Abstract
Medial frontal event-related potentials (ERPs) following rewarding feedback index outcome evaluation. The majority of studies examining the feedback related medial frontal negativity (MFN) employ active tasks during which participants’ responses impact their feedback, however, the MFN has been elicited during passive tasks. Many of the studies examining the MFN show enhanced effects when an error in reward prediction occurs (i.e. expected rewards are not delivered). To clarify the roles of reward prediction error and active responding in producing the MFN, the current study employed a reward prediction design with active and passive task blocks. Following the presentation of a reward predictor, participants (active task) or the computer (passive task) indicated whether participants would receive the outcome associated with a stimulus presented on the left or right of the reward predictor. The MFN was largest when the trial outcome was worse than predicted and that this effect was enhanced when the participant, rather than the computer, made the choice. These results show that both reward prediction error and active choice impact the neural system of outcome monitoring with the largest MFN when the individual’s decision led to the negative outcome.
Evaluating the potential positive and negative outcomes associated with individual choices is a critical aspect of the decision-making process. The neural reward system assesses, updates, and maintains reward values based on current and prior decisions and outcomes. Medial frontal event-related potentials (ERPs), including the error related negativity (ERN) and the feedback related negativity (FRN) components index reward-related neural activity associated with task performance and outcome monitoring. Known generically as the medial frontal negativity (MFN), these ERP components index how “good” or “bad” an action or outcome is within a given context (Hajcak, Moser, Holroyd, & Simons, 2006; Nieuwenhuis, Holroyd, Mol, & Coles, 2004). If sufficient information is present for evaluation at the time of the behavioral response, then an ERN occurs to that response, but if performance feedback is required to evaluate the action outcome, then an FRN occurs to the feedback stimulus (Nieuwenhuis, Holroyd et al., 2004).
A prominent theory of the MFN proposes that these components reflect neural activity associated with reinforcement learning (Holroyd & Coles, 2002; Nieuwenhuis, Holroyd et al., 2004) and outcome evaluation (Gehring & Willoughby, 2002). The reinforcement learning theory posits that the dopaminergic neurons of the ventral tegmental area, which show enhanced firing to unpredicted rewards and suppressed firing when a predicted reward is not delivered (Schultz, Dayan, & Montague, 1997), deliver a ‘learning signal’ to the anterior cingulate cortex (ACC) when the outcome of a choice or action is worse than expected (Holroyd & Coles, 2002). The feedback related MFN has a medial frontal scalp distribution, above the ACC, and has been localized to ventral areas of the ACC using source analysis (Nieuwenhuis, Slagter, Alting von Geusau, Heslenfeld, & Holroyd, 2005; Taylor et al., 2006).
Unlike the response related MFN, behavioral errors and active responses are not necessary to elicit a feedback related MFN (Gehring & Willoughby, 2002). Gehring & Willoughby (2002) demonstrated that negative outcomes in a choice alternative task elicited an MFN even when that choice was not an explicit error. ‘Slot machine’-like tasks also elicit an MFN that is largest when unfavorable outcomes occur, especially when those outcomes are unexpected, even in the absence of a choice or action by the participant (Donkers, Nieuwenhuis, & van Boxtel, 2005; Martin & Potts, 2004; Potts, Martin, Burton, & Montague, 2006). Although passive tasks can elicit an MFN, the MFN is generally larger during active tasks, implying that the outcome monitoring system is differentially engaged by the individual’s actions. Yeung, Holroyd, and Coles (2005) used a four-choice forced alternative design in which either the participant or the computer made the choice that resulted in a monetary gain or loss. An MFN was elicited to choices that resulted in loss whether the choice was made by the participant or by the computer, but the MFN was larger when the participant made the choice. However, unlike the passive ‘slot-machine’ tasks (Donkers et al., 2005; Martin & Potts, 2004; Potts et al., 2006), the design used by Yeung, Holroyd, and Coles (2005) did not manipulate reward prediction probability; the reward probability associated with each choice was the same, thus the relative impact of reward prediction error and active choice on the outcome monitoring system could not be assessed. Given that the MFN is amplified when outcomes are worse than predicted (Holroyd & Coles, 2002) and during active compared to passive tasks (Yeung et al., 2005), the current study sought to investigate the impact of both reward prediction violation and active choice on the neural system of outcome monitoring using the MFN.
In the current study, reward predicting and outcome delivering stimuli were presented in active and passive choice blocks to determine if reward prediction violation would fully determine MFN amplitude or if active choice in a probabilistic reward context would modulate the MFN. A main effect of reward prediction (expected vs. unexpected outcome) but not task (active vs. passive task) on the MFN would indicate that reward prediction error drives the neural outcome monitoring system to a greater degree than the impact of the participant’s choice on that outcome. On the other hand, a main effect of task but not reward prediction violation would indicate that the outcome monitoring system is more devoted to evaluating the individual’s actions than to reward prediction error. However, if reward prediction and task interact on the MFN then the results would indicate that the relationship between reward prediction error and choice outcome evaluation is complex and both aspects should be taken into account when developing models of behavior monitoring in the brain.
Materials and Methods
Participants
Twenty (5 female) Rice University undergraduate psychology students (ages 18–22) participated for course credit. Five participants (1 female) were excluded from the ERP analysis due to excessive eye movement and eye blink artifact. Excessive artifact was defined as fewer than 20 artifact-free trials per condition. All participants provided informed consent prior to participation.
Task and Stimuli
A reward prediction S1/S2 paradigm based on Martin and Potts (2004) and Potts et al. (2006) was modified to incorporate both active and passive responses. Stimuli were images of lemons (associated with no reward value) and gold bars (associated with rewards of $0.25). Each trial consisted of a lemon or gold bar (S1) presented at the center of the screen, a choice (left or right) made by the participant’s keypress (active blocks) or by the computer (passive blocks), followed by two stimuli (S2) presented to the left and right of S1. The participant received the outcome associated with the selected S2.
S1 predicted reward delivery with 75% accuracy. For example, if S1 was a lemon 75% of the time the selected S2 was also a lemon and, as predicted, no reward was delivered. If S1 was a gold bar then 75% of the time the selected S2 was also a gold bar and, as predicted, a reward was delivered. However, on 25% of the trials S1 did not match the selected S2 and unpredicted outcomes occurred. When S1 was a lemon and the selected S2 was a gold bar, an unpredicted reward was delivered. On the other hand, when S1 was a gold bar and S2 was a lemon, the predicted reward was not delivered. Two-thirds of the trials on which predicted outcomes were delivered consisted of congruent S2 stimuli, i.e. the S2 stimuli on the left and right of S1 both matched S1. The remaining predicted outcome trials and all the unpredicted outcome trials consisted of incongruent S2 stimuli on which only one of the S2 stimuli matched S1. Incongruent stimuli were used to increase participants’ levels of engagement on the task by showing that the alternative choice would have resulted in the opposite outcome.
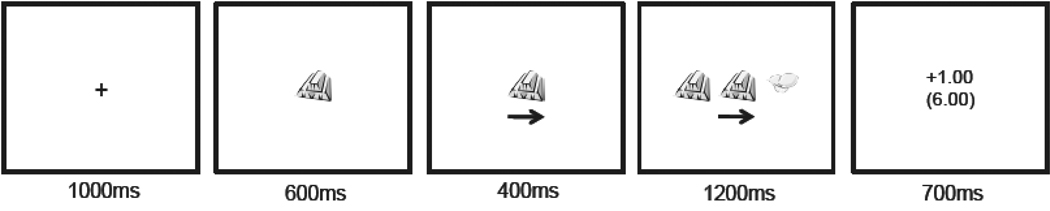
The experiment consisted of eight blocks with 100 trials per block. Four of the blocks were participant choice (active blocks) and four were computer choice (passive blocks). The experiment alternated between active and passive blocks with the order counterbalanced such that half the participants began with an active block followed by a passive block and the other half began with a passive block followed by an active block. Participants began with $0 in their bankroll and $0.25 was added each time a reward was delivered. At the end of the experiment participants were paid the total from one of the eight experimental blocks based on a random draw. Overall participants earned between $9.25 and $14.75. Figure 1 illustrates an example trial.
Figure 1.
An example trial illustrating when an unpredicted no reward trial (i.e. the expected reward was not delivered).
Stimulus presentation and behavioral response collection
Stimulus presentation and behavioral response collection was controlled by E-Prime 1.0 (PST, Pittsburgh). Visual stimuli were presented on an Apple 15" flat-panel active matrix Studio display (Apple Computer, Cupertino, CA). Manual responses were collected with a 4-key microswitch keypad (Electrical Geodesics, Inc., Eugene, OR). Participants sat in an adjustable chair, adjusted for comfort, with their chin in a chinrest placed so that participants’ eyes were approximately 50 cm from the center of the flatpanel screen. Participants were instructed to remain as still as possible, with their eyes on the fixation mark. Breaks were provided after each block.
EEG Data Acquisition and ERP Data Analyses
EEG data were acquired continuously with a 128 channel Electrical Geodesics system (Electrical Geodesics, Inc., Eugene) referenced to the vertex with .1 – 100 Hz analog filtering and digitized at 250 Hz. EEG data were digitally lowpass filtered at 20 Hz and segmented into 1000 ms epochs spanning 200 ms before to 800 ms after the S2 stimulus, which indicated whether or not a reward would be delivered. Data were digitally screened for artifact (eye blinks or movements, subject movement, or transient electronic artifact) and the remaining data were sorted by condition and averaged to create the ERPs. Averaged ERP data were baseline corrected over the first 200 ms of the epoch. The baseline was chosen to be one-fifth of the epoch length (Handy, 2004). Data were then rereferenced into an average reference representation (Dien, 1998). The subject-averaged ERPs were averaged together to produce mean waveforms across participants.
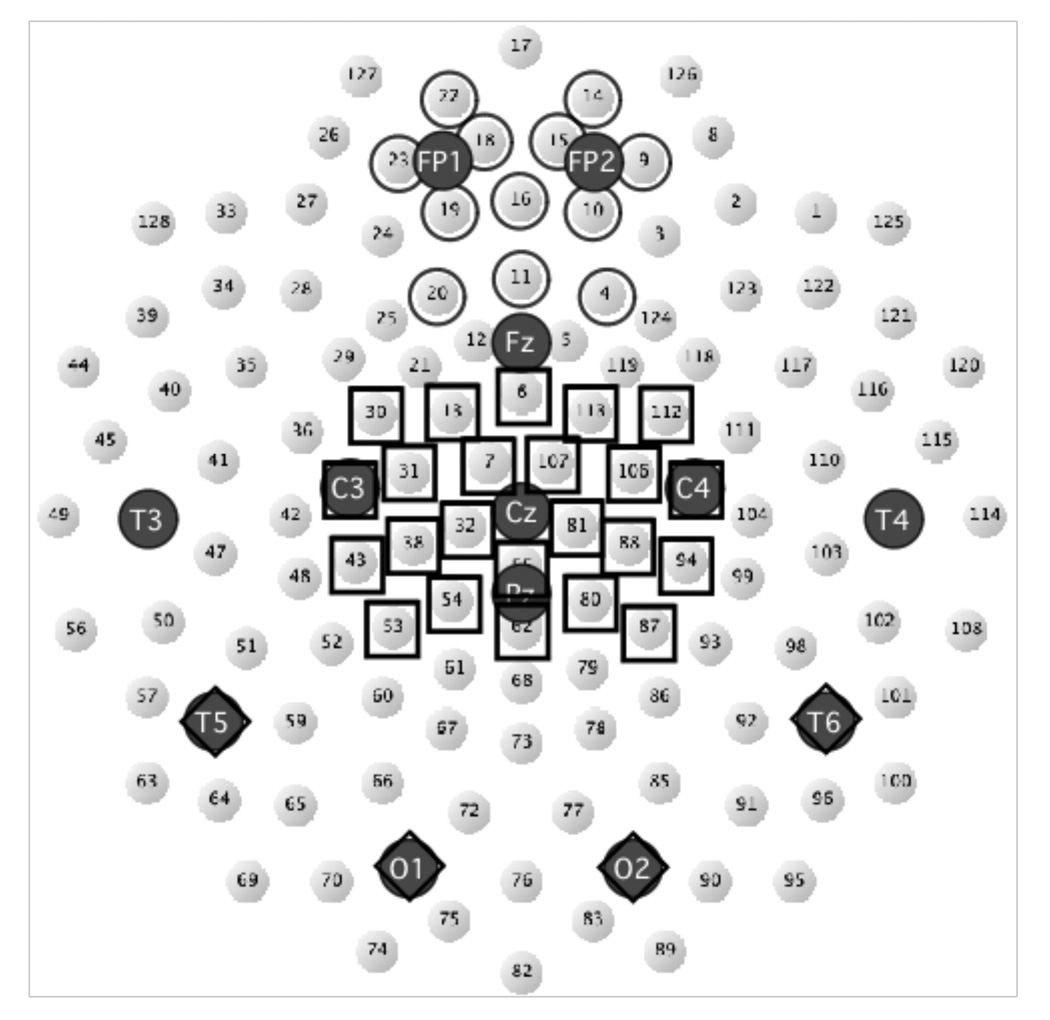
The current analysis reduced the dimensionality of the Electrode factor (with potentially 129 levels) by using region of interest (ROI) electrode averages, one frontal for the MFN and one central for a P3 analysis. The frontal ROI and latency were based on the spatio-temporal distribution of MFN in the current data, determined by visual inspection of the waveform plots and scalp field topographic maps and previous studies examining the MFN using a similar reward prediction task (Martin & Potts, 2004; Potts et al., 2006). Electrodes included in the frontal ROI were centered around FPz (EGI electrodes: 4, 9, 10, 11, 14, 15, 16, 18, 19, 20, 22, and 23; see Figure 2). The MFN was extracted by calculating the average voltage across the 12 frontal electrodes from a temporal window 250–325 ms after S2 (the reward delivering stimulus) onset. Analyses were performed on both unsubtracted and difference waveforms. Following Holroyd and Krigolson (2007), difference waves were computed to isolate the effects of reward probability by creating an Unexpected difference wave (Unpredicted No Reward – Unpredicted Reward) and an Expected difference wave (Predicted No Reward – Predicted Reward).
Figure 2.
Map of the 128 channel electrode net with the 10/20 system locations marked. Electrodes included in the Frontal (MFN) ROI are marked with circles, the centro-parietal (P3) ROI with squares.
Due to the anterior distribution of the MFN in the current study, source analysis was done to isolate the neural responses during the active task when outcomes were worse than expected (i.e. expected reward was not delivered). Source dipoles were estimated using two symmetrical regional sources in the BESA program (MEGIS Software GmbH, Germany). Source modeling used a 4-shell ellipsoid head model.
A preliminary analysis employing a 2 × 2 Congruency (Congruent, Incongruent) × Task (Active, Passive) repeated measures ANOVA revealed no effect of congruency, i.e. there was no significant difference between expected outcomes when the alternative choice would have resulted in an unexpected outcome compared to expected outcome, p=.58, neither did Congruency interact with Task, p=.67, therefore congruent and incongruent trials were collapsed together. A 2 × 2 × 2 repeated measures ANOVA, Prediction (Unexpected, Expected) × Outcome (Reward, No Reward) × Task (Active, Passive), was conducted to examine the unsubtracted MFN effects. A 2 × 2 repeated measures ANOVA, Prediction (Unexpected, Expected) × Task (Active, Passive), was conducted to examine the difference wave MFN effects. Within task effects were analyzed using a 2 × 2 (Prediction × Outcome) repeated measures ANOVA for the unsubtracted waveforms and a one-way ANOVA for the difference waves.
In addition, the P3 was analyzed over a centro-parietal ROI from 290–550ms. Electrodes included in the P3 ROI were centered around Cz and Pz (EGI electrodes 6, 7, 13, 30, 31, 32, 37, 38, 43, 53, 54, 55, 62, 80, 81, 87, 88, 94, 105, 106, 107, 112, 113). A 2 × 2 × 2 repeated measures ANOVA including Prediction (Unexpected, Expected), Outcome (Reward, No Reward) and Task (Active, Passive) was employed to examine P3 effects of both reward prediction and reward outcome.
Results
MFN
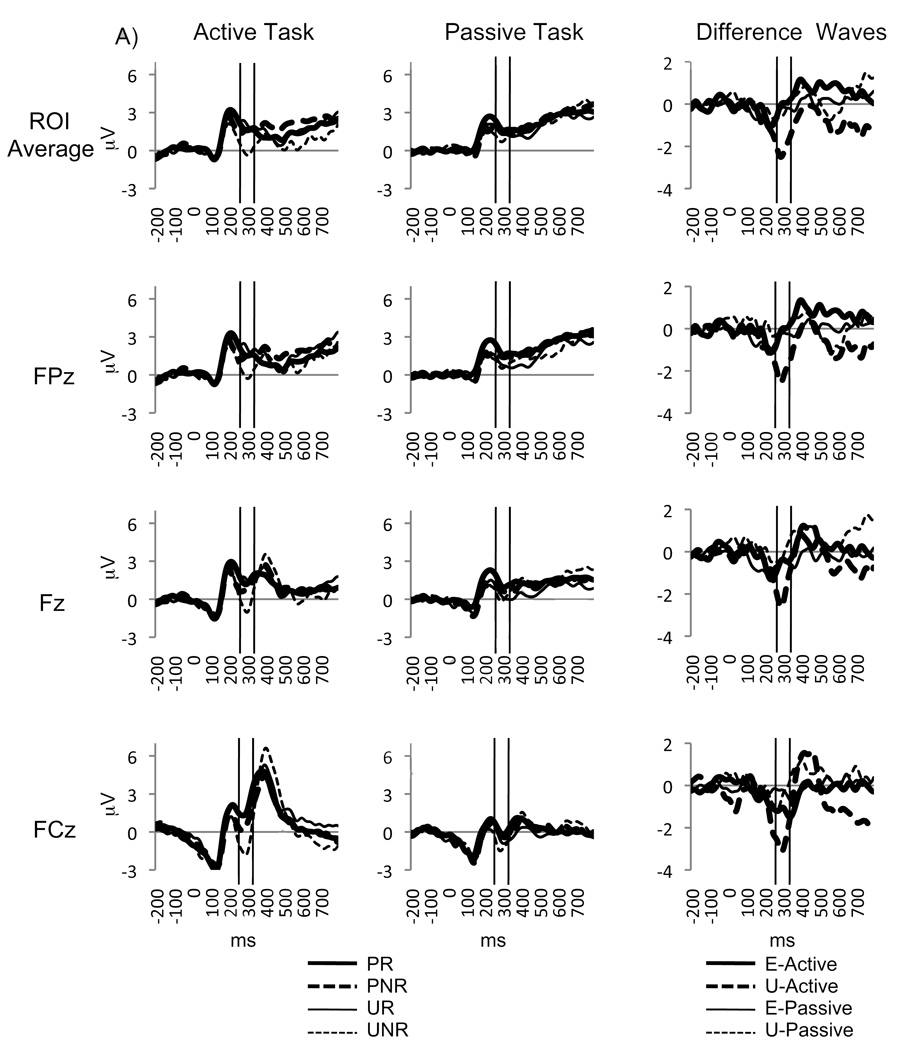
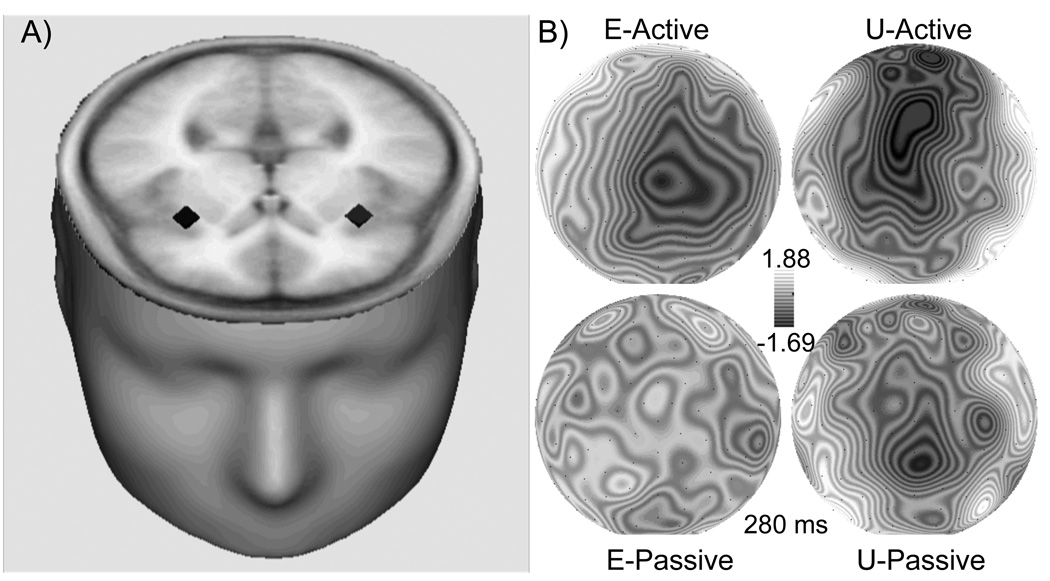
The unsubtracted MFN showed a main effect of Prediction, F(1, 14) = 5.538, p <.05, with a larger MFN when outcomes were unexpected compared to expected. In addition a main effect of Outcome, F(1, 14) = 10.292, p < .01, showed a larger MFN when no rewards were delivered compared to when rewards were delivered. An interaction between Prediction and Outcome, F(1, 14) = 4.606, p < .05, showed the largest MFN when outcomes were worse than expected and no MFN when outcomes were better than expected. Furthermore, an interaction between Prediction, Outcome, and Task, F(1, 14) = 4.765, p < .05, revealed that active responses enhanced the MFN to worse than predicted outcomes. A within task analyses of the active task showed a main effect of Outcome, F(1, 14) = 9.727, p < .01, and a Prediction × Outcome interaction F(1, 14) = 7.079, p < .05, in which the largest amplitudes were found when outcomes were worse than expected (i.e. expected rewards were not delivered) and smallest amplitudes when outcomes were better than expected (i.e. unexpected rewards were delivered). On the other hand, within task analyses of the passive task showed a trend towards a main effect of Prediction, with larger amplitudes when outcomes were unexpected, F (1, 14) = 4.195, p = .06, but did not show an interaction with Outcome (see Figure 3a). Source modeling of the active task revealed an estimated bilateral source in the inferior frontal gyrus (IFG, xyz: 32, 23, 11 and −32, 23, 11; see Figure 4a).
Figure 3.
A) Unsubtracted MFN waveform plots averaged across the ROI and electrodes FPz, Fz, and FCz during the active and passive tasks depicting the following conditions: predicted reward (PR), predicted no reward (PNR), unpredicted reward (UR) and unpredicted no reward (UNR) for the active and Passive tasks. B) MFN difference waves for Unexpected (U; Unpredicted No Reward – Unpredicted Reward) and Expected (E; Predicted No Reward – Predicted Reward) during the active and passive tasks.
Figure 4.
A) Regional source dipoles for the unpredicted no reward (UNR) condition during the active task accounting for 87% of the variance (xyz: 32, 23, 11 and −32, 23). B) Topographic voltage maps displayed with the nose at the top showing (280 ms) of the MFN difference waves for Unexpected (U; Unpredicted No Reward – Unpredicted Reward) and Expected (E; Predicted No Reward – Predicted Reward) during the active and passive tasks.
The MFN difference wave effects were consistent with the unsubtracted waveform results showing a main effect of Prediction, F(1, 14) = 4.606, p < .05, with a larger MFN for unexpected compared to expected difference waves (see Figure 4) and a Prediction × Task interaction, F(1, 14) = 4.765, p < .05, showing enhancement of the Prediction effect in the active task. A within task analyses for the active task showed that the difference MFN was larger for unexpected than expected outcomes F(1, 14) = 7.079, p < .05. However, no significant differences were found between expected and unexpected outcomes in the passive task p = .7 (see Figure 3b). A topographic voltage map of the scalp field distribution at 280 ms following outcome delivery shows the MFN with a fronto-central scalp distribution in the Unexpected subtraction in the Active task only (See Figure 4b). Interactions with electrode site (i.e. FPz, Fz, FCz) were also examined for the MFN difference waveforms (see Figure 3). These analyses indicated that there was no main effect for Electrode (p = .13), no Task × Electrode interaction (p = .58), no Prediction × Electrode interaction (p = .78), and no Task × Prediction × Electrode interaction (p = .69).
P3
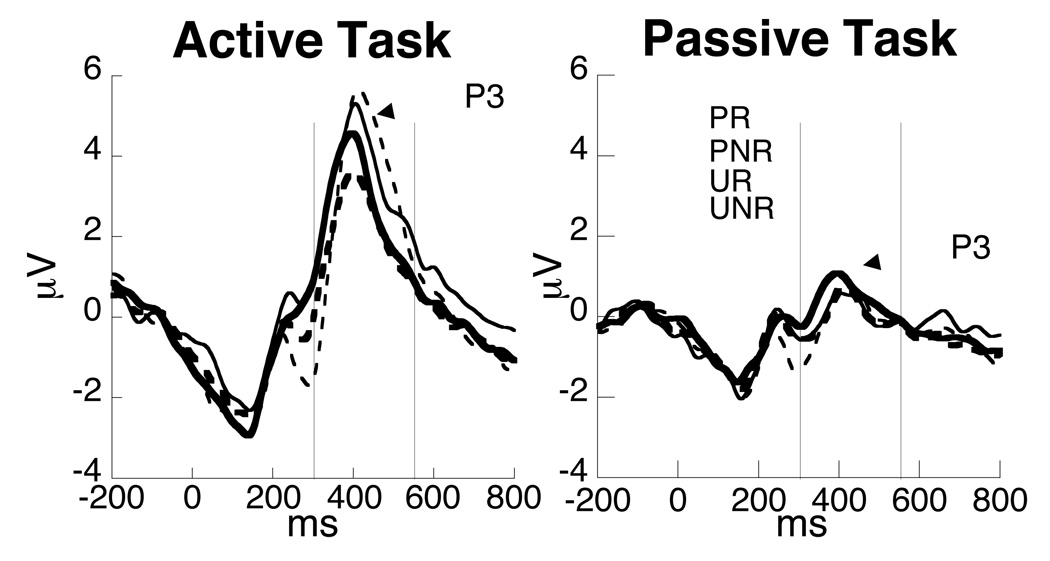
The P3 (Figure 5) was larger in the active compared to the passive task, F(1, 14) = 33.161, p<.001. The P3 was larger when rewards were delivered compared to when rewards were not delivered, F(1, 14) = 4.566, p < .05. The P3 showed a pattern in which amplitudes were larger when outcomes were unexpected, however this difference was not significant, p = .20. In addition, a Prediction × Task interaction, F(1, 14)= 7.011, p<.05 was found. Follow-up analysis of the Active task showed a main effect of Prediction, F(1, 14)=6.159, p<.05, revealing more positive amplitudes when outcomes were unexpected compared to when outcomes were expected, an effect not found for the passive task. No interactions were found between Prediction and Outcome or Outcome and Task, p > .35.
Figure 5.
P3 waveform plots for the active and passive tasks showing the predicted reward (PR), predicted no reward (PNR), unpredicted reward (UR) and unpredicted no reward (UNR) for the active and Passive tasks.
Discussion
The current study examined the influence of active responses on the neural system of outcome monitoring using the MFN in a design that included explicit reward prediction violation. The current results are consistent with previous studies that show the largest MFN amplitudes when outcomes are worse than expected (Martin & Potts, 2004; Potts et al., 2006), a result found during the active task in the current study. This MFN response pattern is similar to the response pattern shown by dopamine neurons in the ventral tegmental area (VTA) in animal studies (Schultz, Dayan, & Montague, 1997) in which VTA neurons show an increase in firing when unexpected rewards are delivered and a decrease in firing when expected rewards are not delivered. Thus the current results from the active task are consistent with the model of the MFN as an index of reward prediction error (Holroyd & Coles, 2002; Nieuwenhuis, Holroyd et al., 2004) in that deviations from expected outcomes resulted in enhanced MFN amplitude. The current results are also consistent with a recent study by Moser and Simons (2009), which showed the largest MFN when participants first predicted they would win and then changed their minds before they received feedback and predicted that they would lose. Moser and Simons (2009) proposed that the MFN was modulated by feelings of regret on the participant’s part because they had changed their mind and then received an unexpected loss. Although the current study cannot directly test the amount of regret participants may feel when playing a game of chance during which they have no control over whether they win or lose, participants most likely experience more negative feelings when they are explicitly told by the preceding stimulus (a gold bar) that they will win, and then they unexpectedly lose. In the current study this disappointment appears to be greater during the active task when participants may feel they have more control over the outcomes.
In contrast to the negative outcome feedback model of the MFN, Oliveira et al. (2007) proposed that that the MFN indexes expectancy violation rather than negative feedback, demonstrated by their finding that an MFN was elicited when participants received positive “correct” feedback after they had made an error. The current findings are not consistent with this hypothesis in that unexpected S2 stimulus was associated with a larger MFN only when the outcome was negative; the MFN was not enhanced when S2 delivered an unexpected positive outcome. However in Oliveria et al. (2007) the unexpected positive stimulus that elicited an MFN was false performance feedback, feedback that was itself an error, while in the current study the unexpected positive stimulus was a reward that violated expectation instantiated by S1 but was not in itself erroneous. Thus Oliveria et al., demonstrated that a positive, but invalid, performance feedback elicited an MFN while the current study showed that an unexpected positive outcome which did not provide an invalid assessment of performance accuracy did not produce an MFN. Therefore, it appears the unexpected feedback does not always elicit an MFN but feedback following behavioral errors does, even when that feedback is inaccurate, although further studies are needed to clarify the relationships between the MFN elicited by behavior accuracy and choice outcome feedback and that to reward prediction violations.
Consistent with Yeung et al. (2005), the current results indicate that active participant responses enhance the MFN. The current study failed to show a significant MFN effect in the absence of participant response (i.e. the passive task) despite previous studies which demonstrated an MFN to negative outcomes during passive tasks (Martin & Potts, 2004; Potts et al., 2006; Yeung et al., 2005). Although not statistically significant, the current waveforms do show a clear negative deflection during the passive task when the outcome was worse than expected, supported by a trend in the unsubtracted waveforms showing larger MFN to unexpected outcomes. These results indicate that prediction error may drive effects seen in passive tasks, whereas the interaction between prediction error and reward delivery indicates stronger involvement of outcome monitoring when the participant’s choices are (apparently) determining the outcome.
The failure to show a significant difference in the difference waveforms during the passive task in the current study may be due to the alternation between active and passive trials. Unlike Yeung et al. (2005) in which all the passive trials were presented contiguously, separated from all the active trials, the current study intermixed blocks of active and passive trials. During debriefing, some participants in the current study reported “boredom” during the passive blocks. The brief duration of an individual passive block and the knowledge that an active choice block was coming soon reduced engagement with the passive task. Thus task engagement may be crucial for producing significant MFN effects, and the level of task engagement is relative to the nature of the task.
Previous studies have manipulated task engagement and shown that participant’s level of engagement can impact the MFN. An MFN is elicited when observing someone else’s feedback that is relevant to oneself during a cooperative or antagonistic task (Itagaki & Katayama, 2008). Yu and Zhou (2006) also found an MFN response when participants received feedback from someone else’s performance, suggesting that individuals can be engaged by the actions of another, however that MFN was smaller than when they received feedback on their own performance (Yu & Zhou, 2006). Yeung et al. (2005) reported that MFN amplitude was positively correlated with participants’ ratings of task involvement, and that participants reported higher levels of engagement when they felt that their responses had an impact on the outcome (active compared to passive tasks).
Similarly, a study by Holroyd et al (2009) tested the impact that learning reward associations had on the MFN. This study showed that the MFN is larger when stimulus-response contingencies are learnable, i.e. when the participant can impact the outcome by modifying their behavior. While the responses in the current study did not actually impact reward outcome, the participants’ perceived connection between their choice and the trial outcome in the active task may have placed additional demand on the outcome monitoring system, enhancing the MFN, whereas the possible perceived lack of control in the passive task reduced this monitoring demand. Overall the current results indicate greatest involvement of the outcome monitoring system in reward expectation violation when there is a perception of some degree of control over the outcome.
The P3 results also suggested a lack of task engagement in the passive condition. Similar to the current study, Yeung et al. (2005) found that the P3 was affected by participants’ self-reported interest in the task, being larger during the more interesting active task compared to the less interesting passive task. The P3 in the current study was also larger during the active, compared to the passive task. Although, the current study did not employ a formal self-report measure of task engagement, several participant reports during unstructured debriefing described the passive task as “boring”, consistent with the P3 as an index of participants’ levels of task engagement.
Although Nieuwenhuis et al (2004), found similar topographies for the MFN when feedback indicated that participants had won or lost money and when feedback indicated that participants had made the “correct” or “error” response, the MFN in the current study was more anterior (around FPz) than is usually reported (around FCz). MFNs are elicited in two primary types of design: monetarily motivated ‘gambling’-like designs where participants win or lose money, and performance feedback designs, where participants receive feedback as to the correctness of their response in a task. The MFN in the current study is consistent with the more anterior topography as reported by Gehring and Willoughby in a gambling-like design (Gehring & Willoughby, 2004). A recent principal component analysis of the error-elicited ERN and the reward prediction violation elicited feedback related MFN demonstrating that, unlike the response related MFN, the reward prediction violation MFN is composed of a central component, perhaps more related to the error related MFN and error detection, and a prefrontal component, possibly more reflecting the updating of goal representations (Potts, Martin, Kamp, & Donchin, In Press). The more anterior topography and the IFG source for the MFN in the current study may reflect greater engagement of this more frontal MFN subcomponent indexing the goal updating operation. The active task, in which that participant’s choice can (supposedly) influence the outcome, shows larger differences between unexpected outcomes, outcomes that would require updating of the representations linking actions to goals when there was an unexpected outcome. Recent functional magnetic resonance imaging (fMRI) studies show increased activation of the IFG when the delivery of rewards changed unexpectedly or reversed to punishments (d'Acremont, Lu, Li, Van der Linden, & Bechara, 2009; Greening, Finger, & Mitchell, 2010). Moreover, the IFG has been implicated in behavior inhibition (Aron, Robbins, & Poldrack, 2004). In light of the current results, the MFN with a source in the IFG may be more robust during the Active task compared to the Passive task as participants plan their future actions and try to avoid future unexpected outcomes.
Additionally, the current study used lateralized stimuli, and the EEG data were acquired with a Geodesic Sensor Net that contains a number of anterior inferior recording sites not found in most recording arrays, factors which may have influenced the topographic distribution of the MFN. A limitation of the current study is that the level of task engagement was not directly assessed, thus the influence of engagement on the MFN when reward prediction error is manipulated is speculative. Future studies are needed to fully address the issues associated with employing both active and passive response tasks during a within subject research design. Despite this limitation, the current study demonstrates that the MFN response is consistent with an index of a neural system of outcome monitoring that receives input from the ventral tegmental reward prediction area but that this system is differentially engaged depending on the kind of task (active or passive). This may guide future studies exploring how reward prediction violation is employed in the brain in the ongoing monitoring of behavior efficacy.
Highlights.
> The MFN was examined during an active and passive reward prediction task. > The MFN was largest when expected rewards were not delivered. > The MFN response was enhanced during the active task. > Both reward prediction error and active choice impact outcome monitoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. TRENDS in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- d'Acremont M, Lu Z-L, Li X, Van der Linden M, Bechara A. Neural correlates of risk prediction error during reinforcement learning in humans. NeuroImage. 2009;47:1929–1939. doi: 10.1016/j.neuroimage.2009.04.096. [DOI] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behavior Research Methods, Instruments, & Computers. 1998;30(1):34–43. [Google Scholar]
- Donkers FCL, Nieuwenhuis S, van Boxtel GJM. Mediofrontal negativities in the absence of responding. Cognitive Brain Research. 2005;25:777–787. doi: 10.1016/j.cogbrainres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain. Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; 2004. pp. 14–20. [Google Scholar]
- Greening S, Finger E, Mitchell D. Parsing decision making processes in prefrontal cortex: Response inhibition, overcoming learned avoidance, and reversal learning. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71:148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Handy T. Basic Principles of ERP Quantification. In: Handy T, editor. Event-Related Potentials A Methods Handbook. MIT Press; 2004. pp. 33–55. [Google Scholar]
- Holroyd C, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C, Krigolson O. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44:913–917. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Holroyd C, Krigolson O, Baker R, Lee S, Gibson J. When is an error not a prediction error? An electrophysiological investigation. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):59–70. doi: 10.3758/CABN.9.1.59. [DOI] [PubMed] [Google Scholar]
- Itagaki S, Katayama J. Self-relevant criteria determine the evaluation of outcomes induced by others. NeuroReport. 2008;19(3):383–387. doi: 10.1097/WNR.0b013e3282f556e8. [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. NeuroReport. 2004;15(9):1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- Moser J, Simons R. The neural consequences of flip-flopping: The feedback-related negativity and salience of reward prediction. Psychophysiology. 2009;46:313–320. doi: 10.1111/j.1469-8986.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MGH. Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neuroscience & Biobehavioral Reviews. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter H, Alting von Geusau N, Heslenfeld D, Holroyd CB. Knowing good from bad: Differential activation of human cortical areas and negative outcomes. European Journal of Neuroscience. 2005;21:3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cerebral Cortex. 2004;14:741–747. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Oliveira F, McDonald J, Goodman D. Performance monitoring in the anteiror cingulate is not all error related: Expectancy deviation and the representation of action-outcome associations. Journal of Cognitive Neuroscience. 2007;19(12):1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: Medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Kamp S-M, Donchin E. Neural response to action and reward prediction errors: Comparing the error-related negativity to behavioral errors and the feedback-related negativity to reward prediction violations. Psychophysiology. doi: 10.1111/j.1469-8986.2010.01049.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Taylor S, Martis B, Fitzgerald K, Welsh R, Ableson J, Liberzon I, et al. Medial frontal cortex activity and loss-related responses to errors. The Journal of Neuroscience. 2006;26(15):4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15:535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhou X. Brain responses to outcomes of one's own and other's performance in a gambling task. NeuroReport. 2006;17:1747–1751. doi: 10.1097/01.wnr.0000239960.98813.50. [DOI] [PubMed] [Google Scholar]