Abstract
Background
Patients with schizophrenia have a decreased ability to interpret the intentions of other individuals, called Theory of Mind (ToM). As capacity for ToM normally advances with brain maturation, research on ToM in individuals at heightened clinical risk for psychosis may reveal developmental differences independent of disease based differences.
Methods
We examined ToM in at clinical high risk and schizophrenia patients as well as healthy controls: 1) 63 clinical high risk (CHR) patients and 24 normal youths ascertained by a CHR program; and 2) in 13 schizophrenia cases and 14 normal adults recruited through a schizophrenia program. ToM measures included first- and second-order false belief cartoon tasks (FBT) and two “higher order” tasks (“Strange Stories Task” (SST) and the “Reading the Mind in the Eyes” task). In the first study, CHR patients and normal youths were also assessed for cognition, “prodromal” symptoms and social function.
Results
Errors on first- and second-order false belief tasks were made primarily by patients. CHR patients and their young comparison group had equivalent performance on higher order ToM, which was not significantly different from the worse ToM performance of schizophrenia patients and the higher performance of normal adult controls. In the combined dataset from both studies, all levels of ToM were associated with IQ, controlling for age and sex. ToM bore no relation to explicit memory, prodromal symptoms, social function, or later transition to psychosis.
Conclusions
Higher order ToM capacity was equally undeveloped in high risk cases and younger controls, suggesting performance on these tasks is not fully achieved until adulthood. This study also replicates the association of IQ with ToM performance described in previous studies of schizophrenia.
Keywords: psychosis, theory of mind, IQ, risk, prodrome
1. Introduction
Theory of Mind (ToM) is the ability to make inferences about others’ thoughts and intentions; it has been studied in developmental disorders characterized by social deficits, such as autism and schizophrenia (Frith, et al., 1999). First-order ToM is the ability to understand that someone can have an inaccurate mental representation of events based on incomplete knowledge (Wellman, et al., 2001). Second-order ToM is the ability to infer deception e.g. the false belief of one individual about the belief of a second individual (Miller, 2009). First- and second-order ToM are assessed using false belief cartoon tasks (FBT’s) and are normally achieved by ages 5–6. More complex ToM includes the comprehension of indirect speech as in irony and metaphor, assessed using the Strange Stories Task (Happe, 1994), which continues to develop throughout childhood and adolescence, as well as the recognition of subtle affect via inference of mental states from looking at an individual’s eyes, assessed using the “Reading the Mind in the Eyes” Task (Baron-Cohen, et al., 2001). Unlike the false belief tasks these higher order ToM tasks continue to develop and mature through adolescence (O'Hare, et al., 2009), Kaland et al., 2008.)
Meta-analyses show large effect sizes for ToM deficits in schizophrenia (e.g. false belief (D = 1.1 – 1.4), indirect speech (D = 1.0 -1.1) and emotion recognition (D = 0.9)), which are associated with disorganized symptoms and IQ, but unrelated to age, sex, education or antipsychotic exposure (Bora, et al., 2009; Sprong, et al., 2007), findings replicated in recent individual studies (Abdel-Hamid, et al., 2009). These deficits in ToM occur as early as the first episode of psychosis i.e. indirect speech comprehension related to IQ (Bertrand, et al., 2007) and affect recognition (Eyes Task) (Kettle, et al., 2008). However, it is unclear if ToM deficits exist in youths at heightened clinical risk for psychosis. When using first and second order ToM tasks, one study found none of the age matched groups (CHR, schizophrenia patients, and healthy controls) differed on performance, i.e. all subjects (including schizophrenia patients) performed at ceiling level (Brüne, et al., 2011). Using developmentally higher order tasks, one study found deficits in both the detection of deception (second-order FBT) and in understanding indirect speech (Happe’s Strange Stories Task), which were associated with full-scale and verbal IQ (Chung, et al., 2008). However, in a second study, there were no deficits in affect recognition (the Eyes Task) in a large cohort of CHR patients (n = 88), as compared to an older comparison group and schizophrenia patients (Couture, et al., 2008).
The current study aimed to comprehensively evaluate ToM (first- and second-order FBT’s, the Strange Stories Task, the Eyes Task) in young CHR patients, as compared to an age-appropriate “young adult” control group, both well-characterized in terms of symptoms, cognition and social function. A small sample of schizophrenia patients and an “older adult” control group were also included for comparison, i.e. to examine whether hypothesized differences between at risk individuals and their age appropriate controls were similar to differences in ToM performance between schizophrenia patients and their age appropriate controls. Secondarily, we investigated whether differences between CHR and healthy subjects were due to developmental differences, as the CHR group and its controls were significantly younger than the schizophrenia patients and their controls. It was hypothesized that across groups, ToM deficits would be related to IQ, as has been found in schizophrenia (Bora, et al., 2009; Sprong, et al., 2007), CHR patients (Chung, et al., 2008) and even in genetic high risk cohorts (for the Hinting Task) (Janssen, et al., 2003; Marjoram, et al., 2006).
2. Methods
2. 1. Participants
Clinical high risk (CHR) patients and comparably aged “young” normal control participants were participants in the Center of Prevention and Evaluation (COPE), a “prodromal” research program at NYSPI at Columbia. Recruitment and ascertainment relied on clinician referrals, Craigslist (a centralized network of online communities, featuring online advertisements with sections devoted to jobs, pooling a wide variety of potential volunteers), the COPE program website and the mailing of brochures. Clinical high risk (CHR) patients were help-seeking individuals ages 12–30 (Corcoran, et al., 2008) who met criteria for being at heightened clinical risk for psychosis using the Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS; (Miller, et al., 2003)). Exclusion criteria included any major medical or neurological disorder, IQ < 70, significant risk of harm to self and others, an inability to speak English, and “prodromal” symptoms occurring solely in the context of substance intoxication or withdrawal. Additional exclusion criteria for healthy controls included any current Axis I disorder within the past two years, as assessed by structured diagnostic interview, and any personal or familial (first degree relative) history of psychosis.
The Diagnostic Interview for Genetic Studies (Nurnberger, et al., 1994) was used for patients (to exclude any history of psychotic disorder) and for control participants over age 18 (to exclude any Axis I disorder in the past two years). The Schedule for Affective Disorders and Schizophrenia for School-age Children - Present and Lifetime version (KSADS-PL) (Kaufman, et al., 1997) was used to evaluate exclusion diagnoses for CHR patients (any psychotic disorder diagnosis) and healthy controls (any Axis I disorder in the past two years) who were younger than 18. The SIPS/SOPS, DIGS and KSADS-PL were all administered by masters level researchers; interrater reliability for all measures and diagnoses was >.70.
Schizophrenia and schizoaffective patients, and comparably aged “older” healthy comparison subjects, were recruited through the Lieber Schizophrenia Research Program at the New York State Psychiatric Institute. Diagnoses were established using the Diagnostic Interview for Genetic Studies (Nurnberger, et al., 1994). Patients were evaluated while clinically stable (i.e. no medication changes for four weeks with stable symptoms) (Rosenfield, et al., 2010). This older comparison group was recruited primarily through the use of fliers posted in the medical center and community. Exclusion criteria again included medical disorder, low IQ, and clinical acuity, and for controls, any Axis I disorder within the past two years. The Diagnostic Interview for Genetic Studies (Nurnberger, et al., 1994) was used to determine diagnoses for patients (schizophrenia or schizoaffective disorder) and for control participants (to exclude any Axis I disorder in the past two years).
Both studies were approved by the Institutional Review Board at NYSPI and Columbia University. For both sets of subjects, participants 18 and older provided written informed consent after they were evaluated for capacity. Participants younger than 18 provided written assent with written informed consent provided by a parent.
2.2. Measures
2.2.1. Theory of Mind Tasks (Studies 1 and 2)
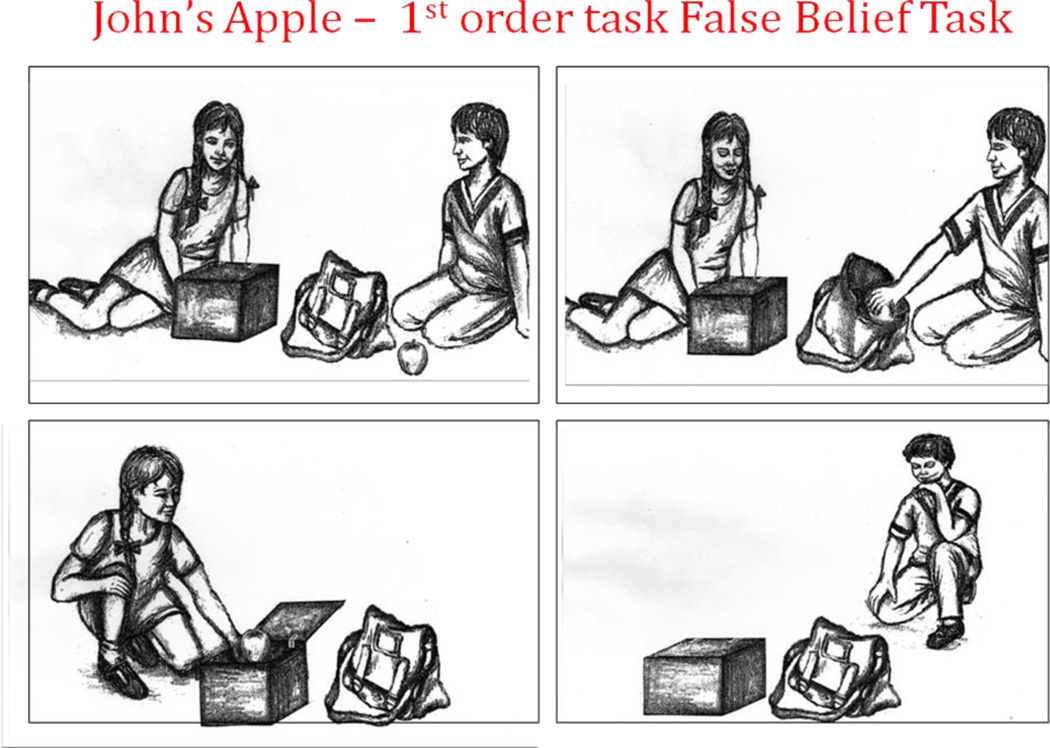
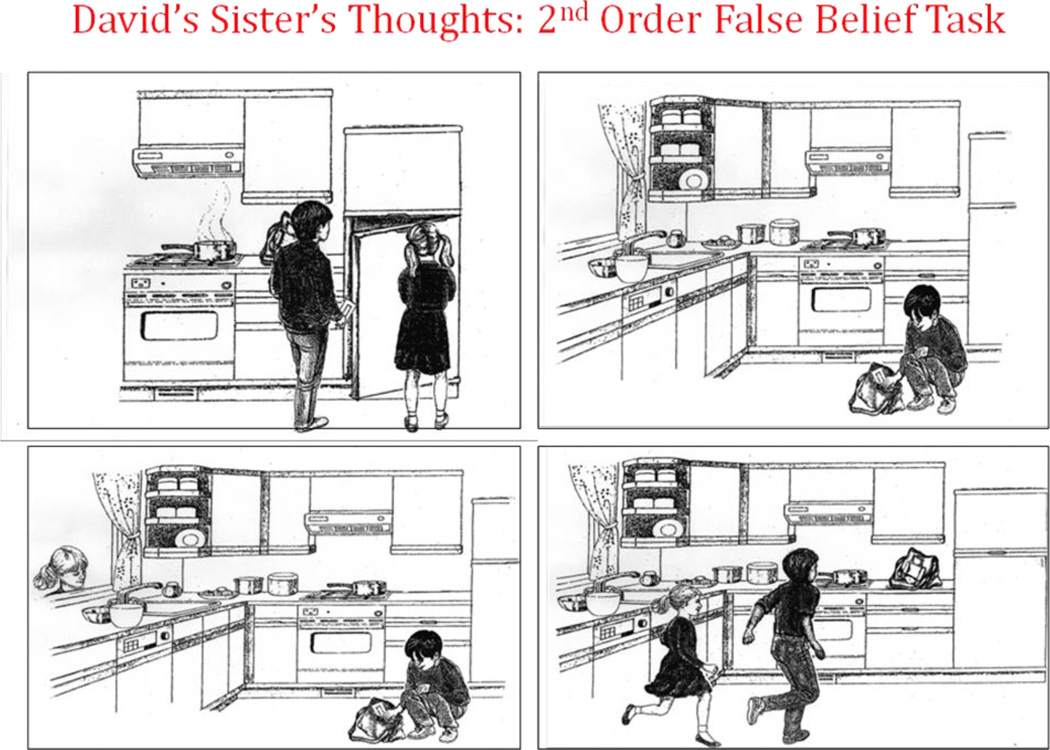
The first-order false belief “Apple Task” (figure 1; courtesy of Baron-Cohen) is a cartoon task that queries whether an individual could know that an object had been moved in his absence. The second-order false belief “Refrigerator Task” (figure 2; courtesy of Baron-Cohen) is a cartoon task that adds in the recognition of deception. The first- and second-order false belief task stories were read to the subjects as they viewed the cartoons. The Strange Stories Task (Happe, 1994) is a verbal task that involves advanced inference and an appreciation of a higher level of cognitive function in others, i.e. telling white lies, sarcasm, pretending (figure 3). After reading each of twelve vignettes aloud, participants were asked if the protagonist was truthful or not and why he or she would say what was said. Scoring relies on giving an inferential response and whether the inference was plausible. The Eyes Task (Baron-Cohen, et al., 2001) is a forced choice mental state recognition task in which participants examine 37 black and white photos of pairs of eyes, one page for each pair of eyes (figure 4). Participants select one of four words surrounding the pair of eyes, which they believe best describes what the person with those eyes is thinking or feeling. They have free access to word list definitions should they not understand a choice. Of note, none of these ToM tasks were timed during administration.
Figure 1. First-Order False Belief Task.
Figure 2. Second-Order False Belief Task.
Figure 3. Strange Stories Task, example.
Figure 4. Reading the Mind in the Eyes Task, example.
2.2.2. Neuropsychological Measures
CHR patients and young healthy controls were assessed for:
IQ, using the Wechsler Adult Intelligence Scale-Revised (WAIS-R) for subjects 18 and older (Arithmetic, Vocabulary, Picture Arrangement, and Block Design) (Wechsler, 1981); and the Wechsler Intelligence Scale for Children- Third Edition (WISC-III) (Wechsler, 1991) for participants ages 12 through 17 (verbal and performance IQ);
Explicit memory, using the Wechsler Memory Scale (Russell, et al., 1975) (immediate and delayed verbal recall and visual memory).
2.2.3. Clinical Measures
“Prodromal” symptoms were assessed using the SIPS/SOPS (Miller, et al., 2003) by raters with interrater reliability of kappa= 1.00 for CHR category and ~.7 to .9 ICC’s for dimensional ratings of positive and other symptoms. Social function was assessed using the Social Adjustment Scale-Self Report (SAS-SR) with a global score calculated according to its manual (Weissman, et al., 1976). Questions from the SAS-SR were read aloud to participants (Corcoran et al., 2010).
Schizophrenia patients’ symptoms were assessed by trained clinical raters with the Positive and Negative Syndrome Scale (Kay, et al., 1987); as for interrater reliability, kappa’s were > .85 for diagnosis and > .75 for individual symptom ratings.
2.3. Data Analysis
Data were analyzed using SPSS for Windows (version 17.0). Group means and proportions for demographics (age and sex) and for ToM assessments were compared for each of the patient subgroups with their respective control groups, using Standard t-tests and chi-square tests. Any identified demographic differences were then included in further analyses. The CHR patients and the “young” control group were compared in terms of ToM, IQ, explicit memory, symptoms and social function. Exploratory correlational analyses and linear regression were done of ToM with symptoms and social function. Spearman correlations were used in lieu of Pearson and Mann Whitney in lieu of Independent samples t-test when data were not normally distributed. Schizophrenia patients were simply described in terms of symptoms and cognition as their comparison group did not have these assessments. Alpha was set at .05 for hypothesized deficits in ToM tasks for each patient group compared to its respective comparison group and for associations of ToM task performance with IQ (controlling for age, sex and diagnosis; examining by subgroup and in the combined dataset from both studies).
3. Results
3.1. Sample
The participants (CHR patients, “young controls”; schizophrenia patients, older controls) are described in terms of demographics and ToM performance in Table 1.
Table 1.
Demographics, Theory of Mind, Cognition, Symptoms and Social Function in Patient Groups and Controls
| CHR patients (n = 63) |
Younger controls (n = 24) |
Statistics | p | Schizophrenia Patients (n = 13) |
Older controls (n = 14) |
Statistics | p | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age | 19.6 (3.6) | 21.0 (3.6) | t40 = 1.24 | NS | 33.6 (10.3) | 29.1 (9.5) | t26 = 1.22 | NS |
| Sex (M/F) | 50/13 | 15/9 | Fisher exact | 0.02 | 6/7 | 6/9 | Fisher exact | NS |
| Years of Education | N/A | N/A | 14.2 (3.9) | 15.2 (2.1) | t26 = −0.84 | NS | ||
| Theory of Mind | ||||||||
| 1st order FBT errors | 21% | 4% (N=1) | Fisher exact | NS | 23% | 0% | Fisher exact | NS |
| 2nd order FBT errors | 11% | 0% | Fisher exact | NS | 15% | 0% | Fisher exact | NS |
| Strange Stories Task | 32.1 (5.2) | 33.7 (3.8) | t32 = 1.30 | NS | 30.6 (7.6) | 36.3 (1.6) | t13 = −2.53 | .02 |
| The Eyes Task | 25.4 (4.8) | 25.7 (4.9) | t40 = 0.25 | NS | 23.9 (5.4) | 27.9 (4.4) | t26 = −2.14 | .04 |
| Cognition, scaled and age-corrected | ||||||||
| Full-scale IQ | 104.2 (19.5) | 107.5 (11.5) | t33 = 0.79 | NS | 93.8 (18.2) | |||
| Verbal IQ | 107.7 (19.0) | 111.6 (12.2) | t33 = 0.75 | NS | 100.2 (21.6) | |||
| Performance IQ | 98.8 (19.5) | 101.8 (11.8) | t33 = 0.70 | NS | 86.8 (12.6) | |||
| General Memory Index | 96.1 (20.8) | 96.6 (20.1) | t40 = 0.075 | NS | 92.7 (16.8) | |||
| Verbal Memory Index | 93.5 (18.6) | 96.3 (18.4) | t29 = .44 | NS | 92.6 (16.6) | |||
| Visual Memory Index | 104.7 (17.1) | 98.2 (16.7) | t29 = −1.11 | NS | 97 (16.6) | |||
| Attention Index | 94.9 (22.2) | 91.8 (17.2) | t29 = −.48 | NS | 88.3 (17.7) | |||
| Delayed Recall | 96.2 (19.9) | 97.9 (21.2) | t29 = 0.24 | NS | 94.7 (17.2) | |||
| Symptoms | ||||||||
| SIPS Total Positive | 13.7 (4.7) | .71 (.81) | t80 = −13.0 | <.001 | ||||
| SIPS Total Negative | 13.0 (6.7) | 1.2 (1.7) | t81 = −8.6 | <.001 | ||||
| SIPS Total Disorganization | 7.0 (4.0) | .54 (1.0) | t81 = −7.9 | <.001 | ||||
| SIPS Total General | 9.3 (4.4) | 0.67 (1.5) | t81 = −9.3 | <.001 | ||||
| PANSS Total | 56.8 (16.5) | |||||||
| PANSS Total Positive | 13.5 (6.3) | |||||||
| PANSS Total Negative | 13.1 (5.7) | |||||||
| PANSS Total General | 30.2 (10.3) | |||||||
| Function | ||||||||
| GAF | 43.6 (7.3) | 80.6 (8.7) | t83 = −20.0 | <.001 | ||||
| Social Adjustment | 2.5(.64) | 1.63(.33) | t69 = −7.65 | <.001 |
The CHR patients were comparable in age and cognition to the young adult comparison group, but had significantly more males. (In the combined group of CHR patients and younger comparison individuals, there was no difference between males and females in age or IQ.) As expected, the CHR patients had greater symptom severity (rarely endorsed by controls) and relative impairment in social function, even controlling for sex (i.e. p < .001). (Of note, years of education completed were not evaluated for these younger participants, as many were still in school.)
Schizophrenia patients were comparable in age, sex and completed education (~ 2 to 3 years education beyond high school) to the older adult comparison group, who were not characterized in terms of symptoms or cognition.
3.2. Theory of Mind performance
Errors in false belief tasks were made primarily by patients i.e. for first-order tasks, 21% of CHR patients and 23% of schizophrenia patients, though the results were not significantly different (Table 1). Commission of errors on the first-order false belief task was unrelated to sex or age, but associated with lower verbal IQ, in both CHR patients (t38=2.7, p<.001) and in schizophrenia patients (t3=4.8, p<.02).
CHR patients had comparable high-order Theory of Mind as their younger comparison group, even accounting for sex: i.e. for Strange Stories Task, p = .20; Eyes Task, p = .47. By contrast, schizophrenia patients had impaired performance in higher-order ToM tasks, as hypothesized, as compared to their comparison group (Table 1), also in analyses accounting for sex: Strange Stories Task (diagnosis: β(SE) = −5.3(1.8), p = .007); Eyes Task (diagnosis: β(SE) = −3.9 (1.9), p = .04). Task performance on the high-order ToM tasks were not normally distributed for each group. Examination of all four groups shows that the two younger groups (CHR patients and the younger controls) had performance intermediate to that of the poor performance of schizophrenia patients and the superior performance of older adults (Table 1). Of note, there were no associations of age or sex in either study with performance on these higher-order tasks.
3.3. Associations between Theory of Mind performance and IQ (Studies 1 and 2)
General linear models were created for the combined dataset for each ToM task to determine its associations with IQ, while controlling for age, sex and diagnosis (i.e., CHR, schizophrenia, and younger controls only as IQ scores were not obtained for the older comparison group). IQ was a statistically significant predictor of performance for every ToM task, whereas age and sex bore no relationship to any ToM domain, and diagnosis was no longer relevant when IQ was considered in these models (Table 2).
Table 2.
Theory of Mind and IQ in the Combined Dataset (CHR patients, schizophrenia patients, younger controls: N = 100)
| Regression Models | Age | Sex | Diagnosis | Full-scale IQ | ||||
|---|---|---|---|---|---|---|---|---|
| Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | |
| 1st order FBT errors | .06 (.08) | .45 | −1.20 (1.10) | .28 | −2.0 (.59) | .73 | −.16 (.05) | .002 |
| 2nd order FBT errors | −.02 (.08) | .76 | .33 (.93) | .72 | .35 (.60) | .56 | −.06 (.03) | .03 |
| Strange Stories Task | −.08 (.11) | .49 | −2.78 (1.6) | .08 | −.07 (.99) | .94 | .16 (.04) | <.001 |
| The Eyes Task | .02 (.10) | .83 | −.78 (1.29) | .55 | −.24 (.78) | .76 | .15 (.03) | <.001 |
| Age | Sex | Diagnosis | Verbal IQ | |||||
| Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | |
| 1st order FBT errors | .28 (.19) | .14 | −3.6 (2.2) | .11 | −1.4 (1.5) | .26 | −.38 (.16) | .02 |
| 2nd order FBT errors | .003 (.08) | .97 | .17 (1.0) | .87 | .26 (.67) | .70 | −.06 (.03) | .04 |
| Strange Stories Task | −.09 (.12) | .46 | −2.2 (1.6) | .19 | −.13 (.98) | .90 | .18 (.04) | <.001 |
| The Eyes Task | .006 (.09) | .95 | .16 (1.2) | .90 | −.20 (.72) | .79 | .18 (.03) | <.001 |
These associations of ToM with IQ held when similar analyses were done within subgroups as well, i.e. in CHR patients, both full-scale (and verbal) IQ predicted performance on the Strange Stories (p’s = .01) and Eyes Tasks (p’s = .002), controlling for age and sex (data not shown).
Educational achievement was associated with performance on the Strange Stories Task (Spearman ρ = .69, p < .02) only (and not the Eyes Task) in the combined older group of schizophrenia patients and their older comparison group (for whom IQ scores were not available).
Regression models for explicit memory comparable to those constructed for full-scale and verbal IQ (with age, sex and diagnosis added) showed no association of explicit memory with ToM performance in the combined cohort of 100 (schizophrenia patients, CHR patients, younger controls).
3.4. Associations of Theory of Mind performance with symptoms and social function in CHR patients
Exploratory correlational analysis of the CHR cohort showed no association of any “prodromal” symptom domain (positive, negative, disorganization and general) with performance on any of the four ToM tasks (first- and second-order FBT’s, Strange Stories Task, the Eyes Task), even when using an alpha of .05 that was uncorrected for multiple testing. Although there was a relative impairment in self-reported social function in CHR patients, there was no association between any measures of ToM and social impairment in - the CHR group, even in analyses which controlled for sex and age (data not shown). None of the ToM measures predicted conversion to psychosis (data not shown).
4. Discussion
4.1. Main findings
This study employed four ToM tasks of escalating difficulty in CHR and schizophrenia cases and in two age-appropriate comparison groups, which were separately ascertained in concert with the respective patient groups. The main finding was that only schizophrenia patients (but not CHR patients) had relative deficits (as compared to age-appropriate controls) in performance of higher-order ToM tasks, which test the appreciation of subtle mental states (Happe, 1994) and subtle facial affect recognition (Baron-Cohen, et al., 2001). The inclusion of a younger control group was instrumental, as an apparent deficit would have been wrongly concluded for the CHR patients had they simply been compared to the older comparison group. In fact, for the two higher-order tasks, both CHR patients and their young comparison group had observed performance intermediate to that of adult controls (best) and of schizophrenia patients (worst), consistent with higher-order ToM not being fully developed until adulthood (Blakemore, 2008). This finding replicates the work by (Gibson, et al., 2010) where no Eyes performance difference was found between genetic high risk individuals and age matched controls even younger than this group This is consistent with imaging studies which demonstrate differential (increased) brain activation in adolescents vs. adults during mentalizing tasks, specifically the medial prefrontal cortex during ToM tasks involving irony and intentional causality, and the anterior cingulate cortex during facial affect recognition (Blakemore, 2008).
The absence of a relative deficit in higher-order ToM tasks in CHR patients is consistent with a previous study by Couture and colleagues, which found no differential performance from controls using the Eyes Task in similarly aged CHR subjects (Couture, et al., 2008) and younger genetic at risk teens (Gibson et al 2010) and no association with symptoms (Couture, et al., 2008). We replicated their findings for the Eyes Task using a measure which specifically probes prodromal, specifically attenuated positive, symptoms (Miller, et al., 2003) and with a control group more closely matched in age. By contrast, our finding that an appreciation of subtle social inferences was equally underdeveloped in CHR patients and healthy younger controls stands in contrast with the findings of Chung and colleagues, who found lower scores on the same Strange Stories Task in a CHR cohort as compared with a comparison group matched for IQ and age (Chung, et al., 2008). (The mean IQ of ~110 and mean age of ~21 to 22 among subjects in the study by Chung and colleagues was quite similar to that in the current study.) Our lack of a finding of deficit for the Strange Stories Task was likely not due to Type 2 error: mean performances on this task by high-risk patients (32.1 (SD 5.2) and their age-appropriate controls (33.7 (SD 3.8)) in our study were nearly identical to one another. Disparate findings from Chung et al could be accounted for by differences in the samples (their sample was more balanced in terms of sex and included some controls who were within “the social networks of hospital staff members”) or methodology (their Strange Stories Task had been translated into Korean and accounted for “relative cultural differences”; scoring strategies vary widely for this task). Further studies of subtle affect recognition in CHR patients may clarify whether a deficit exists, though for this there are also disparate findings (negative - (Pinkham, et al., 2007); positive - (Addington, et al., 2008) in similarly characterized “clinical high risk” cohorts (though IQ unknown) for related measures of face emotion identification and discrimination (Kerr, et al., 1993).
As for lower-order ToM performance, which assesses false beliefs, errors were made in our study only by patients – both schizophrenia and CHR – with a mistake made by only one of the healthy controls. This is consistent with the extant literature on schizophrenia as well as the findings of impaired performance on false belief tasks among CHR patients (Chung, et al., 2008) and in genetic high risk individuals with symptoms (Marjoram, et al., 2006). The one study that did not find deficits on deception (also a low order ToM task) in CHR compared to healthy controls, was performed in an older cohort, and the schizophrenia patients did not differ from controls either (Brüne, et al., 2011), i.e. all subjects performed at ceiling levels. Our replicated finding of error commissions by CHR patients merits further investigation, as first- and second-order false belief ToM is normally achieved before ages 5 to 6, i.e. before the onset of the prodrome (Miller, 2009; Perner, et al., 1985; Wellman, et al., 2001; Wimmer, et al., 1983). However, studies from cognitive neuroscience show the interaction between ToM and executive function continues to develop in late adolescence (Dumontheil, et al., 2010). Of note, in both CHR and schizophrenia patients, subjects who made errors on these false belief tasks had lower verbal IQ than those who did not. Furthermore, while performance levels may not differ, brain function may differ, as has been found in CHR on low order ToM tasks with fMRI (Brüne, et al., 2011).
As hypothesized, we found an association of all levels of ToM with intelligence (in particular verbal IQ) independent of diagnosis, controlling for age and sex, consistent with previous meta-analyses (Bora, et al., 2009). This strong association of ToM with verbal intelligence evident in both schizophrenia (and its risk states) has been hypothesized to be due to overlapping neuroanatomy (Bora, et al., 2009), though could also be a function of the verbal and/or executive function demands intrinsic to the ToM measures commonly employed. By contrast with IQ, symptoms were unrelated to Theory of Mind in the CHR cohort, consistent with the prior study by Couture and colleagues in 2008. No association was found for ToM with specifically disorganization symptoms, as has been demonstrated for schizophrenia in meta-analysis (Sprong, et al., 2007), although this may be due to the low level of these symptoms in the CHR patients in our study. Finally, ToM performance was unrelated to the marked social impairment which characterizes this and other CHR cohorts (Ballon, et al., 2007; Cannon, et al., 2008; Corcoran, et al., 2010; Cornblatt, et al., 2007; Shim, et al., 2008; Velthorst, et al., 2009), which might be expected given the finding of lack of association of ToM with social function in patients with schizophrenia (Badan, et al., 2008). The presence of an association of ToM with IQ and not with social function suggests there may be other social cognitive impairments beyond mentalizing which underlie the marked social dysfunction seen in schizophrenia and its risk states.
Considering the absence of a testable developmental deficit in ToM in CHR patients and the presence of ToM deficits in schizophrenia patients, longitudinal studies would be helpful in understanding how ToM evolves, whether there is a failure to continue to develop ToM in CHR patients who convert to psychosis or if there is a decline in ToM performance with the onset of the illness. Such studies could provide insight into changes in brain function with the disease process. However, ToM performance does not appear to be an appropriate screening tool or marker or transition to psychosis and may not be useful for intervention development at this time.
4.2. Limitations
The relatively small sample of 63 CHR patients is such that Type 2 error may account for the lack of finding of significant ToM deficits in high risk patients, or its correlates in terms of symptoms, cognition and function. In addition, t-tests may be sensitive to the unbalanced groups. Further, the use of Craigslist to ascertain younger normal controls could have obscured a real deficit in the CHR cohort in Theory of Mind, as their performance on the Eyes Test (25.4 (SD 4.8)) is lower than what has been observed for other “normal” individuals: mean score 28.1 in 15-year-olds (Kaland, et al., 2008) and mean score 28.0 in college students (mean age of 20.8) (Baron-Cohen, et al., 2001). However, the performance of our Craigslist controls is similar to that observed in an older (mean age 46.5) “general population” sample i.e. mean score 26.2 (Baron-Cohen, et al., 2001). Also, the Craigslist cohort had average IQ, negligible prodromal types of symptoms, and intact social and global function. Other limitations include the incomplete characterization and smaller sample sizes of the schizophrenia patient and older control subgroups, and the relative excess of males among younger subjects, particularly among CHR patients (although sex was unrelated to ToM, age and IQ).
Finally, this study is largely cross-sectional, as is true of nearly all previous studies of ToM in both schizophrenia and CHR cohorts; only a longitudinal study will begin to clarify the role of ToM in evolving symptoms and functional impairment in emerging psychotic disorder, relevant as deficits in ToM are more clearly evident in first episode psychosis cohorts (as compared to CHR cohorts). Also, the current study employed traditional measures of ToM; future studies in CHR patients could also utilize newer measures of mentalizing developed by cognitive neuroscientists, such as the “Moving Shapes” computer-based paradigm, which shows impairment within first episode schizophrenia patients related not only to verbal IQ and memory but also to concurrent positive symptoms (Koelkebeck, et al., 2010).
4.3. Conclusions
Deficits in ToM were not evident in this study of 63 CHR patients, as compared to young adults similar in age and IQ, who had few symptoms and good social function. Further, ToM at all levels was strongly associated with IQ across patient and comparison groups, consistent with prior findings, and bore little or no association with symptoms or function.
Acknowledgments
We thank Dr. Baron-Cohen for permission to include the figures for the false belief tasks and for the Reading the Mind in the Eyes Task, and Dr. Happe for permission to include a figure describing her Strange Stories Task. We thank Drs. Shamir Khan, Lauren Bodkin and Alex Crumbley in their successive role as the Project Director for the high risk program, for their recruitment, clinical characterization and neuropsychological assessment of all CHR patients. Additionally, we thank Shelly Ben David, who succeeded Ms. Messinger as the Research Coordinator for the high risk program, and continued data collection, cleaning, entry and analysis.
Role of Funding Source
This research was supported by NARSAD (ADS, CMC), the Sackler Institute for Developmental Psychobiology (CMC), the Irving Institute for Clinical and Translational Research (CMC), the Lieber Center for Schizophrenia Research (CMC, ADS) and by NIMH Grants K23 MH066279 (CMC), K23 MH076976 (ADS), R01 MH066428 (DM) and R01 MH59114 (DM)). None of these private benefactors nor NIMH had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors.
Dr. Stanford designed the study with guidance from Drs. Malaspina and Corcoran. Drs. Stanford and Corcoran wrote the protocols for the respective studies with schizophrenia and clinical high risk patients, and conducted the respective case identification and ascertainment of patients and controls. Ms. Messinger was the research coordinator who collected, cleaned and entered the demographic and Theory of Mind data, whereas Dr. Corcoran oversaw the evaluation of high risk patients in terms of symptoms and cognition. Drs. Stanford and Corcoran conducted the literature searches and undertook the statistical analyses. Dr. Stanford wrote successive drafts with editing done by Drs. Corcoran and Malaspina. All authors contributed to and have approved the final manuscript.
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- Abdel-Hamid M, Lehmkamper C, Sonntag C, Juckel G, Daum I, Brune M. Theory of mind in schizophrenia: The role of clinical symptomatology and neurocognition in understanding other people's thoughts and intentions. Psychiatry Res. 2009;165(1–2):19–26. doi: 10.1016/j.psychres.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Brit J Psychiat. 2008;192(1):67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badan M, Zanello A, Varnier M, Koellner V, Merlo MCG. Deficits in neurocognition, theory of mind, and social functioning in patients with schizophrenic disorders - are they related? J Nerv Ment Dis. 2008;196(2):153–156. doi: 10.1097/NMD.0b013e318162aa08. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Kaur T, Marks II, Cadenhead KS. Social functioning in young people at risk for schizophrenia. Psychiat Res. 2007;151(1–2):29–35. doi: 10.1016/j.psychres.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the mind in the eyes" Test revised version: A study with normal adults, and adults with asperger syndrome or high-functioning autism. J Child Psychol Psyc. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bertrand MC, Sutton H, Achim AM, Malla AK, Lepage M. Social cognitive impairments in first episode psychosis. Schizophr Res. 2007;95(1–3):124–133. doi: 10.1016/j.schres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: Meta-analysis. Schizophr Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brüne M, Özgürdal S, Ansorge N, von Reventlow HG, Peters S, Nicolas V, et al. An fmri study of "Theory of mind" In at-risk states of psychosis: Comparison with manifest schizophrenia and healthy controls. NeuroImage. 2011;55(1):329–337. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk. Arch Gen Psychiat. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Kang DH, Shin NY, Yoo SY, Kwon JS. Deficit of theory of mind in individuals at ultra-high-risk for schizophrenia. Schizophr Res. 2008;99(1–3):111–118. doi: 10.1016/j.schres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Biol Psychiat. 2008;63(7):127s–127s. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Kimhy D, Parrilla-Escobar MA, Cressman VL, Stanford AD, Thompson J, et al. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2010:1–11. doi: 10.1017/S0033291710000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Addington J, Woods SW, Perkins DO. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophr Res. 2008;100(1–3):237–241. doi: 10.1016/j.schres.2007.12.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Apperly IA, Blakemore SJ. Online usage of theory of mind continues to develop in late adolescence. Dev Sci. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Prinstein MJ, Perkins DO, Belger A. Social skill and social cognition in adolescents at genetic risk for psychosis. Schizophr. Res. 2010;122(1–3):179–184. doi: 10.1016/j.schres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe FG. An advanced test of theory of mind: Understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Jolles J, van Os J. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatr Scand. 2003;108(2):110–117. doi: 10.1034/j.1600-0447.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Kaland N, Callesen K, Moller-Nielsen A, Mortensen EL, Smith L. Performance of children and adolescents with asperger syndrome or high-functioning autism on advanced theory of mind tasks. J Autism Dev Disord. 2008;38(6):1112–1123. doi: 10.1007/s10803-007-0496-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kerr SL, Neale JM. Emotion perception in schizophrenia - specific deficit or further evidence of generalized poor performance. J Abnorm Psychol. 1993;102(2):312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- Kettle JWL, O'Brien-Simpson L, Allen NB. Impaired theory of mind in first-episode schizophrenia: Comparison with community, university and depressed controls. Schizophr Res. 2008;99(1–3):96–102. doi: 10.1016/j.schres.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Koelkebeck K, Pedersen A, Suslow T, Kueppers KA, Arolt V, Ohrmann P. Theory of mind in first-episode schizophrenia patients: Correlations with cognition and personality traits. Schizophr. Res. 2010;119(1–3):115–123. doi: 10.1016/j.schres.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Miller P, McIntosh AM, Owens DGC, Johnstone EC, Lawrie S. A neuropsychological investigation into 'theory of mind' and enhanced risk of schizophrenia. Psychiat Res. 2006;144(1):29–37. doi: 10.1016/j.psychres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Miller SA. Children's understanding of second-order mental states. Psychol Bull. 2009;135(5):749–773. doi: 10.1037/a0016854. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bull. 2003;30(2):218–218. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, Yorkcooler C, Simpson SG, Harkavyfriedman J, et al. Diagnostic interview for genetic-studies - rationale, unique features, and training. Arch Gen Psychiat. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- O'Hare AE, Bremner L, Nash M, Happe F, Pettigrew LM. A clinical assessment tool for advanced theory of mind performance in 5 to 12 year olds. Journal of Autism and Developmental Disorders. 2009;39(6):916–928. doi: 10.1007/s10803-009-0699-2. [DOI] [PubMed] [Google Scholar]
- Perner J, Wimmer H. "John thinks that mary thinks that…" Attribution of 2nd-order beliefs by 5-year-old to 10-year-old children. J Exp Child Psychol. 1985;39(3):437–471. [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: A comparison of individuals "At-risk" For psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12(3):198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- Rosenfield PJ, Kleinhaus K, Opler M, Perrin M, Learned N, Goetz R, et al. Later paternal age and sex differences in schizophrenia symptoms. Schizophr Res. 2010;116(2-3):191–195. doi: 10.1016/j.schres.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell PN, Bannatyne PA, Smith JF. Associative strength as a mode of organization in recall and recognition - comparison of schizophrenics and normals. J Abnorm Psychol. 1975;84(2):122–128. doi: 10.1037/h0076984. [DOI] [PubMed] [Google Scholar]
- Shim G, Kang DH, Chung YS, Yoo SY, Shin NY, Kwon JS. Social functioning deficits in young people at risk for schizophrenia. Aust Nz J Psychiat. 2008;42(8):678–685. doi: 10.1080/00048670802203459. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, Van Engeland H. Theory of mind in schizophrenia - meta-analysis. Brit J Psychiat. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Becker HE, van de Fliert R, Dingemans PM, Klaassen R, et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109(1–3):60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the wechsler adult intelligence scale revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiat. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Dev. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13(1):103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]