Abstract
Prenatal cannabis exposure can complicate in utero development of the nervous system. Cannabis impacts the formation and functions of neuronal circuitries by targeting cannabinoid receptors. Endocannabinoid signaling emerges as a signaling cassette to orchestrate neuronal differentiation programs through the precisely timed interaction of endocannabinoid ligands with their cognate cannabinoid receptors. By indiscriminately prolonging the ‘switched-on’ period of cannabinoid receptors, cannabis can hijack endocannabinoid signals to evoke molecular rearrangements, leading to the erroneous wiring of neuronal networks. Here, we formulate a hierarchical network design necessary and sufficient to describe molecular underpinnings of cannabis-induced neural growth defects. We integrate signalosome components deduced from genome- and proteome-wide arrays and candidate analyses to propose a mechanistic hypothesis on how cannabis-induced ectopic cannabinoid receptor activity overrides physiological neurodevelopmental endocannabinoid signals, affecting the timely formation of synapses.
Keywords: drug abuse, metabolome, transcriptome, synapse
Endocannabinoids: gatekeepers of neuronal development
Molecular cloning of the CB1 cannabinoid receptor (CB1R) [1], and its functional characterization as the major target of Δ9-tetrahydrocannabinol (THC) from cannabis [2] led to a sea-change in the understanding of the molecular mechanisms of this psychoactive drug s actions on neuronal structure and function in brain regions controlling memory, cognition, movement and pain perception [3]. These findings, coupled with the discovery that the CB1R functions as an essential signal transducer in an elaborate molecular network relying on “endogenous cannabinoids” (endocannabinoids) to modulate the plasticity of many synapses [4] prompted a remarkably vibrant discipline of contemporary neurobiology. Ensuing developmental biology studies concerned with the formation of endocannabinoid signaling networks [5–9], the role of endocannabinoids [10–19] and, consequently, the molecular blueprint of prenatal cannabis abuse [16,20–23] in the developing nervous system soon followed. Our understanding so far is that endocannabinoids can act as focal instructive signals that affect neural progenitor proliferation [18] and neuron vs. glia fate decisions [24], as well as the differentiation programs of forebrain neurons (including but not restricted to cell migration, axonal growth and synapse development) [24].
Here we ask: “Are the molecular mechanisms of endocannabinoid signaling (that is, the dynamic arrangements to the enzymatic control of focal endocannabinoid availability and signaling at the CB1R) necessary and sufficient to establish cannabis sensitivity in developing neurons?” We present a series of arguments to pinpoint the nascent axon as a structural substrate of cannabis action [6,13,15,17], and to suggest a causal link between compartmentalized endocannabinoid signaling and cannabis (or cannabinomimetic)-driven modifications to the wiring of emergent neuronal networks in the fetal brain [6,7,13]. Our molecular model reconciles competing and provocative hypotheses on the mode(s) of action of endocannabinoids, the cellular configuration of their metabolic machinery, and signaling by intra- vs. extracellular CB1Rs.
The ‘high-way’ of brain development: clinical considerations
THC can enter the fetal circulation with rapid onset [25] via efficient transfer through the placenta [26]. THC levels in the amniotic fluid and fetus remain elevated up to 5h, followed by gradual clearance within 48h after exposure [25]. Exceptionally high THC doses (>100 mg/kg) may be teratogenic and induce in utero death [25]. However, cannabis use during pregnancy can lead to growth retardation [27] and is associated with adverse neurodevelopmental outcomes [28]. The delay in nervous system development upon in utero cannabis exposure in humans can impair cognitive performance [29–31], visual-motor coordination [32,33], and social behaviors [29,34], and increase the incidence of drug seeking [35], attention deficit [36], anxiety and depression [37] among affected neonatal or adolescent offspring.
It is becoming evident that not only THC but any plant-derived or synthetic drug – alone [16,38] or in mixture [39] –, which displays significant potency and efficacy at the CB1R might evoke significant modifications of neuronal differentiation [12,16] and synapse physiology [40,41] by disrupting normal patterns of endocan signaling.
Endocannabinoids in the nervous system
In addition to the best known ligands, 2-arachidonoyl glycerol (2-AG) [42–44] and N-arachidonoylethanolamine (AEA) [45], the list of possible endocannabinoids includes a growing number of structurally related ligands with appreciable pharmacological efficacy at the CB1R or CB2 cannabinoid receptor (as well as having other targets; Box 1, Table 1). However, the concentration, regionalized distribution, metabolic and signaling interactions of these endocannabinoid-like substances remain as yet largely elusive.
Box 1. Molecular complexity and diversity of endocannabinoid metabolism.
2-Arachidonoyl glycerol (2-AG) selectively activates CB1R and CB2R with high efficacy, typically as a full agonist [62]. 2-AG is produced by Ca2+-dependent sn-1-diacylglycerol lipase (DAGL) α and β following the PLC-dependent hydrolysis of membrane phospholipid precursors to PIP2 anddiacylglycerol (DAG) [54] (Table 1). Other pathways to generate 2-AG include sequential reactions by phospholipase A1 and lysophosphatidylinositol-specific phospholipase C (lyso-PLC), and phosphatase-mediated conversion of 2-arachidonoyl lysophosphatidic acid to 2-AG [46]. Monoacylglycerol lipase (MAGL) primarily catalyzes 2-AG hydrolysis, with limited contributions by α/β-hydrolase domain-containing 6 and 12 (ABHD6, ABHD12) hydrolases [92]. N-arachidonoylethanolamine, (anandamide, AEA) be-haves as an agonist at the CB1R, CB2R and transient receptor potential cation channel V1 (TRPV1) channels. Several pathways are involved in AEA synthesis [46,93] (Table 1). First, Ca2+-stimulated N-acyltransferase generates N-arachidonoyl phosphatidylethanolamine (NAPE), followed by N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD)-mediated NAPE hydrolysis yielding AEA. Second, ABHD4 deacylates NAPEs [93] with subsequent conversion of glycerophospho-NAEs by the glycerophosphodies-terase GDE1 to generate AEA. Third, PLC-mediated cleavage of NAPE can yield bioactive phospho-AEA intermediates that are dephosphorylated by phosphatases (PTPN22). AEA is primarily degraded by fatty-acid amide hydrolase (FAAH) [46]. FAAH can also hydrolyze the ester bond in 2-AG in vitro but its in vivo contribution to 2-AG hydrolysis seems limited. In addition, N-acylethanolamine-hydrolyzing acid amidase (NAAA) can degrade AEA [94]. Alternatively, cyclooxygenase-2 (COX-2) - and lipoxygenases - can oxidize both 2-AG and AEA to prostaglandin-glycerol esters and ethanolamines, respectively.
Other putative neuroactive lipids recovered from adult mammalian brain and exhibiting some efficacy at the CB1R [46] are also listed at the bottom of Table 1.
Table 1.
Endogenous neuroactive lipids with agonist potency at the CB1 cannabinoid receptor (CB1R), and their metabolic pathways.
| Precursor | Biosynthesis | Endocannabmoids and some related lipids | Transporter1 | Degradation | Receptor |
|---|---|---|---|---|---|
| DAG 2- Arachidonoy1-lysophospholipid 2-Arachidonoyi-sn-glycero3-phosphate |
DAGLα and β PhospholipaseA,/ Lyso-PLC Uncharactertized LPA phosphatase |
2-arachidonoyl glycerol (2-AG) | Facilitated diffusion Membrane transporter |
MAGL1/2 ABHD6.ABH012 COX-2 FAAH1/2 |
CB,R CB,R orphan GPCRs |
| NAPEs | NAPE-PLDor ABHD4/GDE1 or PLC/phosphatase (PTPN22) pathways | N-anchidonoylethanolamine2 (anandamide, AEA) | Facilitated diffusion Membrane transporter |
FAAH1/2 NAAA COX-2 |
CB,R CB.R TRPV1 GPR55 ion channels |
| NAPEs acyl-CoA +amino acid |
2-Arachidonyl glycerol ether O- Arachidonoylethanolamine (Virodhamine) N-Palmitoylethanolamide (PEA) N-Arachidonoyl-L-serine N-Arachidonoyl -L-doparo«no N-Arachidonoyl -L-gryane N-Acyl-taurines |
shared with AEA (?) | shared with AEA (?) NAAA |
CB,R CB,R GPR55 ion channels TRPV1 GPR18 |
Although a membrane transport mechanism facilitating endocannabinoid liberation and uptake has been proposed, the existence and molecular identity of this transporter remain to be elucidated.
In addition to N-acyl ethanolamines, such as AEA, N-acyl-taurines are also FAAH substrates.
Endocannabinoids function as retrograde messengers to modulate the plasticity of many synapses in the adult brain [3]. Coupled with in-depth neurophysiology analyses at many brain regions [46], the understanding of the molecular principles governing activity-dependent endocannabinoid synthesis and utilization have rapidly expanded. However, the molecular architecture of endocannabinoid signaling networks in developing neurons substantially differs from their adult counterparts.
This review focuses on 2-AG, because most available data emphasize the involvement of 2-AG in both developmental processes [13,18] and the control of synaptic neurotransmission [3]. This is largely due to the relative lack of information on the molecular identity, use-dependent recruitment, and cellular localization of metabolic enzymes controlling AEA bioavailability in the nervous system (Box 1, Table 1). However, recent data functionally implicating AEA in retrograde signaling [47] or AEA-induced activation of Ca2+-permeable TRPV1 channels to regulate 2-AG synthesis [48] may soon change this view. This latter pathway may be controlled by feedback from AEA/2-AG-activated CB1R to limit Ca2+ flux through TRPV1 channels [49] (Figure 1b).
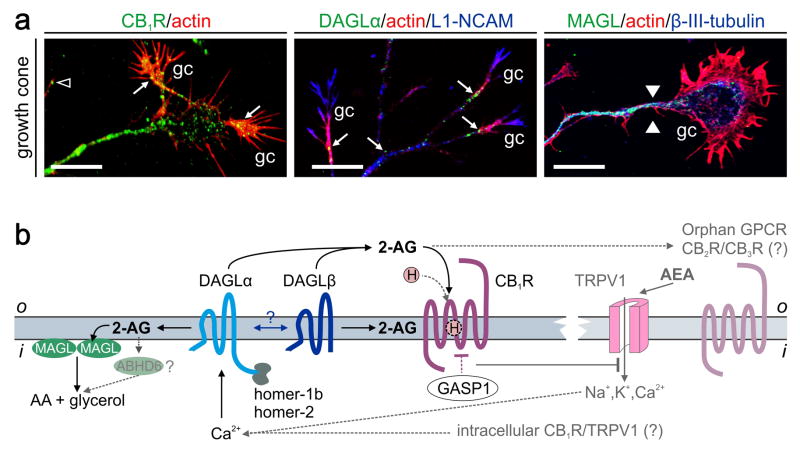
Figure 1. Molecular organization of 2-AG signaling networks in developing neurons.
(a)Receptor and metabolic enzyme components of 2-AG signaling coexist in developing neurons and are preferentially targeted towards their axons and growth cones (gc). The CB1R and DAGLα both accumulate in the central domain and actin-rich filopodia of growth cones (arrows) [15]. By contrast, MAGL accumulates in the tubulin-rich axon stem with its levels descending towards the growth cone forming a decreasing gradient of 2-AG hydrolysis activity (arrowheads indicate the start of this gradient) [13]. (b) Molecular organization of 2-AG signaling in developing neurons. 2-AG, produced by DAGLα or β, can activate the CB1R either from the extracellular space or by lateral diffusion in the plasmalemma. Homer scaffolds can anchor DAGLα at preferred signaling positions [51]. The lack of homer binding sites in DAGLβ suggests that DAGLα and DAGLβ may directly associate, anchoring DAGLβ and spatially restricting 2-AG biosynthesis. CB1R trafficking and/or signaling might be modulated by hemopressin (H) [95], or C-terminal interacting proteins. GPCR-associated sorting protein 1 (GASP1) is one such example [96]. MAGL [56] or ABHD6 [92] can hydrolyze 2-AG into arachidonic acid (AA) and glycerol to terminate signaling. Potential, yet controversial, involvement of other endocannabinoids (e.g., AEA), CB2Rs, orphan GPCRs and TRPV1 are shown in grey. AEA-activated TRPV1s are particularly intriguing because they could serve on either the plasmalemma or intracellular membranes as Ca2+ sensors controlling DAGL activity [48]. Scale bars = 10 μm.
Molecular organization of endocannabinoid signaling in developing neurons
Neurogenic commitment differentially regulates the expression of the CB1R [7], sn-1-diacylglycerol lipase α and β isoforms (DAGLα/β) [11] and monoacylglycerol lipase (MAGL) [50], 2-AG synthesizing and degrading enzymes, respectively. Neuronal differentiation up-regulates CB1R expression [7], whereas cell-cycle exit of neural stem cells represses DAGLs [11]. During neuronal polarization, this signaling triad is co-transported along the nascent axon (Figures 1a,b) [6,13,15,16]. Although the CB1R can be expressed on the surface of the axon stem, they are preferentially trafficked to the growth cone, including motile filopodial extensions, positioned to sense 2-AG [15,17]. Activation of the CB1R elicits growth cone collapse [15,17] suggesting that endocannabinoids can act as chemotropic guidance cues.
Both DAGLα and DAGLβ are expressed in the developing brain. Scaffolding proteins (e.g., homer-1/-2b) are likely to determine the subcellular sites of 2-AG synthesis by anchoring DAGLα [51]. Because DAGLβ lacks a homer-binding domain and is redistributed upon DAGL knock-out [18], a candidate mechanism to limit the subcellular dispersion of DAGLβ is its association with DAGLα. The axonal growth cone is particularly enriched in DAGLs. Here, the enzymatic activity of Ca2+-dependent DAGLs may be spatially confined (“domain-specific”) because Ca2+ signaling is lateralized in the growth cone during turning (that is, [Ca2+] is increased in the domain facing the attractive gradient [52]). Thus, DAGLs may function as coincidence detectors by focally controlling 2-AG availability and autocrine signaling at the CB1R to limit the process of growth cone turning.
MAGL is a cytosolic enzyme that can be recruited to the inner leaflet of the plasmalemma to inactivate 2-AG at the plasma membrane [53]. MAGL undergoes focal and rapid proteasomal degradation in the motile neurite tip [13]. The physiological significance of MAGL microgradients tailing off in the distal axon segment may be to enrich the growth cone in signaling-competent 2-AG.
Synaptogenesis coincides with MAGL entering into the growth cone [13], where it may act as a “stop” signal by eliminating growth-promoting 2-AG. Once synaptogenesis concludes, DAGLs redistribute into the somatodendritic domain of neurons to provide 2-AG for retrograde signaling [54]. In contrast, both the CB1R and MAGL remain localized to presynaptic terminals [13].
Endocannabinoids: intracellular or extracellular signals?
Endocannabinoids are viewed as lipophilic ligands whose ability to disperse in an aqueous extracellular environment may be limited. However, this view is challenged by the complete lack of retrograde synaptic signaling in DAGLα−/− mice [18,55]. Because retrograde signaling relies on facilitated transsynaptic 2-AG diffusion to activate presynaptic CB1Rs, data from DAGLα−/− models unequivocally identify 2-AG as an extracellular retrograde messenger. 2-AG can also signal intracellularly [46]. Therefore, 2-AG s physicochemical properties could suffice to sustain both cell-autonomous and intercellular signaling mechanisms in the developing nervous system.
Is endocannabinoid signaling indispensable for brain development?
The answer might be ambiguous for several reasons. First, developmentally redundant signaling cassettes rely on promiscuous ligand-receptor interactions to sustain signaling efficacy even if a component of a signaling system is compromised. Therefore, using constitutive and global gene knock-outs to determine the function of a single gene may be limited to decipher the absolute contribution of a candidate mechanism to homeostatic control pathways [18,55,56]. Second, DAGL functions may be more essential for neuronal metabolism than previously thought, because in addition to lower levels of 2-AG, DAGLα−/ − and DAGLβ−/ − mice have significantly lower arachidonic acid (AA) and AEA levels (~80% and ~40% for DAGLα−/ −, respectively) in the brain [18]. AA is often a metabolite of 2-AG [18]. Therefore, the decrease of AA in the absence of 2-AG highlights the close relationship between 2-AG, AA and AEA metabolism, likely incorporating yet unidentified metabolic routes. This emphasizes that only a fraction of 2-AG might be used for inter-cellular signaling. Instead, the bulk of 2-AG could be immobilized in, e.g., the plasmalemma or lipid droplets [57], for intermediary metabolism or other processes. Third, genetic disruption of endocannabinoid signaling networks may modify, yet not fully repress, a specific developmental response pattern [18]. This may be interpreted such that endocannabinoids are gatekeepers of developmental processes, and impairments of their signaling must coincide with secondary insults (e.g., maternal deprivation [58], stress or seizures) to impose enduring modifications to neuronal circuitry.
CB1R can diversify the ontogenic impact of endocannabinoid signals
The CB1R can exhibit tremendous signaling complexity. The simplest functional unit of a GPCR, (including the CB1R), is a homodimer that recruits a heterotrimeric G protein [59]. However, the CB1R is physiologically “dominant” because it can becoupled to Gi/o-proteins even in the absence of an agonist (“constitutive activity”), thereby depleting the common intracellular Gi/o pool and limiting the biological signals of other Gi/o-coupled receptors [60]. GPCRs can also heterodimerize with other receptors, leading to a combinatorial recruitment of second messengers [61]. Evidence exists for the assembly of CB1R-μ-opioid, CB1-OX1 orexin, CB1-β2 adrenergic, CB1R-D2 dopamine, CB1R-A2A adenosine and CB1R-tyrosine kinase B (TrkB) receptor dimers, among others [62].
The C-terminus of the CB1R contains phosphorylation sites for GPCR kinases (GRKs) leading to β-arrestin-dependent desensitization (AAs 426/430) [63], as well as an internalization (AA460–473) domain [64]. Once internalized, the CB1R is either recycled to the membrane or tagged by GPCR-associated sorting protein-1 (Figure 2) and targeted for proteasomal degradation [65]. The AA465–473 extremity of the CB1R can bind adaptor proteins [66] such as CB1R interacting protein 1a (CRIP1a) and 1b. This latter interaction with the CB1R may be restricted to the adult brain because CRIP1a is targeted to the somatodendritic domain of neurons, and its protein expression profile is different from that of the CB1R in fetal brain [13].
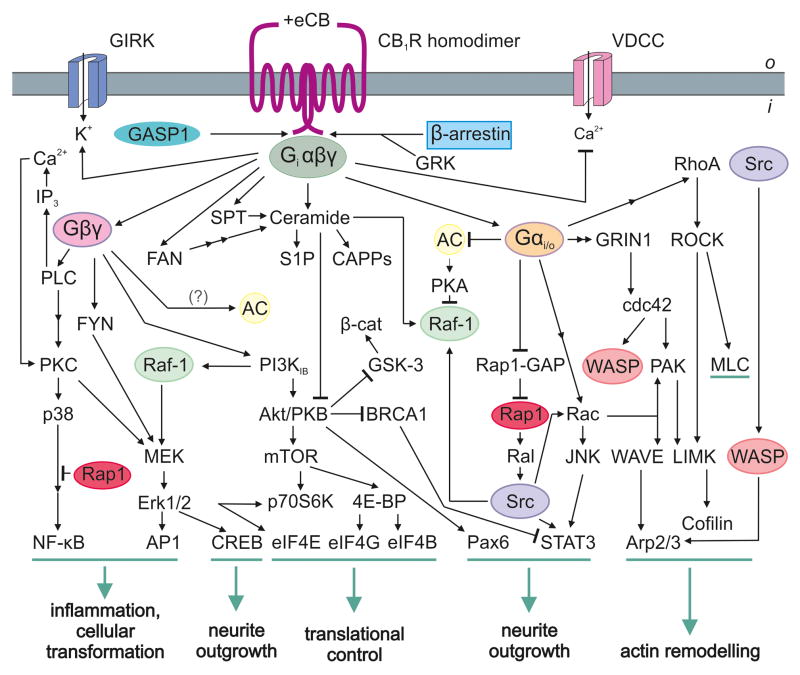
Figure 2. Agonist-induced combinatorial second messenger signaling at the CB1R.
The contemporary view of GPCR signaling identifies receptor homodimers as signaling units [59]. Recruitment of signal effectors is cell state-specific in developing neurons with the active signaling cascade directly determining the physiological outcome (green). The CB1R can inhibit voltage-dependent Ca2+-channels (VDCC) or activate G protein-coupled inward rectifying potassium channels (GIRKs) through Gβγ subunits [62]. β-Arrestins participate in CB1R internalization and desensitization, while GPCR-associated sorting protein 1 (GASP1) can direct this receptor towards lysosomal degradation [96]. It is being recognized that the CB1R can recruit mTOR signaling to regulate protein translation through activation of mammalian elongation initiation factors (eIF4E/B/G) [97]. Abbreviations: 4E-BP, eukaryotic translation initiation factor 4E-binding protein 1; AC, adenylyl cyclase; Akt/PKB, protein kinase B; AP1, activator protein-1; Arp2/3, actin related protein 2/3; β-cat, β-catenin; BRCA1, breast cancer susceptibility protein 1; CAPPs, ceramide activated protein phosphatases; Cdc42, cell division control protein 42 homolog; CREB, cAMP response element-binding protein; EGF, epidermal growth factor; Erk1/2, extracellular signal-regulated kinase 1/2; FAN, factor associated with neutral sphingomyelinase activation; FYN, member of Src family tyrosine kinases; GRIN1, G protein-regulated inducer of neurite outgrowth 1; GRK, G protein-coupled receptor kinase; GSK-3, glycogen synthase kinase-3; IP3, inositol 1,4,5 triphosphate; JNK, c-Jun N-terminal kinase; LIMK, LIM motif-containing protein kinase; MEK, Erk kinase; MLC, myosin light chain; mTOR, mammalian target of rapamycin; Nf-κB, nuclear factor κB; p38, mitogen-activated protein kinase (MAPK); p70S6K, serine/threonine kinase; Pak, p21 activated kinase; Pax6, paired box gene 6; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; Rac, member of Rho family GTPases; Raf-1, MEK kinase; Ral, Ras-like protein; Rap-1, Ras related protein-1; RAP1-GAP, RAP1 GTPase activating protein; RhoA, Ras homolog gene family, member A; ROCK, Rho-associated protein kinase; S1P, sphingosine 1-phosphate; SPT, serine palmitoyltransferase; Src, v-src sarcoma viral oncogene homolog; STAT3, signal transducer and activator of transcription 3; WAVE/WASP, Wiskott-Aldrich syndrome family protein.
Signaling complexity at the CB1R is further enhanced by endogenous agonists or antagonists, whose synthesis and degradation are distinct from those of the endocannabinoids. These compounds can modulate the availability and signaling competence of the CB1R. Hemopressin is an endogenous nonapeptide derived from α-hemoglobin (Figure 1b). Although hemopressin itself is recognized as an inverse agonist, its truncation products reportedly function as CB1R agonists [67]. Hemopressin derivatives structurally and energetically fit the ligand-binding pocket of CB1R [68], affect CB1R trafficking, and modulate CB1R-induced neurite outgrowth [67]. The lack of hemopressin effects in CB1R−/− mice suggest that hemopressin may efficiently modulate neuronal CB1Rs in vivo [69]. Although full-length hemopressin was isolated from brain homogenates, it remains uncertain whether short peptide fragments cleaved from the parent peptide might instead confer its biological activity [68,69].
Finally, the fetal brain can be perceived as a rapidly changing kaleidoscope of neural activity, as a plethora of molecular control switches are turned “on” and “off” in a precise sequence. Maternal cannabis abuse can be viewed as a pathogenic stimulus to derail the physiological output of the CB1R by forcing it to signal in (a)synchrony with other receptors [70], thus overriding physiological endocannabi-noid signal cascades.
Signaling pathways linking the CB1R to cell proliferation/survival and neurite outgrowth
Classical Gi protein-mediated signaling at the CB1R is well suited to activate effectors that couple endocannabinoid signaling to cell survival, proliferation, and differentiation (Figure 2). Here we discuss novel facets of understanding of cell state- specific signaling events and emphasize the importance of CB1R signaling in relation to axonal growth and guidance [15,17,71,72], which have evolved since a recent survey of available data [62].
A major branch of CB1R signaling is directed towards controlling the population size of neural progenitors, including those of cerebellar granule cells and olfactory neurons [73]. This is achieved upon Akt/PKB-mediated inactivation of glycogen synthase kinase-3 through phosphorylation [74], thus allowing anti-apoptotic signal progression through the nuclear import of stabilized β-catenin to regulate cell survival (Figure 2). CB1R activation can also couple to the accumulation of ceramide, a lipid mediator critical to control senescence, as well as differentiation [62].
It is becoming clear that neuronal CB1 activates a hierarchical signaling network to induce neurite outgrowth (Figure 2). The classical signaling pathway that couples nthe CB1R to neurite outgrowth is through the Gβγ subunit-dependent, sequential recruitment of phospholipase C (PLC), protein kinase C(ε) [75] or Fyn, a Src-family tyrosine kinase, to activate the extracellular signal-regulated kinase pathway [15,76]. Signaling through Gβγ can also activate Akt/PKB kinase, with cAMP response element-binding (CREB) and Pax6 transcription factors acting as activators coupling the CB1R to axonal growth [20]. Alternatively, Akt/PKB can suppress BRCA1 signaling to disinhibit signal transducer and activator of transcription 3 (STAT3) phosphorylation, regulating transcription [20].
Gαi/o activation downstream from the CB1R can induce STAT3 upon upstream activation of Ras-like protein (Ral) or Rac GTPase/c-Jun N-terminal kinase pathways converging at the level of Src [77] (Figure 2). The CB1R induces focal cytoskeletal remodeling [17] by coupling to small Rho GTPases [15]. In particular, Gαi-dependent induction of G protein-regulated inducer of neurite outgrowth 1 (GRIN1) can signal via cdc42 to activate Arp2/3 or cofilin [77]. Otherwise, Gαi- dependent guanine nucleotide exchange factor induces cofilin-dependent actin remobilization through RhoA. The physiological outcome of CB1-stimulated Rho GTPase-dependent signaling events may be the CB1R-dependent collapse of axonal growth cones [13,15,17].
Receptor interactions sensitizing developing neurons to endocanna-binoids
Molecular arrangements exerting upstream control upon endocannabinoid signaling during neurite outgrowth are essential to define the ultimate physiological outcome. An appealing hypothesis is to implicate receptor tyrosine kinases (Trks) (e.g. the fibroblast growth factor receptor) as they induce Ca2+ mobilization through PLCγ [78]. Elevated intracellular Ca2+ can activate DAGLs to generate 2- AG and trigger CB1R activation [24]. The robustness of Trk signaling is epitomized by i) the ability of brain-derived neurotrophic factor (BDNF) to increase cellular CB1R mRNA content through TrkB receptors [50], ii) the ability of BDNF to sensit- ize neurons to endocannabinoids (that is, in the presence of BDNF subphysiologi- cal endocannabinoid concentrations promote Akt phosphorylation) [50], iii) the fact that TrkB can assemble into signaling multimers with the CB1R, can be phospho-rylated in a BDNF-independent manner upon CB1R activity, and can recruit Src kinases [14], iv) the finding that BDNF-stimulated endocannabinoid release at inhibitory cortical synapses [79]. The latter mechanism is well poised to link endocannabinoid-driven growth cone turning responses to inhibitory synapse formation in vivo [15].
The coincidence of endocannabinoid and interleukin-6 (IL-6) signaling was recently highlighted as a means to modify the developmental efficacy of CB1R activation. The simultaneous presence of a CB1R agonist and IL-6 at subthreshold concentrations synergizes to activate CREB and STAT3 [19]. This signal convergence in turn inhibits SHP2 phosphatase to unmask morphogenic PLC signaling. Thus, integration of multiple protein kinases and transcription factors from GPCRs and cytokine receptors is sufficient to evoke neurite outgrowth. This interaction might be significant during intrauterine infection or autoimmune pathologies by modifying cell-cycle control, neuronal migration and neurite growth in response to IL-6-like cytokines (e.g., IL-11, ciliary neurotrophic factor or leukemia inhibitory factor) [80].
The cell-surface receptor DCC (deleted in colorectal cancer) functions as an integral component of a receptor complex that mediates the axonal chemoattractive response towards netrin-1 [81] in a Fyn kinase-dependent fashion [82]. Within the visual system, netrin-1/DCC interactions are particularly important to guide retinal ganglion cell (RGC) axons to innervate relay neurons in the dorsolateral geniculate nucleus [17]. Ocular dominance and non-overlapping topology of the terminal fields of individual RGCs in the geniculate nucleus are established through progressive pruning of their axons. Recently, the CB1R was found to modulate RGC growth cone morphology through the cAMP/protein kinase A (PKA) pathway [17]. An endocannabinoid-netrin signaling interplay to control the growth cone morphology of RGC axons was suggested since CB1R activity affected PKA-dependent targeting of DCC to the plasma membrane (CB1R antagonists increase DCC s insertion into the plasma membrane) [17]. However, the morphological outcome of this CB1R/DCC interaction is ultimately growth arrest; this also manifests in the lack of eye-specific segregation of retinal projections in CB1R−/− mice in vivo because the CB1R drives this interaction by decreasing intracellular cAMP levels via Gi/o proteins.
Compartmentalized signaling by the CB1R
Any instructive signal must be spatially restricted to encode positional information. This can be achieved either by limiting the expression, cell-surface presentation and extracellular spread of a ligand or by compartmentalizing ligand-receptor interactions. Neurotrophin receptors, DCC, and probably the CB1R can be clustered by function as “dependence” (or survival) receptors because they create cellular states of dependence towards their cognate ligands by inducing apoptosis if left unoccupied [81]. A common characteristic of these receptors is their association with cholesterol- and sphingolipid-enriched membrane microdomains, that is, lipid rafts, to initiate second messenger signaling [81–83]. Lipid rafts are particularly abundant in regions of high membrane turnover, (e.g. growth cones [84]). They can sequester many kinases, particularly those belonging to the Src family (e.g., Src, Fyn, focal adhesion kinase (Fak)) and are required for axon guidance [82]. The timing and signaling properties of a receptor may be substantially different in raft and non-raft portions of the plasma membrane [82]. Compartmentalized signaling upon focal enrichment of the CB1R in lipid rafts may be particularly efficacious to control growth cone steering decisions by promoting the formation of submembrane signal transduction complexes specialized for fast signal coupling.
THC can hijack physiologically silent CB1Rs
In developing cortical axons, MAGL forms a 2-AG-inactivating barrier in the established axon segment, and can function as a switch-off system’ to terminate 2-AG signaling (Figure 3a) [13]. We postulate that the role of MAGL in elongating axons is to prevent lipophilic 2-AG from accessing and prematurely engaging intracellular CB1R in the axon stem as they undergo axonal transport.
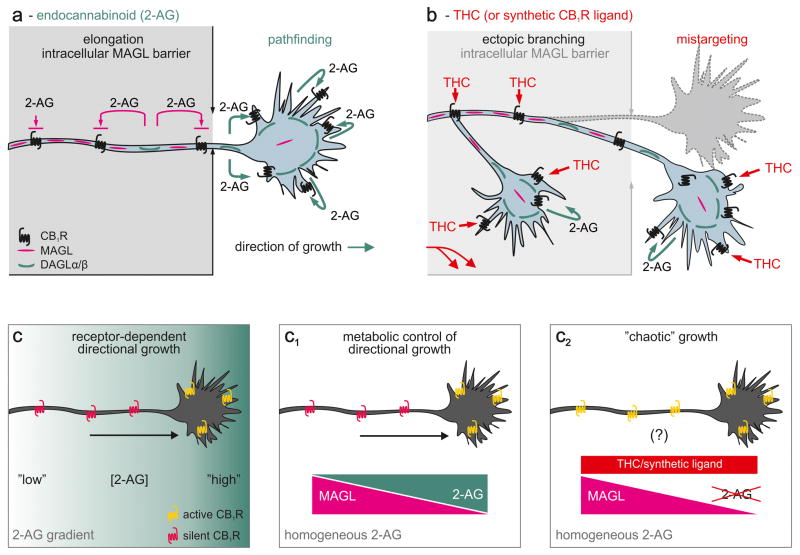
Figure 3. Hypothetical model of phytocannabinoid or cannabinomimetic-induced neuronal wiring defects.
(a) During cortical axonal development, MAGL forms an intracellular enzymatic barrier to prevent 2-AG-driven activation of the CB1R transported along the axon [13]. Thus, we recognize MAGL as a metabolic checkpoint to control 2-AG-dependent formation of axon collaterals. A decrementing MAGL gradient towards the motile growth cone will allow sufficient 2-AG accumulation to activate the CB1R to gain signal competence towards 2-AG, thus impacting growth cone steering decisions. (b) Prenatal exposure to phytocannabinoids, particularly Δ9-tetrahydrocannabinol (THC), or other cannabinomimetics can override this endogenous mechanism since these ligands are resistant to MAGL. Thus, THC can hijack axonal CB1R en route to their signaling positions and induce errant second messenger signaling. (c–c1) An extracellular 2-AG gradient may be dispensable for CB1R-mediated axon guidance. (c) Polarized axonal distribution of the CB1R is sufficient to induce directional growth on an extracellular 2-AG microgradient. (c1) Compartmentalized 2-AG degradation by MAGL within the axon stem will be sufficient to focally restrict 2-AG-induced CB1R activity on a homogeneous 2-AG background. (c2) THC might alter the trajectory of growth and synapse formation by ectopic CB1R activation irrespective of the pattern of 2-AG presentation.
The differential recruitment of DAGLs and MAGL along the axon might serve several independent modes of endocannabinoid signaling, whose outcome - irrespective of the underlying molecular sequence of events–will be neurite outgrowth. The putative molecular cascade of cell-autonomous 2-AG signaling during axonal growth and guidance has recently been reviewed [24]. Here, we discuss whether intercellular communication in the presence or absence of extracellular 2-AG gradients can be equally successful.
The canonical paradigm of axon guidance implies the presentation of chemotropic guidance cues as local gradients. In the developing brain, the expression of DAGLs shows considerable regionalization [13,16,54] suggesting that 2-AG concentrations may substantially vary whilst an axon traverses to innervate its target cell. 2-AG can either be presented by cells lining the path of the axonal trajectories or originate from DAGL+ processes, when multiple neighboring axons extend simultaneously in the process of fasciculation [6,13]. These cellular arrangements can establish a 2-AG gradient “map” to preferentially activate the CB1R within forward-facing filopodia (Figure 3a,c), thus inducing rapid cytoskeletal reorganization within the growth cone. This model suggests that CB1Rs transported along the axon and distal to the chemotropic 2-AG gradient might remain silent even if left unprotected by insufficient 2-AG-degrading activity (MAGL/ABHD6).
Compartmental MAGL localization during axonal growth [13] suggests that an alternative scenario may be physiologically favored. When axons navigate over surfaces that present quasi-homogeneous 2-AG micro-patterns, the enzymatic activity of MAGL might be necessary and sufficient to establish a cell-autonomous 2-AG gradient. This is because the 2-AG concentration within the axonal plasmalemma and intracellularly inversely correlates with the focal 2-AG-degrading capacity of MAGL (Figures 3a,c1). Thus, 2-AG concentrations within the growth cone may be equivalent to that in the immediate microenvironment of the developing axon (Figure 3c1). Inhibition of axonal growth upon disrupting the intracellular gradient of MAGL emphasizes the physiological significance of the above metabolic arrangements [13]. By making extracellular endocannabinoid gradients dispensable, this model reconciles the discrepancy between the limited propensity of endocannabinoids to diffuse over considerable distances and gradient requirements of axonal growth (Figure 3c1).
Unlike 2-AG, THC is not degraded by MAGL. Therefore, prenatal THC exposure can have at least two cellular foci of action: i) it can displace 2-AG from the CB1R in motile growth cones (Figure 3b), thus modifying (or occluding) second messenger signaling to alter directional axonal growth [15], and ii) THC can bypass the axonal MAGL barrier to hijack CB1Rs as they are being trafficked in axons, thus disrupting the spatial specificity of endocannabinoid signaling by activating “silenced” CB1Rs (Figure 3b,c2). Notably, this molecular scenario can also explain how metabolically stable synthetic CB1R antagonists (e.g., SR141716A [16]) can induce axonal defasciculation and mistargeting, and delay synapse formation [12,16] by compromising the spatial and temporal precision of morphogenic endocannabinoid signals. We propose that the cellular basis of THC-induced axonal growth and guidance errors is the indiscriminate activation of CB1Rs otherwise kept muted during the physiological process of neurite outgrowth (Figure 3b).
The molecular fingerprint of prenatal cannabis abuse
If THC affects developmental processes, then its molecular fingerprint must involve developmentally regulated genes. The cumulative complexity of this gene/protein network will be reflective of the relative “power” of THC to affect developmental processes. Global genome and proteome profiling after exposure to THC or CB1R agonists in utero (or during adolescence) increasingly support this notion by identifying a largely invariable cluster of target molecules (Figure 4).
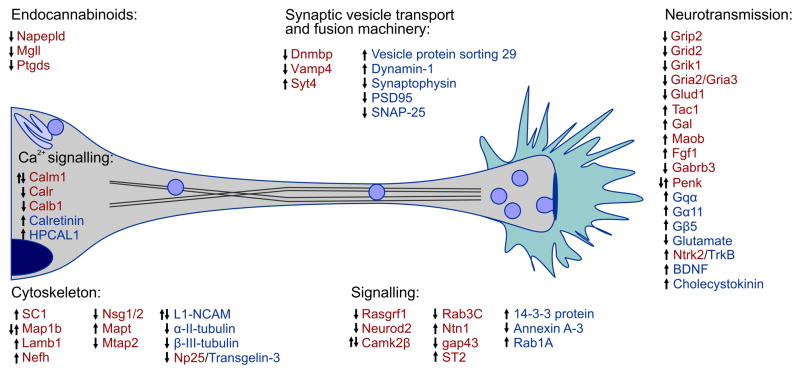
Figure 4. Molecular blueprint of CB1R activation by THC or synthetic agonists in relation to axonal growth and synapse development, deduced from genome and proteome-wide arrays.
Red and blue colors indicate molecular entities identified through genomic [21,22,85–87,89,98–100] and proteomic approaches [14,16,87,88,90,91], respectively. Corresponding gene and protein clusters are indicated. Arrows indicate the direction of regulation upon drug exposure. Double arrows suggest divergent responses due to experimental conditions [21,22], age [89,99], sexual dimorphism [100] or length [89,98] of treatment.
Disrupting the temporal precision of CB1R activity can affect 2-AG degradation by repressing MAGL expression [85,86], reinforcing the functional backbone of our model of THC sensitivity in developing neurons. Cannabinomimetics can up-regulate the expression or enhance the stability of G protein subunits (including Goα1) [87] and growth-associated second messengers [87,88], suggesting altered signal coupling of GPCRs and growth responses, respectively. THC increases the expression of neurotrophins (BDNF [50], Fgf1 [21]) whose signal transduction cascades (e.g., TrkB) can significantly alter CB1R-mediated growth responses [14,50,78]. These changes, together with THC s potency to alter the expression of cell-adhesion molecules (L1-NCAM) [89] and cytoskeletal proteins (β-III-tubulin) [88] essential to maintain neuronal polarity and cell-cell interactions, further emphasize its impact on neuronal morphology. The precise dynamics of Ca2+ signaling in neurons are integral to regulate growth cone steering decisions and to maintain synaptic neurotransmission at mature synapses. Disrupted signaling down-stream from the CB1R changes the expression of Ca2+-binding proteins [22,85,87,89], which act as either intracellular Ca2+ buffers or sensors, members of the presynaptic vesicle trafficking machinery – including proteins implicated in both synaptic vesicle docking/exocytosis (SNAP-25, synaptophysin) [88] and endocytosis (dynamin) [90] – and postsynaptic scaffolding proteins (PSD95; [90], suggest significant modifications to the structural establishment and function of synapses. Glutamatergic (excitatory) neurotransmission may be particularly affected at multiple levels (glutamate metabolism, AMPA receptor subunit expression [21,91]) recapitulating findings from earlier neurophysiology studies linking altered CB1R function to deficits of hippocampal long-term potentiation, memory encoding and glutamate release.
Concluding remarks
Whether THC is an agonist or antagonist at the CB1R during development, when neurons require a high intrinsic endocannabinoid tone to sustain growth processes, is hotly debated [24]. Nevertheless, the concept that THC exerts its detrimental effects by disrupting the temporal and spatial cohesion of endocannabinoid signaling has recently gained significant momentum [12,14,24]. Based on the evidence from cell and systems biology presented herein, we conclude that the functional redundancy of the many endocannabinoid ligands, receptors, metabolic and signaling pathways has evolved to enable endocannabinoids to drive context- and cell state-dependent specification programs in the developing nervous system. This redundancy may be particularly important to prevent developmental defects when the contribution of one or more molecular endocannabinoid components is compromised. Prenatal cannabis exposure can lead to growth defects during formation of the nervous system. The cellular basis of errant neuronal wiring upon cannabis exposure may be due to the ability of THC and related phytocannabinoids to circumvent the spatially precise metabolic control of 2-AG signaling (Figure 3a,b,c2), thus altering positional signaling downstream from cannabinoid receptors. Ectopic CB1R activity, whether on the cell surface or intracellularly (Figure 1), appears to powerfully perturb nervous system patterning and intercellular communication. Understanding the functional significance of molecular changes upon exposure to cannabis in utero or during the adolescent critical period of brain development might not only provide new insights in endocannabinoid functions but also prompt future investigations to decipher the molecular basis of cannabis-induced psychiatric illnesses in affected offspring.
Acknowledgments
The authors thank P. Doherty and R.A. Ross for constructive criticisms and discussions. This work was supported by the Scottish Universities Life Science Alliance (SULSA, TH), Swedish Medical Research Council (TH), European Commission (HEALTH-F2-2007-201159, TH) and National Institutes of Health grants (DA023214 (TH), DA11322 (KM), and DA021696 (KM)).
Glossary
- Cell cycle exit
This term refers to the event when a cell permanently leaves the cell cycle to adopt a terminal differentiation program. To achieve this, cells become refractory to proliferative signals
- Fate decision
The point when a progenitor cell commits towards and initiates an intrinsic specification program to generate a terminally differentiated cell
- Lateral geniculate nucleus
This nucleus resides within the thalamus and functions as the primary relay center for visual information received from the retina of the eye
- Growth cone
A specialized region at the tip of a growing neurite that is responsible for sensing the local environment and for guiding the axon through the transduction of attractive and repulsive extracellular guidance cues toward a target cell
- Intercellular (synaptic) communication
Information exchange between neurons via neurotransmitter release at a specialized junction, the synapse
- Neurite outgrowth
Sequential process including the specification, elongation, and branching of developing axons and dendrites
- Neurogenesis
The process by which neurons are created irrespective of the specific region where these cells are generated or their specific functions within the nervous system
- Progenitor cell
An early descendant of a stem cell that can proliferate and differentiate. A progenitor cell is more limited than a stem cell in the lineages of cells it can generate
- Retrograde signaling
A phenomenon during which a signal molecule travels from the postsynaptic neuron towards the presynaptic one with its direction opposing that of the relevant synaptic neurotransmitter
- Synaptogenesis
The formation of functional synapses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Devane WA, et al. Determination and characterization of a canna-binoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 3.Regehr WG, et al. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 5.Berrendero F, et al. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Wu CS, et al. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32:693–706. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begbie J, et al. Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J Anat. 2004;205:213–218. doi: 10.1111/j.0021-8782.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez O, et al. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia. 2010;58:1913–1927. doi: 10.1002/glia.21061. [DOI] [PubMed] [Google Scholar]
- 9.Morozov YM, et al. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;19(Suppl 1):i78–i89. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncalves MB, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Walker DJ, et al. Down-regulation of diacylglycerol lipase-alpha during neural stem cell differentiation: Identification of elements that regulate transcription. J Neurosci Res. 2010;88:735–745. doi: 10.1002/jnr.22251. [DOI] [PubMed] [Google Scholar]
- 12.Watson S, et al. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci. 2008;38:89–97. doi: 10.1016/j.mcn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Keimpema E, et al. Differential subcellular recruitment of monoacyl-glycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J Neurosci. 2010;30:13992–14007. doi: 10.1523/JNEUROSCI.2126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berghuis P, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berghuis P, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 16.Mulder J, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argaw A, et al. Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance. J Neurosci. 2011;31:1489–1499. doi: 10.1523/JNEUROSCI.4134-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neu-rosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorina Y, et al. Cannabinoid 1 receptor and interleukin-6 receptor together induce integration of protein kinase and transcription factor signaling to trigger neurite outgrowth. J Biol Chem. 2010;285:1358–1370. doi: 10.1074/jbc.M109.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberg KD, et al. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campolongo P, et al. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol. 2007;12:485–495. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 22.Economidou D, et al. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta9-tetrahydrocannabinol. Toxicol Appl Pharmacol. 2007;223:73–85. doi: 10.1016/j.taap.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Trezza V, et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl) 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 24.Harkany T, et al. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbison RD, Mantilla-Plata B. Prenatal toxicity, maternal distribution and placental transfer of tetrahydrocannabinol. J Pharmacol Exp Ther. 1972;180:446–453. [PubMed] [Google Scholar]
- 26.Hutchings DE, et al. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- 27.El Marroun H, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the generation R study. J Am Acad Child Adolesc Psychiatry. 2009;48:1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 28.Hurd YL, et al. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol. 2005;27:221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Goldschmidt L, et al. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 2004;26:521–532. doi: 10.1016/j.ntt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Fried PA, et al. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 32.Willford JA, et al. Effects of prenatal tobacco, alcohol and marijuana exposure on processing speed, visual-motor coordination, and interhemis-pheric transfer. Neurotoxicol Teratol. 2010;32:580–588. doi: 10.1016/j.ntt.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AM, et al. Effects of prenatal marijuana on visuospatial working memory: an fMRI study in young adults. Neurotoxicol Teratol. 2006;28:286–295. doi: 10.1016/j.ntt.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Day NL, et al. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33:129–136. doi: 10.1016/j.ntt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day NL, et al. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–1322. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- 36.Leech SL, et al. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 37.Leech SL, et al. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry. 2006;45:223–230. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- 38.Li L, et al. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron. 2009;64:537–549. doi: 10.1016/j.neuron.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atwood BK, et al. CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.01.066. in press (available online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castaldo P, et al. Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Delta(9)-THC. Pharmacol Res. 2010;61:334–341. doi: 10.1016/j.phrs.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman AF, et al. Opposing actions of chronic {Delta}9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 43.Sugiura T, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 44.Stella N, et al. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 45.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 46.Kano M, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 47.Lourenco J, et al. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–3248. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maccarrone M, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- 49.Mahmud A, et al. Cannabinoid 1 receptor activation inhibits transient receptor potential vanilloid type 1 receptor-mediated cationic influx into rat cultured primary sensory neurons. Neuroscience. 2009;162:1202–1211. doi: 10.1016/j.neuroscience.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Maison P, et al. BDNF regulates neuronal sensitivity to endocanna-binoids. Neurosci Lett. 2009;467:90–94. doi: 10.1016/j.neulet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Roloff AM, et al. Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. J Neurosci. 2010;30:3072–3081. doi: 10.1523/JNEUROSCI.4603-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin M, et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–2347. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labar G, et al. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. Chembiochem. 2010;11:218–227. doi: 10.1002/cbic.200900621. [DOI] [PubMed] [Google Scholar]
- 54.Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanimura A, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Schlosburg JE, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neuros-ci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaczocha M, et al. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem. 2010;285:2796–2806. doi: 10.1074/jbc.M109.058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suarez J, et al. Early maternal deprivation induces changes on the expression of 2-AG biosynthesis and degradation enzymes in neonatal rat hippocampus. Brain Res. 2010;1349:162–173. doi: 10.1016/j.brainres.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 59.Kasai RS, et al. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192:463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rios C, et al. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharma-col. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Jin W, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh C, et al. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- 65.Smith TH, et al. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol. 2010;160:454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niehaus JL, et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- 67.Gomes I, et al. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scrima M, et al. Binding of the hemopressin peptide to the cannabinoid CB(1) receptor: structural insights. Biochemistry. 2010;49:10449–10457. doi: 10.1021/bi1011833. [DOI] [PubMed] [Google Scholar]
- 69.Dodd GT, et al. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci. 2010;30:7369–7376. doi: 10.1523/JNEUROSCI.5455-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, et al. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909–915. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Oudin MJ, et al. Endocannabinoids regulte the migration of subventricular zone-derived neuroblasts in the post-natal brain. J Neurosci. 2011;31:4000–4011. doi: 10.1523/JNEUROSCI.5483-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vitalis T, et al. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur J Neurosci. 2008;28:1705–1718. doi: 10.1111/j.1460-9568.2008.06484.x. [DOI] [PubMed] [Google Scholar]
- 73.Trazzi S, et al. CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3beta/beta-catenin signaling. J Biol Chem. 2010;285:10098–10109. doi: 10.1074/jbc.M109.043711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozaita A, et al. Regulation of PI3K/Akt/GSK-3 pathway by cannabi-noids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 75.Asimaki O, Mangoura D. Cannabinoid receptor 1 induces a biphasic ERK activation via multiprotein signaling complex formation of proximal kinases PKCvarepsilon, Src, and Fyn in primary neurons. Neuro-chem Int. 2011;58:135–144. doi: 10.1016/j.neuint.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Derkinderen P, et al. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He JC, et al. The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J Biol Chem. 2005;280:33426–33434. doi: 10.1074/jbc.M502812200. [DOI] [PubMed] [Google Scholar]
- 78.Williams EJ, et al. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hatta T, et al. The Role of gp130 in cerebral cortical development: in vivo functional analysis in a mouse exo utero system. J Neurosci. 2002;22:5516–5524. doi: 10.1523/JNEUROSCI.22-13-05516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furne C, et al. Netrin-1 is a survival factor during commissural neuron navigation. Proc Natl Acad Sci U S A. 2008;105:14465–14470. doi: 10.1073/pnas.0803645105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petrie RJ, et al. Compartmentalized DCC signalling is distinct from DCC localized to lipid rafts. Biol Cell. 2009;101:77–90. doi: 10.1042/BC20070108. [DOI] [PubMed] [Google Scholar]
- 83.Rimmerman N, et al. Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line. Br J Pharmacol. 2008;153:380–389. doi: 10.1038/sj.bjp.0707561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guirland C, et al. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 85.Parmentier-Batteur S, et al. DNA microarray analysis of cannabinoid signaling in mouse brain in vivo. Mol Pharmacol. 2002;62:828–835. doi: 10.1124/mol.62.4.828. [DOI] [PubMed] [Google Scholar]
- 86.Khare M, et al. Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Mol Hum Reprod. 2006;12:321–333. doi: 10.1093/molehr/gal036. [DOI] [PubMed] [Google Scholar]
- 87.Colombo G, et al. Transcriptomic and proteomic analyses of mouse cerebellum reveals alterations in RasGRF1 expression following in vivo chronic treatment with delta 9-tetrahydrocannabinol. J Mol Neurosci. 2009;37:111–122. doi: 10.1007/s12031-008-9114-2. [DOI] [PubMed] [Google Scholar]
- 88.Quinn HR, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsy-chopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 89.Kittler JT, et al. Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics. 2000;3:175–185. doi: 10.1152/physiolgenomics.2000.3.3.175. [DOI] [PubMed] [Google Scholar]
- 90.Rubino T, et al. Changes in hippocampal morphology and neurop-lasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 91.Suarez I, et al. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and peri-natal delta9-tetrahydrocannabinol exposure. Cerebellum. 2004;3:66–74. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- 92.Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst. 2010;6:1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, et al. Expression and secretion of N-acylethanolamine-hydrolysing acid amidase in human prostate cancer cells. J Biochem. 2008;144:685–690. doi: 10.1093/jb/mvn122. [DOI] [PubMed] [Google Scholar]
- 95.Heimann AS, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martini L, et al. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 97.Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 98.Grigorenko E, et al. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids. 2002;121:257–266. doi: 10.1016/s0009-3084(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 99.Gomez M, et al. The activation of cannabinoid receptors during early postnatal development reduces the expression of cell adhesion molecule L1 in the rat brain. Brain Res. 2007;1145:48–55. doi: 10.1016/j.brainres.2007.01.102. [DOI] [PubMed] [Google Scholar]
- 100.Perez-Rosado A, et al. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Res Dev Brain Res. 2000;120:77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]