% of the outcome variables.
RESULTS
A total of 2069 STI patients were approached by 62 physicians about taking part in this study. Eight STI patients were younger than 18 years old and not eligible. Among those eligible, a total of 271 (13.1%) participants refused both tests and their physicians filled out a ten item refuser form. The remainder of the patients (1790, 86.9%) filled out a more complete survey instrument. Among all those eligible (n = 2061), 1681 (81.6%) had syphilis test uptake and 1673 (81.2%) had HIV test uptake. All of the patients who received an HIV test also received a syphilis test, but eight STI patients only received a syphilis test. This was the first syphilis or HIV test for the large majority (1388, 77.7%) of participants. More detailed information about syphilis testing compared to HIV testing can be found in Table 1. The most highly correlated measures were syphilis/HIV tests performed (correlation coefficient 0.957, p-value < 0.001) and willing to be tested for syphilis/HIV (correlation coefficient = 0.939, p < 0.001). Having a doctor offer syphilis testing was associated with having the doctor offer HIV testing (p < 0.001); patient returning for syphilis test results was also associated with the patient returning for HIV test results (p < 0.001).
TABLE 1.
Correlation between syphilis test variables and HIV test variables among those who completed the long Plum Blossom survey (n = 1790)
| Variable | Syphilis test variable (%) | HIV test variable (%) | Correlation coefficient* | p-value |
|---|---|---|---|---|
| Patient not tested in the past | 1280 (71.4) | 1316 (73.4) | 0.719 | <0.001 |
| Doctor test offer | 1758 (98.1) | 1749 (97.6) | 0.637 | <0.001 |
| Patient willing to be tested | 1705 (95.1) | 1697 (94.7) | 0.939 | <0.001 |
| Patient accepted testing | 1702 (95.0) | 1693 (94.5) | 0.815 | <0.001 |
| Test performed | 1699 (94.8) | 1691 (94.4) | 0.957 | <0.001 |
| Patient returned for test results | 1681 (93.8) | 1673 (93.4) | 0.887 | <0.001 |
Kendall rank correlation coefficient comparing responses to respective syphilis and HIV items.
Several individual and interpersonal factors were significantly associated with HIV test refusal in the most parsimonious multilevel model (Table 2). No physician level factors significantly improved the most parsimonious model. The final model predicting HIV test refusal included the following factors: being married, having a previous HIV test, being unaccompanied, and participating in the last two months of the study. Being married and having prior HIV testing were the strongest predictors of HIV test refusal. Married participants had an HIV uptake of 80.3% compared to unmarried participants who had an HIV uptake of 98.0% (aOR 14.59). Participants who received HIV testing in the past had an HIV uptake of 67.3% compared to those who never received HIV testing who had an HIV test uptake of 95.7% (aOR 6.49). Among those who reported HIV testing in the past, 213 (37.6%) reported testing in the past six months, 208 (36.7%) reported testing between 6 months and 12 months ago, and 146 (25.7%) reported being tested between one and five years ago. Unaccompanied participants had an HIV test uptake of 84.5% compared to those who were accompanied (significant other, spouse, or other) who had an HIV test uptake of 91.2% (aOR 1.82). Participants who enrolled in the last two months of the study had a mean HIV test uptake of 83.7% compared to those who enrolled in the first three months of the study who had an HIV test uptake of 88.8% (aOR 1.46). Age, local residency, occupation, education, and sex did not improve the most parsimonious model. Given the high concordance between syphilis test uptake and HIV test uptake, the factors associated with HIV test uptake were also included in the most parsimonious model predicting syphilis test uptake.
TABLE 2.
Correlates of HIV test uptake from the most parsimonious multilevel model* (n=1960)
| Variable | Total study (%) | HIV test uptake (%) | Adjusted odds ratio, 95% credible interval | |
|---|---|---|---|---|
| Overall | 1960 (100) | 1689 (82.0) | NA | |
| Marital Status | Unmarried | 636 (32.4) | 623 (98.0) | 14.59, 6.55–32.79 |
| Married | 1263 (64.4) | 1014 (80.3) | 1.00 | |
| Prior HIV testing | Never tested | 1294 (66.0) | 1239 (95.7) | 6.49, 4.44–9.40 |
| Tested | 643 (32.8) | 210 (67.3) | 1.00 | |
| Annual Income | ≤ 4000 USD | 949 (48.4) | 879 (92.6) | |
| >4000 USD | 793 (40.5) | 649 (81.8) | ||
| Age | >40 years old | 1563 (79.7) | 1378 (88.2) | |
| ≤40 years old | 363 (18.5) | 285 (78.5) | ||
| Accompanied at STI clinic | Accompanied | 511 (26.1) | 466 (91.2) | 1.82, 1.16–2.92 |
| Unaccompanied | 1398 (71.3) | 1181 (84.5) | 1.00 | |
| Education completed | ≤ High school | 1338 (68.3) | 1182 (88.3) | |
| > High school | 599 (30.6) | 489 (81.6) | ||
| Study month | First 3 months | 1076 (54.9) | 955 (88.8) | 1.46, 1.02–2.05 |
| Last 2 months | 778 (39.7) | 651 (83.7) | 1.00 | |
| Local Resident | ≤ 1 year | 242 (13.1) | 217 (89.7) | |
| > 1 year | 1610 (86.9) | 1377 (85.5) | ||
| Occupation | Entertainment | 76 (3.9) | 69 (90.8) | |
| Non-entertainment | 1848 (94.3) | 1597 (86.4) | ||
| Sex | Female | 687 (35.1) | 600 (87.3) | |
| Male | 1269 (64.9) | 1088 (85.7) |
Individual level variables are included in this table. None of the level-2 physician variables or interaction terms improved the most parsimonious model.
At level two, physician-level variance accounted for 28.4% of the total variance, assuming the patient-level variance corresponds to that of a standard logistic distribution. None of the physician-level predictors improved the model: physician city, physician age, physician sex, physician medical degree, physician HIV training, physician HIV stigma measured using a validated survey, patients evaluated per day by the physician. Neither of the following patient-physician interaction variables substantially improved the model: sex of patient/physician, age of patient/physician.
DISCUSSION
This study reveals an opportunity for scaling up integrated syphilis/HIV testing at STI clinics in China. 77% of this patient sample had never had a syphilis test or HIV test before this visit, suggesting that STI clinic-based syphilis/HIV screening is uncommon. In the context of China where 59% of HIV infections are thought to be sexually transmitted,7 scaling up STI clinic based HIV testing represents a feasible, logical extension of other testing efforts. To our best knowledge, this study is the first to analyze multilevel predictors of HIV test uptake in a clinical setting. While multilevel modeling has been widely applied in the fields of education and sociology, there have been far fewer studies in medicine and sexual health in particular.26,27 Multilevel modeling presents advantages in describing clustered observations since it provides more accurate standard errors, accounts for patient-physician interactions, and permits analysis of physician-level predictors. Previous studies of HIV testing have either ignored clustering of observations or analyzed them using generalized estimating equations.
The high level of integrated syphilis/HIV test uptake, 81%, found at Chinese STI clinics represents marked improvement compared to lone HIV testing at STI clinics and the current system of voluntary counseling and testing (VCT). One study of isolated HIV testing at STI clinics did not measure HIV test uptake but found that approximately 60% of Chinese STI patients accepted a lone HIV test, compared to 94.5% of STI patients in our sample accepting an HIV test.10 Studies from the general population,8 sex workers,28 and migrants29 have noted infrequent attendance and poor HIV test uptake at government-designated VCT centers. The high level of integrated syphilis/HIV test acceptance observed corresponds to high levels of integrated HIV test acceptance reported in Chinese HIV/TB integrated programs (99%)30 and antenatal care/HIV integrated programs (84%).31 The prenatal care system in China has launched programs to expand integrated HIV/HBV/syphilis testing31 that could provide useful guidance for HIV test integration more generally. Our research and these related integrated HIV testing programs highlight the benefits of provider-initiated HIV testing in China.
The association between being married and refusing HIV testing has been noted in quantitative HIV uptake studies from Malawi32 and the United States.33 Married individuals may decline HIV testing since a positive test result could have consequences for their spouse and marriage. In China a large number of Chinese men who have sex with men are married34 and this may also decrease HIV test uptake in this population. The association between prior HIV testing and test refusal has also been noted elsewhere,32 but may be particularly important in China where few education programs have focused on promoting HIV testing and a one-time massive HIV testing program was implemented in 2003–2004 with minimal counseling.35 There have been no studies on HIV test uptake as it relates to accompaniment. Other studies of couples VCT have been encouraging,36 and our finding that those who are accompanied have higher HIV test uptake is promising for developing couple-based testing programs or re-instating pre-marital syphilis screening in China.
The large portion of physician-level variance supports that multi-level modeling is an effective tool for interpreting clinic-based HIV test uptake compared to either ignoring clustering or using marginal models. The lack of measured physician-level factors and patient-physician interactions in explaining HIV test uptake suggests the need for further research in this field. A more comprehensive assessment of physician attitudes to testing and testing-related variables could help explain the large portion of physician-level variance. While previous research has shown that Chinese physicians have substantial HIV stigma,19,37 at least within urban developed areas such as the Pearl River Delta where this study took place, the level of HIV stigma among STI physicians seems unlikely to influence the expansion of HIV testing. Furthermore, physician HIV/STI training, which has been found deficient in other Chinese studies,38,39 appears to be sufficient for rolling out integrated syphilis/HIV testing in this context.
Integration of syphilis/HIV testing also offers a potential opportunity to quantify sexual risk in a subsection of individuals who refuse HIV testing. The high number of HIV test refusers with a positive rapid syphilis test (four out of eight) and the common correlates of syphilis test positive and HIV test refusal (married, history of prior HIV testing) underscore the need for more studies of those who decline HIV testing. Improved patient counseling and physician training on the frequency of HIV testing and counseling for married STI patients may improve HIV test uptake.
This study has several limitations. First, the study was done at six STI clinics in the Pearl River Delta and so may not be representative of STI clinics in other parts of the province or region. Second, self-reporting bias regarding same sex behaviors precludes a more formal assessment of men who have sex with men in this sample, similar to other studies in STI clinics.36 Finally, this study was done as part of a research project and so ordinary clinical practice may result in lower uptake rates, consistent with our finding that the HIV test uptake rate slightly decreased after three months of the program.
This pilot supports the utility of integrated syphilis/HIV testing, suggesting the importance of integrated syphilis and HIV programs. Many individuals at increased risk of both syphilis and HIV infection are evaluated at STI clinics in China and care should be taken to evaluate those who decline HIV testing. The Chinese Ministry of Health recently released a ten-year syphilis prevention and control plan that establishes explicit goals for syphilis/HIV integration.40 This political commitment should help translate research findings from this study into specific guidelines and practice likely to find substantial numbers of patients with unrecognized HIV infection.
Supplementary Material
Acknowledgments
Financial support for this research came from an NIH Fogarty K01 Award (US NIH 1K01TW008200-01A1; JT), the UNC Fogarty AIDS International Research and Training Program (NIH FIC D43 TW01039), the UNC Social Science Research on HIV/AIDS in China (NIH NICHD R24 HD056670-01), the UNC Center for AIDS Research, the China-Australia Health and HIV/AIDS Facility Project (HIV 04), the WHO Rapid Syphilis Test Project (UNICEF/UNDP/World Bank/WHO A70577), the Harvard Institute for Global Health, and Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Wellcome Postdoctoral Fellowship in Tropical Infectious Diseases.
We would like to thank all the participants and members of the Plum Blossom team who made this possible: Dr. Tinglu Ye, Dr. Xuqi Ren, Dr. He-Kun Lu, Dr. Bao-Yuan Zhang, Dr. Shu-Jie Huang, Dr. Xue-Ling Tan, Dr. Wei-Jun Deng, Dr. Jian-Xin Yu, Dr. Yun Feng, Dr. Jing-Feng Huang, Dr. Xiao-Xiong Huang, Dr. Hua Peng, Dr. Sen-Miao Zhang, Dr. Fang-Mei Chen, and Dr. Xiao-xi Zhang. Thanks to Professor Baoxi Wang (National STD Control Center), Dr. Heather Ribaudo (Harvard), Professor Gail Henderson (UNC Chapel Hill), Professor Sten Vermund (Vanderbilt), Professor Arthur Kleinman (Harvard), Dr. Rochelle Walensky (Harvard), Professor Martin K. Whyte (Harvard), Professor Rosanna Peeling (London School of Hygiene and Tropical Medicine), Professor James Maguire (Harvard), and Dr. Sarah Hawkes (University College London).
Footnotes
Part of this work was presented at the XVIII International AIDS Conference (Vienna, Austria) as late-breaker oral presentation FRLBE106 on July 23rd, 2010 and at the World Health Organization Rapid Syphilis Test Conference (Shenzhen, China) as an oral presentation on October 22nd, 2010.
References
- 1.Chen ZQ, Zhang GC, Gong XD, et al. Syphilis in China: results of a national surveillance programme. Lancet. 2007 Jan 13;369(9556):132–138. doi: 10.1016/S0140-6736(07)60074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tucker JD, Chen XS, Peeling RW. Syphilis and social upheaval in China. N Engl J Med. 2010 May 6;362(18):1658–1661. doi: 10.1056/NEJMp0911149. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Jia M, Ma Y, et al. The changing face of HIV in China. Nature. 2008 Oct 2;455(7213):609–611. doi: 10.1038/455609a. [DOI] [PubMed] [Google Scholar]
- 4.HIV Infection--Guangdong Province, China 1997–2007. MMWR Morb Mortal Wkly Rep. 2009 Apr 24;58(15):396–400. [PubMed] [Google Scholar]
- 5.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008 Sep;8(9):553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rou K, Sullivan SG, Liu P, Wu Z. Scaling up prevention programmes to reduce the sexual transmission of HIV in China. Int J Epidemiol. 2010 Dec;39(Suppl 2):ii38–46. doi: 10.1093/ije/dyq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.China 2010 UNGASS Country Progress Report. Beijing: 2010. [Google Scholar]
- 8.Ma W, Detels R, Feng Y, et al. Acceptance of and barriers to voluntary HIV counselling and testing among adults in Guizhou province, China. AIDS. 2007 Dec;21(Suppl 8):S129–135. doi: 10.1097/01.aids.0000304708.64294.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS/StateCouncil. A Joint Assessment of HIV/AIDS Prevention, Treatment and Care in China 2007. [Google Scholar]
- 10.Chen S-M, Liu D-C, Zhang FR. STD Clinic Provider-Initiated HIV Testing and Counseling Analysis. Paper presented at: 5th National STD Conference; May 2010; Haikou, China. 2010. [Google Scholar]
- 11.Bassett IV, Walensky RP. Integrating HIV screening into routine health care in resource-limited settings. Clin Infect Dis. 2010 May 15;50(Suppl 3):S77–84. doi: 10.1086/651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCSTD/CCDC. Epidemiology. 2008 2007. STD Epidemiology. [Google Scholar]
- 13.Yang LG, Tucker JD, Yang B, et al. Primary syphilis cases in Guangdong Province 1995–2008: Opportunities for linking syphilis control and regional development. BMC Public Health. 2010;10:793. doi: 10.1186/1471-2458-10-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan N, Messina JP, Yang L, et al. A Spatial Analysis of County-level Variation in Syphilis and Gonorrhea in Guangdong Province, China. PloS ONE. 2011 doi: 10.1371/journal.pone.0019648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang LG. Annual STD Surveillance for Guangdong Province, China. China National STI Control Conference; Haikou. 2010. [Google Scholar]
- 16.Tucker JD, Yang LG, Zhu ZJ, et al. Integrated syphilis/HIV screening in China: a qualitative analysis. BMC Health Services Research. 2010;10:58. doi: 10.1186/1472-6963-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand S, Fan VY, Zhang J, et al. China’s human resources for health: quantity, quality, and distribution. Lancet. 2008 Oct 17; doi: 10.1016/S0140-6736(08)61363-X. [DOI] [PubMed] [Google Scholar]
- 18.Arbelaez C, Wright EA, Losina E, et al. Emergency Provider Attitudes and Barriers to Universal HIV Testing in the Emergency Department. J Emerg Med. 2009 Oct 13; doi: 10.1016/j.jemermed.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Wu Z, Zhao Y, Lin C, Detels R, Wu S. Using case vignettes to measure HIV-related stigma among health professionals in China. Int J Epidemiol. 2007 Feb;36(1):178–184. doi: 10.1093/ije/dyl256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parish WL, Laumann EO, Cohen MS, et al. Population-based study of chlamydial infection in China: a hidden epidemic. JAMA. 2003 Mar 12;289(10):1265–1273. doi: 10.1001/jama.289.10.1265. [DOI] [PubMed] [Google Scholar]
- 21.Conover WJ. Practical Non-Parametric Statistics. 2. New York: John Wiley and Sons; 1980. [Google Scholar]
- 22.Goldstein H. Multilevel statistical models. 3. London New York: E. Arnold; Oxford University Press Distributor; 2003. [Google Scholar]
- 23.Raudenbush SW, Chan WS. Application of a hierarchical linear model to the study of adolescent deviance in an overlapping cohort design. J Consult Clin Psychol. 1993 Dec;61(6):941–951. doi: 10.1037//0022-006x.61.6.941. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian SV, Jones K, Kaddour A, Krieger N. Revisiting Robinson: the perils of individualistic and ecologic fallacy. Int J Epidemiol. 2009 Apr;38(2):342–360. doi: 10.1093/ije/dyn359. author reply 370–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browne WJ. MCMC Estimation in MLwiN (Version 2.10) Centre for Multilevel Modelling: University of Bristol; 2009. [Google Scholar]
- 26.Diez Roux AV, Aiello AE. Multilevel analysis of infectious diseases. J Infect Dis. 2005 Feb 1;191(Suppl 1):S25–33. doi: 10.1086/425288. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson LM, Basu A. Multilevel modeling and practice-based research. Ann Fam Med. 2005 May–Jun;3(Suppl 1):S52–60. doi: 10.1370/afm.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Li B, Zheng J, et al. Factors Related to Female Sex Workers’ Willingness to Utilize VCT Service: A Qualitative Study in Jinan City, Northern China. AIDS Behav. 2008 Sep 4; doi: 10.1007/s10461-008-9446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Li X, Stanton B, McGuire J. Correlates of HIV/STD Testing and Willingness to Test among Rural-to-Urban Migrants in China. AIDS Behav. 2008 Oct 25; doi: 10.1007/s10461-008-9482-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang XW, Liu Y, Dong BQ, Liu FY, Chen Q. Provider-initiated testing and counselling for human immunodeficiency virus among tuberculosis patients in Guangxi. Int J Tuberc Lung Dis. 2010 Jul;14(7):921–923. [PubMed] [Google Scholar]
- 31.Zhou Z, Meyers K, Qian H, Chen Q, Lao Y, Zhang X. Integrating HIV, hepatitis B, and syphilis screening and prevention in antenatal care and labor and delivery services in Yunnan Province, China. XVIII International AIDS Conference; Vienna, Austria. 2010. [Google Scholar]
- 32.Kranzer K, McGrath N, Saul J, et al. Individual, household and community factors associated with HIV test refusal in rural Malawi. Trop Med Int Health. 2008 Nov;13(11):1341–1350. doi: 10.1111/j.1365-3156.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 33.Freeman AE, Sattin RW, Miller KM, Dias JK, Wilde JA. Acceptance of rapid HIV screening in a southeastern emergency department. Acad Emerg Med. 2009 Nov;16(11):1156–1164. doi: 10.1111/j.1553-2712.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong FY, Huang ZJ, Wang W, et al. STIs and HIV among men having sex with men in China: a ticking time bomb? AIDS Educ Prev. 2009 Oct;21(5):430–446. doi: 10.1521/aeap.2009.21.5.430. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Sun X, Sullivan SG, Detels R. Public health. HIV testing in China. Science. 2006 Jun 9;312(5779):1475–1476. doi: 10.1126/science.1120682. [DOI] [PubMed] [Google Scholar]
- 36.Cheng JQ, Zhou H, Hong FC, et al. Syphilis screening and intervention in 500,000 pregnant women in Shenzhen, the People’s Republic of China. Sex Transm Infect. 2007 Aug;83(5):347–350. doi: 10.1136/sti.2006.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hesketh T, Duo L, Li H, Tomkins AM. Attitudes to HIV and HIV testing in high prevalence areas of China: informing the introduction of voluntary counselling and testing programmes. Sex Transm Infect. 2005 Apr;81(2):108–112. doi: 10.1136/sti.2004.009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou H, Chen XS, Hong FC, et al. Risk factors for syphilis infection among pregnant women: results of a case-control study in Shenzhen, China. Sex Transm Infect. 2007 Oct;83(6):476–480. doi: 10.1136/sti.2007.026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou P, Qian Y, Xu J, Gu Z, Liao K. Occurrence of congenital syphilis after maternal treatment with azithromycin during pregnancy. Sex Transm Dis. 2007 Jul;34(7):472–474. doi: 10.1097/01.olq.0000246314.35047.91. [DOI] [PubMed] [Google Scholar]
- 40.Tucker JD, Cohen MS. China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011 Feb;24(1):50–55. doi: 10.1097/QCO.0b013e32834204bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.