Abstract
Ethnopharmacological relevance
Because of its virulence and antibiotic resistance, Staphylococcus aureus is a more formidable pathogen now than at any time since the pre-antibiotic era. In an effort to identify and develop novel antimicrobial agents with activity against this pathogen, we have examined Gynoxys verrucosa Wedd (Asteraceae), an herb used in traditional medicine in southern Ecuador for the treatment and healing of wounds.
Materials and Methods
The sesquiterpene lactones leucodine (1) and dehydroleucodine (2) were extracted and purified from the aerial parts of G. verrucosa, and their structure was elucidated by spectroscopic methods and single-crystal X-ray analysis. The in vitro anti-microbial activity of G. verrucosa extracts and its purified constituents was determined against six clinical isolates including S. aureus and Staphylococcus epidermidis strains with different drug-resistance profiles, using the microtiter broth method.
Results
Compound 1 has very low activity, while compound 2 has moderate activity with MIC50s between 49 and195 μg/mL. The extract of G. verrucosa has weak activity with MIC50s between 908 and 3290 μg/mL.
Conclusions
We are reporting the full assignment of the 1H-NMR and 13C-NMR of both compounds, and the crystal structure of compound 2, for the first time. Moreover, the fact that compound 2 has antimicrobial activity and compound 1 does not, demonstrates that the exocyclic conjugated methylene in the lactone ring is essential for the antimicrobial activity of these sesquiterpene lactones. However, the weak activity observed for the plants extracts, does not explain the use of G. verrucosa in traditional medicine for the treatment of wounds and skin infections.
Keywords: Gynoxys verrucosa, dehydroleucodine, leucodine, Staphylococcus aureus, Staphylococcus epidermidis, MRSA
1. Introduction
Staphylococcus aureus is a devastating bacterial pathogen capable of causing a broad array of infections in both humans and animals. The ability to treat these infections is compromised by the continued emergence of antibiotic-resistant strains. This includes strains resistant to methicillin which in recent years have become predominant even among community-acquired isolates (Deurenberg and Stobberingh, 2009). For example, in 2005, invasive infections caused by methicillin resistant S. aureus (MRSA) affected 94,630 patients in the US alone, resulting in approximately 18,650 deaths – exceeding the national mortality rates for HIV/AIDS (Klevens et al., 2007).
Most ominous is the appearance of high-level vanA-mediated vancomycin resistance even among methicillin-resistant S. aureus (MRSA). Although these isolates remain rare (Zhu et al., 2008), this is likely to change given, the increasing reliance on vancomycin as a consequence of the continued emergence of MRSA. New agents with activity against these strains exist, but resistance to these has also begun to appear (Lee, 2008). This emphasizes the urgent need to identify and develop novel antimicrobial agents with activity against MRSA. One possible source of such agents is natural products derived from various biological sources including plants. In this context it has been recently emphasized that natural products, by acting through different mechanisms from that of conventional antibiotics, could be of clinical value in the treatment of resistant bacteria (Simoes et al., 2009).
G. verrucosa is a shrub belonging to the Senecionea tribe of the Asteraceae family. The aerial parts of this plant, commonly know as guangalo, are used in traditional medicine in southern Ecuador for the treatment of skin infections and wound healing by direct application to the skin (Tene et al., 2007).
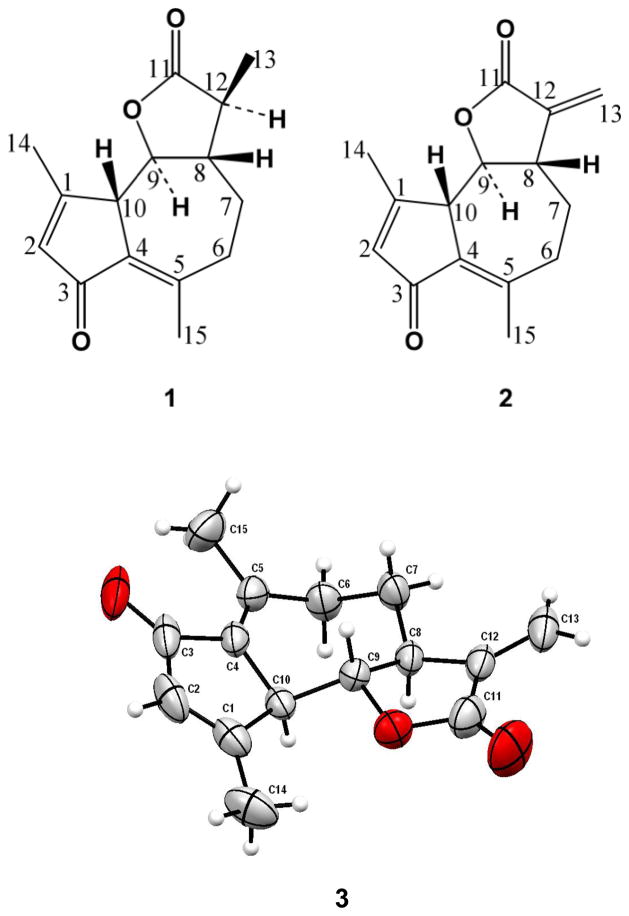
Phytochemical investigation of the ethyl-acetate extract from the aerial parts of G. verrucosa led to the isolation of two known sesquiterpene lactones, of the guainolide group, leucodine (1) and dehydroleucodine (2) (Fig. 1). These compounds have not been previously reported in this plant species but they have been reported in other members of the Asteraceae family, including Artemisia douglasiana Besser (Wendel et al., 2008), Picris koreana Vorosch. (Michalska et al., 2007), Warionia saharae Benthem ex Coss. (Hilmi et al., 2003), Kaunia rufescens (P.W. Lund ex DC.) R.M. King & H. Rob. (Rucker et al., 1997), Achillea collina (Becker ex Rchb. f.) Heimerl (Trendafilova et al., 2006) and Stevia pilosa Lag. (Martínez and Muñoz-Zamora, 1988). However, the full assignment of the 1H-NMR and 13C-NMR of either compound or the crystal structure of compound 2 have not been reported. Although, the biological activity of compound 2 has been demonstrated in various biological systems (Wendel et al., 2008; Vega et al., 2009), its activity against Gram-positive bacteria, in general, or Staphylococcus sp., in particular, has not been previously reported.
Fig. 1.
Structures of leucodine (1), dehydrolecudodine (2) and 3-D-ORTEP projection of the X-ray crystal structure of dehydroleucodine with 50% probability ellipsoids (3).
2. Materials and Methods
2.1. General Experimental Procedures
Melting points were measured with a capillary melting point apparatus and are uncorrected. IR spectra and optical rotation were measured on a Magna IR 550 spectrometer and an Autpol III (Rudolph) polarimeter, respectively. UV spectra were recorded on a Hewlett-Packard 8452A diode array spectrophotometer. NMR spectra were recorded in CDCl3, at 25°C, on a Varian Unity NMR spectrometer, operating at 500 MHz for 1H and 125 MHz for 13C spectra, equipped with a Nalorac 3 mm inverse detection probe with a z axis gradient coil. Each sample consisted of ca. 5 mg of compound dissolved in 1 mL of CDCl3 containing TMS as an internal reference. All 1D and 2D spectra were acquired and processed with standard Varian software. Mass spectral data was obtained in an Agilent 5975 GC/MSD instrument operating under EI conditions at 70 eV. Column chromatography was carried out using 60 – 230 mesh silica gel. Preparative TLC was performed on pre-coated silica gel 60 F254 plates (0.2 mm thick, Merck).
2. 2. Plant material
The aerial parts of G. verrucosa were collected in June 2007 in the locality of Yangana, in the Loja province of Ecuador. Voucher specimens (PPN-as-11) are deposited at the Herbarium of the Applied Chemistry Institute of the Universidad Técnica Particular de Loja, Loja Ecuador, and at the Herbarium Reynaldo Espinoza of the Universidad Nacional de Loja.
2.3. Extraction and isolation
The air-dried plant material (200g) was extracted with ethyl acetate (dynamic maceration for 5 h) at room temperature and concentrated under reduced pressure. The extract (14 g) was filtered through a reverse phase C18 column (LiChroprep Merck 25–40 μm) with a mixture MeOH/H2O 85:15, for the removal of chlorophylls. The filtrate (7g) was fractioned by column chromatography using a hexane-EtOAc gradient. Compound 2 was eluted in the hexane: EtOAc (85:15) fraction (0.7g) and recrystallized from EtOAc as a white crystalline solid. Compound 1 was isolated from the mother liquid using preparative TLC hexane: EtOAc (70:30) and recrystallized from EtOAc.
2.4. Physical and spectroscopic data
Leucodine (1): colorless crystals (EtOAc), mp 199–200°C; [α]25D +61.7 (c 0.41, CH2Cl2); UV (MeOH) λsh (log ε) 214 (3.78) nm, λmax (log ε) 256 (4.22) nm; IR (KBr) vmax 2940, 1780, 1686, 1637, 1620 cm−1; EI-MS: m/z (rel. int.) 246 (100), 231 (10), 217 (26), 173 (31), 159 (16), 145 (19), 135 (14), 115 (9), 105 (16), 91 (42), 77 (19); 1H NMR (CDCl3, 500 MHz) see Table 1; 13C NMR (CDCl3, 125 MHz) see Table 1.
Table 1.
NMR Spectroscopy data at 500 MHz for 1H and 125 MHz for 13C for compounds 1 and 2.
| Leucodine (1) | Dehydroleucodine (2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Position | δH | δC | HMBC | COSY | δH | δC | HMBC | COSY |
| 1 | 169.92 | 169.4 | ||||||
| 2 | 6.16 (q,1.2) | 135.55 | 195.9, 169.9, 131.9, 52.6, 19,8 | 3.41, 2.29 | 6.17 (q, 1.2) | 135.8 | 195.7, 131.9, 53.0, 169.4, 19.8 | 2.33 |
| 3 | 195.91 | 195.7 | ||||||
| 4 | 131.85 | 131.9 | ||||||
| 5 | 152.10 | 151.8 | ||||||
| 6 | 2.42 (ddd, 14.5, 12.5, 2) 2.33 (ddd, 14.5, 6, 2) |
37.55 | 152.1, 131.9, 56.4, 26.0, [21.6]a | 2.33, 1.99, 1.36 2.42, 1.99, 1.36 |
2.52 (ddd, 1.5, 12.5, 2) 2.39 (ddd, 14.5, 6, 2) |
37.3 | 151.9, 131.9, 53.1, 24.5 151.9, 131.9, 53,1, 21.8 |
2.39, 2.22, 1.44 2.52, 2.22, 144 |
| 7 | 1.99 (m) 1.36 (dddd, 14, 12,10, 2) |
25.98 | 152.1, 84.2, 56.4, 37,5 | 2.42, 2.33, 1.95, 1.36 2.42, 2.33, 1.99, 1,95 |
2.22 (dddd, 14, 6, 3,2) 1.44 (dddd, 14, 12, 10, 2) |
24.5 | 151.9, 84.4, 53.1, 37.3 151.9, 84.4, 53.1, 37.3 |
2.89, 2.52, 2.39, 1.44 2.89, 2.52, 2.39, 2.22 |
| 8 | 1.96 (m) | 56.37 | 84.2, 52.6, 41.1, 12.3 | 2.25, 1.99, 1.36 | 2.89 (ddd, 12, 10, 3) | 53.1 | 138.5, 119.0, 84.4, 37.3 | 6.18, 5.47, 3.62, 2.22, 1.44 |
| 9 | 3.62 (t,10.0) | 84.20 | 163.9, 131.9, [56.4], 41.1, 26.0 | 3.41, 1.95 | 3.62 (t,10) | 84.4 | [138.5], 131.9, 53.0, 24.5, [169.4] | 3.52, 2.89 |
| 10 | 3.41 (bd, 10) | 52.56 | [195.9], 169.9, 152.1, 135.6, 131.9, 84,6 | 6.16, 3.62, [2.44], 2.29 | 3.52 (bd, 10) | 53.0 | [151.9], 135.8, 131.9, 84.4, 53.1, 169.4 | 3.62, 2.44, 2.33 |
| 11 | 177.54 | 169.1 | ||||||
| 12 | 2.25 (dq, 12.5, 6.9) | 41.13 | 177.5, 56.4, 26.0, 12.3 | 1.95, 1.27 | 138.5 | |||
| 13 | 1.27 (d,6.9, 3H) | 12.28 | 177.5, 56.4, 41.1 | 2.25 | 6.18 (d,3.3) 5.47 (d,3.3) |
119.0 | 169.1, 138.5, 53,1 169.1, [138.5], 53.1 |
5.47, 2.89 6.18, 2.89 |
| 14 | 2.29 (t, 1.2, 3H) | 19.80 | 169.9, 135.6, 52.6 | 6.16, 3.41 | 2.33 (t,1.2, 3H) | 19.8 | 135.8, 53.0, 169.4 | 6.17, 3.52 |
| 15 | 2.44 (bs, 3H) | 21.61 | 152.1, 131.9, 37.6 | [3.4] | 2.44(bs,3H) | 21.8 | 151.9, 131.9, 37.3 | 3.52 |
Figures in brackets detonate weak long range correlations
Dehydroleucodine (2): colorless crystals (EtOAc), mp 128–130°C; [α]25D +48.98 (c 1.42, CH2Cl2); UV (MeOH) λmax (log ε) 216 (3.91) nm, 256 (4.20) nm; IR (KBr) vmax 3098, 2970, 1780, 1681, 1631, 1615 cm−1; EI-MS: m/z (rel. int.) 244 (100), 183 (13), 173 (13), 159 (15), 145 (18), 133 (10), 115 (13), 91 (46), 77 (22), 53 (22);. 1H NMR (CDCl3, 500 MHz) see Table 1; 13C NMR (CDCl3, 125 MHz) see Table 1.
X-ray Data of compound 2: Crystal data: C15H16O3. Mw = 244.29, orthorhombic, space group P212121, a = 7.5784(5) Å, b = 11.1203(7)Å, c = 15.3702(11)Å, V = 1295.31(15) Å3, Z = 4, crystal dimensions: 0.22 × 0.37 × 0.6 mm, T = 223 K Rigaku SCXmini system, Mercury 2 CCD detector, Mo-Kα radiation (λ = 0.71075A), 9029 total measured reflections, 2969 unique measured reflections, Rsym = 0.025, exposure time:10 sec/image, total collection time: 4hrs 12min, crystal-to-detector distance:50.1 mm, 2θSwing:30.0°, number of Scans: 2, data Images:360, 2θmax = 55.0°. Rfactor (all unique reflections) = 0.054. CCDC 730479 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk).
2.5. Antimicrobial activity
2.5.1. Bacteria and Culture Conditions
Six staphylococcal isolates were used in the determination of bacteriostatic activity. Two strains of S. epidermidis (UAMS-302 & UAMS-1037) and four strains of S. aureus (UAMS-1, UAMS-1625, UAMS-1864, & UAMS-1865) were included in the study. The antibiotic resistance profile for each strain is detailed in Table 2. Staphylococcal isolates were grown in tryptic soy broth (TSB) overnight at 37°C and diluted to the equivalent of OD600nm = 0.05 in TSB for MIC50 testing.
Table 2.
MIC50 and MIC90 values for compounds 1, 2, and the ethyl acetate extract of Gynoxys verrucosa against various strains of methicillin resistant Staphylococcus aureus (MRSA), methicillin sensitive Staphylococcus aureus (MSSA), methicillin resistant Staphylococcus epidermidis (MRSE), and methicillin resistant Staphylococcus epidermidis (MSSE).
| Organism | S. aureus (UAMS-1625) | S. aureus (UAMS-1864, ATCC 33593) | S. aureus (UAMS-1865, NRSa 385) | S. (UAMS-1, ATCCb 49230) | S. epidermidis (UAMS-1037, NRSa 101, ATCCd 35984) | S. epidermidis (UAMS-302, O-47c) | |
|---|---|---|---|---|---|---|---|
| Phenotype | MRSA | MRSA | MRSA | MSSA | MRSE | MSSE | |
|
| |||||||
| Source | Brain abscess (Sifri et al., 2007) | Bloodstream | Bloodstream | Osteomyelitis (bone) | Catheter sepsis | Unknown | |
|
| |||||||
| Antibiotic Resistance Profiled | AMP; CIP; ERY; KAN; LVX; NEO; OX; PEN | AMP; CLI; ERY; GM; KAN; OX; PEN; SXT; TET | AMP; CIP; CLI; CZe; ERY; GM; KAN; LVX; OX; PEN; SXT; TET | AMP; PEN | AMP; CIP; CLI; ERY; GM; KAN; LVXe; OX; PEN | AMP; PEN | |
|
| |||||||
| MIC50 (μg/mL) | Extract | 908 | 1460 | 3290 | 980 | 2190 | 1300 |
| 1 | >246 | >246 | >246 | >246 | >246 | >246 | |
| 2 | 49 | 49 | 147 | 98 | 195 | 195 | |
| OX | 36 | 26 | 92 | 3 | 19 | 0.08 | |
| VAN | 2.7 | 3.2 | 4 | 3.2 | 2.8 | 2.3 | |
|
| |||||||
| MIC90 (μg/mL) | 2 | 450 | 280 | 400 | 440 | 680 | 490 |
| OX | 48 | 80 | 132 | 5 | 88 | 0.75 | |
| VAN | 4.9 | 4.7 | 5 | 4.8 | 4.6 | 4.5 | |
NRS: Network for Antibiotic Resistant Staphylococcus aureus
ATCC: American Type Culture Collection
O-47 was kindly provided by the F. Gotz’s collection of clinical isolates from Germany
AMP: Ampicillin; CIP: Ciprofloxacin; CLI: Clindamycin; CZ: Cefazolin; ERY: Erythromycin; GM: Gentamicin; KAN: Kanomycin; LVX: Levofloxacin; NEO: Neomycin; OX: Oxacillin (Methicillin); PEN: Penicillin; SXT: Sulfamethoxazole/Trimethroprim; TET: Tetracycline; VAN: Vancomycin
Intermediate resistance
2.5.2. Determination of Antibiotic Resistance Profile
Staphylococcal isolates included in this study were examined for their antibiotic resistance profile using the disc diffusion method following established protocols (Isenberg, 2004). Briefly, Mueller-Hinton agar was seeded with bacteria and antibiotic disks: 1 μg Oxacillin, 10 μg Ampicillin, 5 μg Ciprofloxacin, 2 μg Clindamycin, 30 μg Cefazolin, 15 μg Erythromycin, 10 μg Gentamicin, 30 μg Kanomycin, 30 μg Neomycin, 10 units Penicillin, 25 μg Sulfamethoxazole/Trimethroprim, 30 μg Tetracycline and 30 μg Vancomycin (Becton Dickinson, BBL Antibiotic Susceptibility Discs, NJ, USA) were applied to the agar. Following incubation, the diameter of the zone of inhibition was measured and compared to the antibiotic kit’s susceptibility chart for S. aureus and S. epidermidis.
2.5.3. Determination of MICs
Minimum inhibitory concentrations (MICs) were determined by the microtiter broth method (Amsterdam, 1996) in sterile flat-bottom 96-well polystyrene plates. Bacteria were suspended in TSB and added to microtiter plates. Serial dilution techniques were employed to examine the activity of compounds 1 and 2 (purity > 99%), and extract at concentrations ranging from 3–1000 μM. In addition, negative (growth) controls (cells + TSB), vehicle controls (cells + TSB + DMSO), and positive controls (TSB+ antibiotic; vancomycin or oxacillin) were included. All tests were performed in triplicate. Microtiter plates were placed in a 37°C incubator on a shaker for 18 hours. Optical density readings (λ = 600 nm) were taken using a Biotek microplate reader at 0 and 18 hours post-inoculation. Results are reported as the MIC50 and MIC90 for growth at 18 hours post-inoculation.
3. Results and discussion
From the ethyl acetate extract of G. verrucosa, two sesquiterpene lactones were isolated and identified by spectroscopy methods and by single-crystal X-ray analysis. Table 1 shows the NMR analysis of both isolated compounds.
The X-ray crystal structure of compound 2, was established and reported for the first time for this molecule, and is fully consistent with the previously reported structure (Wendel et al., 2008). The molecular structure of 2 is illustrated in Fig. 1, the crystal structure shows that both H-8 and H-10 are below the approximate plane of the seven membered ring, while H-9 is above the plane, hence, the configuration is confirmed as being 8α-H, 9β-H, and 10α-H. The seven-membered ring shows an approximated chair conformation with the atoms C-4, C-5, C-6, and C-10 approximately coplanar, and the atoms C-7, C-8, and C-9 above that plane. The full assignment of the 1H-NMR and 13C-NMR spectra for compounds 1 and 2 are reported for the first time and are fully consistent with the proposed structures and the X-ray crystal structure of compound 2. For compound 1 we observed a large (anti) H-8/H-12 coupling, a ROESY peak between H-12 and H-9 indicating that these hydrogens are on the same face and a ROESY peak between C(13)H3 and H-8 indicating that these hydrogens are also on the same face.
The isolated constituents were tested in vitro against six staphylococcal isolates strains, compound 2 was found to have antimicrobial activity against all staphylococcal isolates, including four methicillin-resistant strains. The antimicrobial activities and the characteristics of the strains tested are presented in Table 2, and range from 49 to 195 μg/mL for the MIC50 and 280–680 for the MIC90. Although, the MRSA activity of compounds 2 is not very high, these results suggest its potential use as a template for further development. Furthermore, the significant activity of compound 2 against the Gram-negative Helicobacter pylori recently reported by Vega et al (2009) suggests that this compounds has a wide spectrum of antimicrobial activity. The fact that compound 2 showed activity against clinical isolates from brain abscesses, suggest that this compound may have a potential clinical use in this area. This is relevant because Staphylococcal brain abscesses are difficult to treat.
Although, no toxic effects have been reported for dehydroleucodine or G. verrucosa, caution should be advised, given that some sesquiterpene lactones have been reported to produce toxic effects to humans and animals, including contact dermatitis and neurotoxicity (Schmidt, 1999).
Compound 2 is a non-ionizable molecule, has a molecular weight smaller than 300, and showed the highest activity against two MRSA clinical isolates resistant to a broad range of antibiotics. All of these facts suggest that compound 2 may have potential for clinical applications as an antimicrobial or may serve as a template for the development of more potent antimicrobials. The establishment of the X-ray structure of compound 2 (Fig. 1) should assist in such efforts.
At a maximum test concentration of 1 mM, compound 1 showed very low level of activity, ranging from 0 – 5% inhibition against strains of S. aureus and 5% and 33% inhibition for the strains of S. epidermidis. Thus, these results demonstrate that the exocyclic conjugated methylene in the lactone ring is essential for the antimicrobial activity of these sesquiterpene lactones. These results are in contrast with the previously reported assertion that the exocyclic α-β-unsaturated methylene moiety is not required for the antimicrobial activity of the sesquiterpene lactones (Lee et al., 1977).
It is significant that three related sesquiterpene lactones structurally similar to compound 2 (arnicolide C, xanthatin and a sesquiterpene ketolactone) have also been found to have significant antimicrobial activity against MRSA and MSSA (Gibbons, 2004). Compound 2 and these compounds have, in addition to the carbonyl of the lactone ring, a carbonyl in the opposite side of the cycloheptane ring, suggesting that perhaps a secondary hydrogen binding point is necessary for the activity.
Acknowledgments
This research was supported by the University of Arkansas for Medical Sciences College of Pharmacy Research Fund, the Universidad Técnica Particular de Loja Faculty Exchange Program, and the National Center for Complementary and Alternative Medicine (grant number F32 AT005040 to CLQ). PEO thanks to Ecuador’s Higher Education, Science and Technology Ministry (SENESCYT) for a graduate studies scholarship. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Loman V, editor. Antibiotics in Laboratory Medicine. 4. Williams and Wilkins; Baltimore, MD: 1996. pp. 52–111. [Google Scholar]
- Deurenberg RH, Stobberingh EE. The molecular evolution of hospital and community associated methicillin-resistant Staphylococcus aureus. Current Molecular Medicine. 2009;9:100–15. doi: 10.2174/156652409787581637. [DOI] [PubMed] [Google Scholar]
- Gibbons S. Anti-staphylococcal plant natural products. Natural Products Reports. 2004;21:263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- Hilmi F, Sticher O, Heilmann J. New cytotoxic sesquiterpene lactones from Warionia saharae. Planta Medica. 2003;69:462–464. doi: 10.1055/s-2003-39703. [DOI] [PubMed] [Google Scholar]
- Isenberg HD. Clinical Microbiology Procedures Handbook. 2004;2:5.1–5.1.15. [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GF, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Journal American Medical Association. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Lee C. Therapeutic challenges in the era of antibiotic resistance. International Journal of Antimicrobial Agents. 2008;54:5197–5199. doi: 10.1016/S0924-8579(09)70002-0. [DOI] [PubMed] [Google Scholar]
- Lee KH, Ibuka T, Wu RY, Geissman TA. Structure-antimicrobial activity relationships among the sesquiterpene lactones and related compounds. Phytochemsitry. 1977;16:1177–1181. [Google Scholar]
- Martínez M, Muñoz-Zamora A. Conformational analysis of achillin and leukodin. Journal of Natural Products. 1988;51:221–228. [Google Scholar]
- Michalska K, Szneler E, Kisie W. Sesquiterpenoids of Picris koreana and their chemotaxonomic significance. Biochemical Systematics and Ecology. 2007;35:459–461. [Google Scholar]
- Rucker G, Schenkel EP, Manns D, Mayer R, Hausen BM, Heiden K. Allergenic Sesquiterpene Lactones From Eupatorium cannabinum L. and Kaunia rufescens (Lund ex de Candolle) Journal of Natural Toxins. 1997;5:223–227. doi: 10.1002/(sici)1522-7189(1997)5:6<223::aid-nt1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ. Toxic Activities of sesquiterpene lactones: Structural and biochemical aspects. Current Organic Chemistry. 1999;3:577–608. [Google Scholar]
- Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clinical Infectious Disease. 2007;45:113–7. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- Simoes M, Bennettb RN, Rosab EAS. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Natural Products Reports. 2009;26:746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- Tene V, Malagón O, Vita Finzi P, Vidari G, Armijos C, Zaragoza T. An ethnobotanical survey of medicinal plants used. in Loja and Zamora-Chinchipe, Ecuador. Journal of Ethnopharmacology. 2007;111:63–81. doi: 10.1016/j.jep.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Trendafilova A, Todorova M, Mikhova B, Vitkova A, Duddeck H. Sesquiterpene lactones from Achillea collina J. Becker ex Reichenb. Phytochemistry. 2006;67:764–770. doi: 10.1016/j.phytochem.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Vega AE, Wendel GH, Maria AOM, Pelzer L. Antimicrobial activity of Artemisia douglasiana and dehydroleucodine against Helicobacter pylori. Journal of Ethnopharmacology. 2009;124:653–655. doi: 10.1016/j.jep.2009.04.051. [DOI] [PubMed] [Google Scholar]
- Wendel GH, María AOM, Guzman JA, Giordano O, Pelzer LE. Antidiarrheal activity of dehydroleucodine isolated from Artemisia douglasiana. Fitoterapia. 2008;79:1–5. doi: 10.1016/j.fitote.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrobial Agents and Chemotherapy. 2008;52:452–7. doi: 10.1128/AAC.00908-07. [DOI] [PMC free article] [PubMed] [Google Scholar]