Abstract
Objective
The cellular substrate of hippocampal dysfunction in schizophrenia remains unknown. We tested the hypothesis that hippocampal interneurons are abnormal in schizophrenia, but that the total number of hippocampal neurons in the pyramidal cell layer is normal.
Methods
We collected whole hippocampal specimens of 13 subjects with schizophrenia and 20 matched healthy control subjects to study the number of all neurons, the somal volume of neurons, the number of somatostatin- and parvalbumin-positive interneurons and the messenger RNA levels of somatostatin, parvalbumin and glutamic acid decarboxylase 67.
Results
The total number of hippocampal neurons in the pyramidal cell layer was normal in schizophrenia, but the number of somatostatin- and parvalbumin-positive interneurons, and the level of somatostatin, parvalbumin and glutamic acid decarboxylase mRNA expression were reduced.
Conclusions
The study provides strong evidence for a specific defect of hippocampal interneurons in schizophrenia and has implications for emerging models of hippocampal dysfunction in schizophrenia.
Keywords: hippocampus, interneurons, GABA, somatostatin, parvalbumin, schizophrenia
1. Introduction
The psychotic symptoms and cognitive deficits of schizophrenia have been linked to abnormalities of the hippocampus (Heckers and Konradi, 2010). However, in contrast to disorders of the hippocampus that lead to psychosis and impaired cognition, such as epilepsy and dementia, schizophrenia is not associated with hippocampal cell loss (Heckers et al., 1991; Schmitt et al., 2009; Walker et al., 2002).
Each human hippocampus contains approximately 10 million neurons, of which 90% are large, pyramidal-shaped, glutamatergic neurons and the remaining 10% are small, non-pyramidal, gamma-amino-butyric acid (GABA)ergic neurons (Freund and Buzsaki, 1996; Olbrich and Braak, 1985; West and Gundersen, 1990). Increasing evidence points to abnormalities of GABAergic hippocampal neurons in schizophrenia. Initial studies revealed a regionally-specific upregulation of GABA(A) receptor binding (Benes et al., 1996; Benes et al., 1997) and decreased density of non-pyramidal cells (Benes et al., 1998). More recent studies have focused on the hippocampal expression of the genes coding for glutamic acid decarboxylase (GAD); GAD1 encoding GAD67 and GAD2 encoding GAD65. An initial in-situ hybridization study revealed significant decreases of GAD2 mRNA expression in bipolar disorder, but non-significant changes in schizophrenia (Heckers et al., 2002). Similarly, two subsequent gene expression microarray studies did not find any changes of overall hippocampal GAD1 and GAD2 mRNA expression in schizophrenia (Konradi et al., 2004a; Straub et al., 2007).
Some studies have shown that abnormal GABAergic hippocampal neurons in schizophrenia are limited to certain regions and cell types. A recent laser-capture microdissection study revealed regionally specific decreases of GAD67 expression in hippocampal sector CA2/3, but not in the large sector CA1 (Benes et al., 2007). Additional evidence for selective changes of hippocampal interneurons in schizophrenia comes from the study of calcium binding proteins, which are differentially expressed in subpopulations of hippocampal interneurons (Freund and Buzsaki, 1996; Seress et al., 1993). For example, a significantly decreased density of parvalbumin-positive neurons was observed in all hippocampal regions, while the density of calretinin-positive cells was normal (Zhang and Reynolds, 2000). This pattern was corroborated by subsequent studies (Eyles et al., 2002; Torrey et al., 2005).
Here we tested the hypothesis that hippocampal interneurons are abnormal in schizophrenia, but that the total number of hippocampal neurons in the pyramidal cell layer is normal. We collected whole hippocampal specimens, which allowed us to estimate the total number of all neurons and the number of anatomically defined subsets of interneurons. In addition, we used a novel real-time quantitative polymerase chain reaction (PCR) approach for fixed human tissue to study gene expression levels of selected populations of hippocampal interneurons.
2. Methods
2.1. Sample
Brains were collected at the Harvard Brain Tissue Resource Center (HBTRC; McLean Hospital, Belmont, MA). The HBTRC is funded by NIH and follows all regulations implemented by the Office for Human Research Protections.
For all the subjects included in this study, two psychiatrists established DSM-IV diagnoses based on the review of a questionnaire filled out by legal next of kin and a review of all available medical records. Control cases had sufficient information from next of kin and medical records to rule out major medical, neurologic, and psychiatric conditions. All brains underwent a complete neuropathological exam and cases with histopathological abnormalities were excluded from this study.
Two diagnostic groups, comprised of 20 healthy control subjects and 13 subjects with schizophrenia, were matched for gender, age, post-mortem interval, and hemisphere (Table 1). One schizophrenia subject did not yield reliable somatostatin immunostaining and four subjects (two each from the two study groups) did not yield reliable parvalbumin immunostaining (Table 1) and were excluded from the immunohistochemical study. A subset of 13 control subjects and 11 subjects with schizophrenia had acceptable RNA quality and were included in the real-time, quantitative PCR (Q-PCR) experiments (Table 1). Schizophrenia samples of the present study were newly collected and did not overlap with samples used in a previous study (Heckers et al., 2002).
Table 1. Demographics of all study subjects.
Control [p] indicates control samples used for the initial paired analysis. Hemi = brain hemisphere; PMI= post mortem interval; CPZ eq = approximate equivalent of chlorpromazine in total grams during the last 6 months (Baldessarini and Tarazi, 2006); SOM = samples used for the somatostatin cell count; PARV = samples used for parvalbumin cell counts; Q-PCR = samples used in the qPCR analysis. All samples shown were used for Nissl cell counts and volume estimates of areas and neurons. Data mean ± SD
| Dx | Gender | Hemi | PMI | Age | Fresh brain weight (g) | Cause of death | CPZ eq | SOM | PARV | Q-PCR |
|---|---|---|---|---|---|---|---|---|---|---|
| SZ | F | L | 23.0 | 49 | 1240 | pulmonary embolism | 219.0 | x | ||
| SZ | F | L | 22.0 | 48 | 1300 | chronic obstruction pulmonary disease | 68.5 | |||
| SZ | F | R | 18.4 | 40 | 1450 | suicide (OD) | 1.8 | x | x | x |
| SZ | F | R | 22.0 | 44 | 1265 | cardiopulmonary arrest | 37.1 | x | x | x |
| SZ | F | R | 18.7 | 56 | 1185 | cardiac arrest | 36.1 | x | x | x |
| SZ | M | R | 33.3 | 41 | 1610 | cardiac arrest | 22.2 | x | x | x |
| SZ | F | L | 28.6 | 74 | 1325 | pneumonia | 82.1 | x | x | x |
| SZ | F | L | 15.7 | 85 | 1200 | cardiopulmonary arrest | 27.0 | x | x | x |
| SZ | M | R | 14.8 | 52 | 1280 | cardiac arrest | 54.0 | x | x | x |
| SZ | M | R | 25.3 | 62 | 1340 | sepsis | 162.0 | x | x | x |
| SZ | M | R | 18.0 | 36 | 1480 | suicide (OD) | 84.2 | x | x | x |
| SZ | M | L | 32.4 | 58 | 1160 | chronic obstruction pulmonary disease | 81.0 | x | x | x |
| SZ | M | R | 21.4 | 68 | 1255 | cardiac arrest | 216.5 | x | x | x |
| control [p] | F | L | 23.0 | 74 | 1100 | pneumonia | x | x | x | |
| control | F | L | 21.1 | 58 | 1280 | myocardial infarct | x | |||
| control [p] | M | R | 14.8 | 30 | 1570 | suicide | x | x | x | |
| control | M | L | 25.7 | 35 | 1530 | acute coronary artery thrombosis | x | x | ||
| control | M | R | 21.5 | 22 | 1360 | myocardial infarct | x | x | ||
| control | F | R | 23.9 | 68 | 1390 | cardiac obstructive pulmonary disorder | x | x | ||
| control [p] | M | L | 13.1 | 52 | 1450 | heart disease | x | x | x | |
| control [p] | M | R | 27.2 | 41 | 1815 | cardiac arrest | x | x | x | |
| control [p] | M | L | 18.4 | 68 | 1520 | heart failure | x | x | x | |
| control | F | L | 12.5 | 60 | 1160 | breast cancer | x | x | ||
| control [p] | F | R | 23.1 | 51 | 1375 | cardiac arrest | x | x | x | |
| control [p] | M | R | 28.3 | 61 | 1510 | myocardial infarct | x | x | x | |
| control [p] | F | R | 27.5 | 55 | 1245 | cardiopulmonary arrest | x | x | x | |
| control [p] | F | L | 6.9 | 86 | 1440 | cardiac arrest | x | |||
| control [p] | F | R | 17.8 | 60 | 1220 | cardiac dysrythmia | x | x | x | |
| control [p] | F | R | 17.4 | 81 | 1135 | colon cancer | x | x | x | |
| control | M | L | 24.6 | 77 | 1190 | cardiac arrest | x | x | ||
| control [p] | F | L | 20.3 | 42 | 1480 | myocardial infarct | x | x | x | |
| control [p] | F | R | 18.1 | 36 | 1390 | cardiac arrest | x | x | x | |
| control [p] | M | L | 30.3 | 60 | 1190 | cardiac arrest | x | x | x | |
|
| ||||||||||
| SZ | 6M / 7F | 5L/ 8R | 22.6±5.9 | 54.8±14.4 | 1314.6±129.3 | 84.0±71.5 | 12 | 10 | 11 | |
| control | 9M/ 11F | 10L/ 10R | 20.8±6.0 | 55.9±17.5 | 1367.5±179.6 | 20 | 18 | 13 | ||
2.2. Tissue collection and processing
The entire hippocampus was dissected from one hemisphere of each case. Tissue was immersion-fixed in 4.0% paraformaldehyde (0.1 M phosphate buffer (PBS), pH 7.4) at 4.0°C for 3 weeks. Hippocampi were placed in cryoprotectant (0.1 M PBS, pH 7.4/ 0.1% sodium azide/ 30.0% ethylene glycol/ 30.0% glycerol), immersed in agar and cut into 2.5 mm thick coronal slabs using an antithetic tissue slicer. Sections were cut from the top-most portion of each slab using a freezing microtome (American Optical Company, Buffalo, NY), with a thickness of 100 μm for Nissl-stain, or 50 μm for immunocytochemistry. Sections were mounted on gelatin-coated glass slides and stained with 0.1% cresyl violet (Nissl stain) or used for immunocytochemistry.
2.3. Immunocytochemistry
The immunocytochemical procedure was performed as described previously (Pantazopoulos et al., 2007) and is detailed in the supplemental material.
The somatostatin antibody was diluted 1:500 (monoclonal rat anti synthetic cyclic somatostatin peptide corresponding to amino acids 1-14; Cat # MAB354, Millipore, Billerica, MA); the parvalbumin antibody was diluted 1:10,000 (monoclonal mouse anti frog muscle parvalbumin, clone PARV-19; Cat #P3088, Sigma). Secondary antibodies were biotinylated, goat anti-rat IgG for somatostatin, and goat anti-mouse IgG for parvalbumin (Vector laboratories). Secondary antibodies were diluted 1:500.
2.4. Morphometric analysis
All cases were coded and data collection was performed without knowledge of diagnosis. Morphometric analysis was carried out using a Zeiss Axioskop 2 Plus microscope (Germany) equipped with a LEP MAC 5000 automated stage (Ludl Electronic Products, Hawthorne, NY). The microscope was interfaced with the Stereo Investigator stereological software (v 6.55, Microbrightfield, Colchester, VT) via an Optronics DEI-750 video camera (Goleta, CA). For the analysis of total neuron number and somal volume, we identified one pyramidal cell layer in three sectors (CA1, CA2/3 and CA4) and two non-pyramidal cell layers in sectors CA1 and CA2/3 (Figure 1). For the analysis of somatostatin- and parvalbumin-positive neurons, we delineated three hippocampal sectors (CA1, CA2/3 and CA4) without further separation into layers (Figures 3 and 4).
Figure 1. Pyramidal layer is normal in SZ.
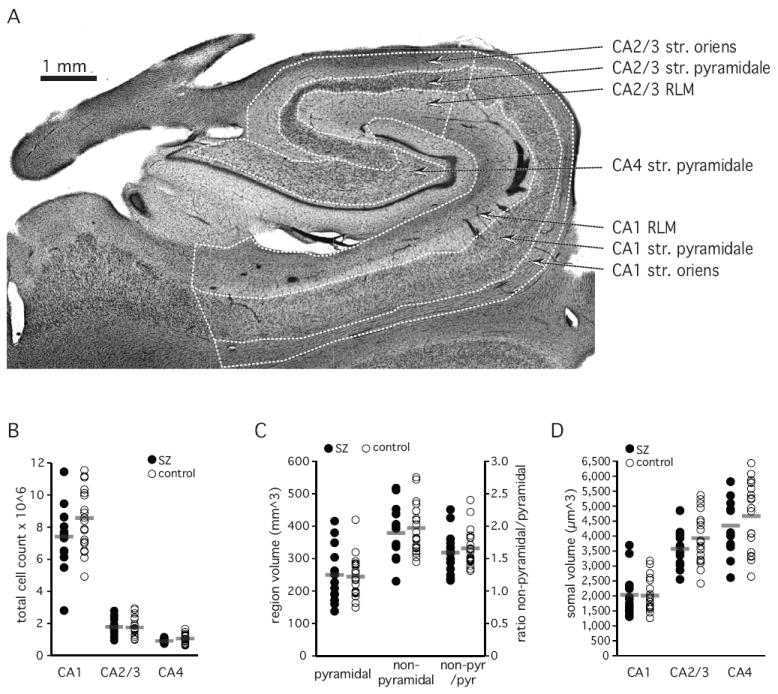
A. Anatomical organization of the hippocampus body. Hippocampal sectors CA1, CA2/3 and CA4 and the pyramidal and non-pyramidal (oriens, radiatum/lacunosum/moleculare (RLM)) layers are delineated. Str=stratum
B. Total neuron number in stratum pyramidale
C. Volume of the pyramidal and non-pyramidal layers.
D. Volume of neuronal cell somata
Grey bar indicates average of 13 schizophrenia subjects and 20 control subjects.
Figure 3. Somatostatin-positive neurons.
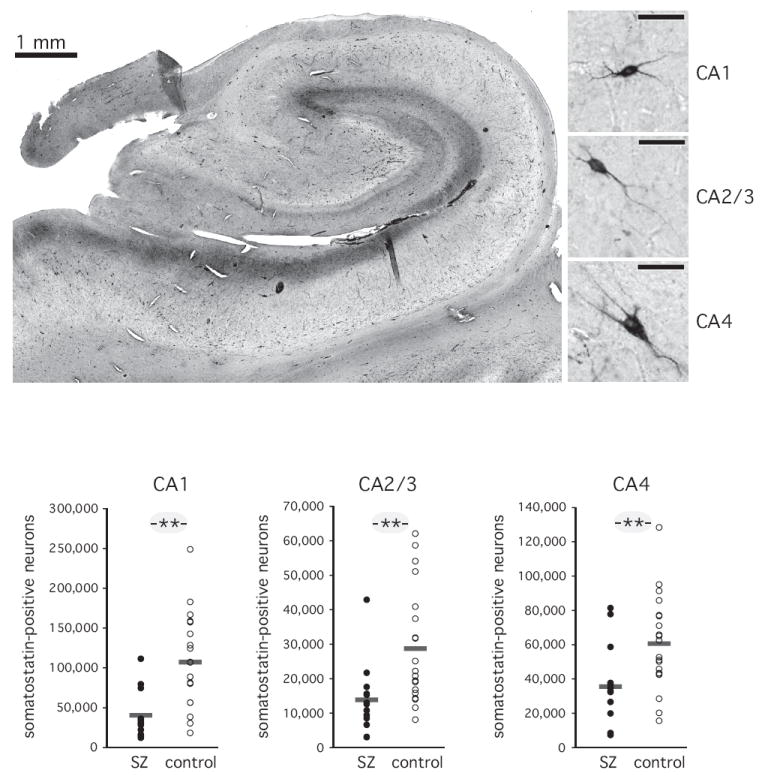
A. Photomicrograph of a representative section through the body of the hippocampus. Somatostatin-positive processes in the stratum lacunosum/moleculare are visible as a dark band. Somatostatin-positive cell bodies are dispersed throughout the stratum pyramidale and the stratum oriens, particularly along the border with the pyramidal cell layer. Immunohistochemically stained neurons for each of the three sectors are shown on the right. Right bar: 50 μm.
B. Total number of somatostatin-positive neurons. Horizontal bars indicate average of 12 schizophrenia subjects and 20 normal control subjects. ** p<=0.01.
Figure 4. Parvalbumin-positive neurons.
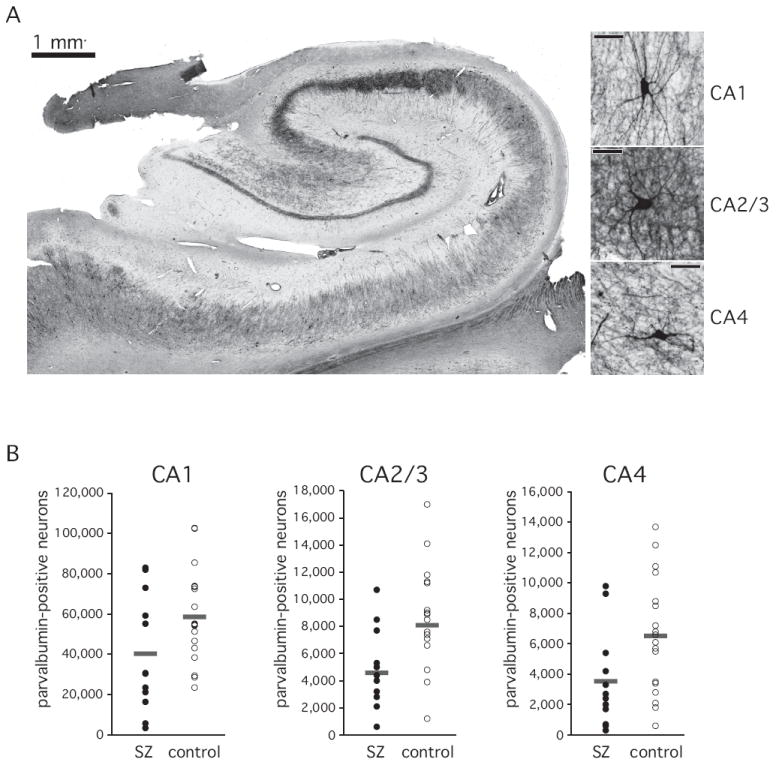
A. Photomicrograph of a representative section through the body of the hippocampus. Parvalbumin-positive processes in the stratum pyramidale are visible as a dark band. A lighter band of processes extends into the stratum radiatum. Parvalbumin-positive cell bodies are dispersed throughout the stratum pyramidale and the stratum oriens. Immunohistochemically stained neurons for each of the three sectors are shown on the right. Right bar: 50 μm.
B. Total number of parvalbumin-positive neurons. Horizontal bars indicate average of 12 schizophrenia subjects and 20 normal control subjects.
2.5. Volume and total neuron number estimates
Uniformly random sampling of CA neurons was conducted in the pyramidal cell layer throughout the entire hippocampal formation. Sections were sampled at a fixed interval of 2.5 mm with a random starting point in the coronal plane (average of 17 sections per hippocampus). Volume estimates of layers in the CA sectors were calculated from the product of known intervals between sections and contour measurements. Weighted means for section thicknesses were determined at every sampling site by differential focusing using a 100X oil-immersion objective (Zeiss, Plan-Apochromat, NA 1.40). The vertical movement of the stage was determined by a microcator (Heidenhain, Germany).
The stereological procedure is detailed in the supplemental methods and resulted in the following average cell count (Q), counting sites (F) and average estimated coefficents of errors (CE): CA1, schizophrenia = 317/129/0.06 (Q/ F/CE); CA1, control = 316/134/0.06; CA2/3, schizophrenia = 315/122/0.06; CA2/3, control = 274/113/0.07; CA4, schizophrenia = 167/125/0.08; CA4, control = 171/129/0.08.
2.6. Somal volume estimates
The nucleator method (Gundersen, 1988; Gundersen et al., 1988) was used to estimate somal volume of neurons in each of the CA sectors. The nucleator probe superimposed four isotropic rays emanating from the nucleolus of each sampled neuron. Estimates of area and volume were calculated from the recorded distance between nucleolus and cell wall for each ray. Neurons were sampled from sections at 7.5 mm intervals. The counting frame dimensions were 2500 × 2500 μm for the CA1 sector and 1000 × 1000 μm for the CA2/3 and CA4 sectors. This resulted in the following average cell count (Q), counting sites (F) and estimated CE per sector: CA1 = 55.1/17.7/0.01 (Q/F/CE); CA2/3 = 59.6/15.5/0.01; CA4 = 43.2/17.7/0.01.
2.7. Total immunopositive neuron estimates
The somatostatin- and parvalbumin-positive neurons were assessed in sections taken from every other slab throughout the whole hippocampus, i.e, at a distance of 5 mm intervals. First, volume estimates of the three CA sectors were calculated from the product of known intervals between sections and contour measurements. Second, using the automated stage of the microscope, each section was systematically scanned through the full x, y, and z axes using a 40x objective to count each parvalbumin- and somatostatin-labeled element with a cell body and at least one process clearly identifiable within each of the three CA sectors (see Figure 2). The average regional CE was 0.02 (CA1 and CA4) and 0.03 (CA2/3). Third, the total number of somatostatin- and parvalbumin-positive neurons was calculated as total number of cells counted/50 μm * 5000 μm.
Figure 2. Comparison of Nissl, somatostatin, and parvalbumin staining in CA1.
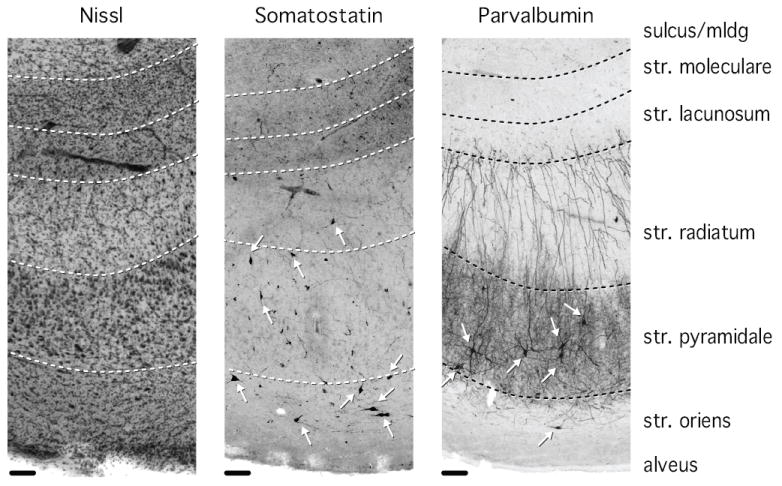
Cell bodies stained for somatostatin are located predominantly in stratum pyramidale and stratum oriens, with occasional appearance of cell bodies in stratum radiatum. Somatostatin processes are particularly dense in stratum lacunosum/moleculare. Cell bodies stained for parvalbumin are located predominantly in stratum pyramidale and to a lesser extent in stratum oriens. Parvalbumin-positive processes are densely distributed in stratum pyramidale and less densely in stratum radiatum. Arrows point to stained neuronal cell bodies. Str = stratum, mldg = molecular layer of the dentate gyrus. Scale bar: 100 μm.
2.8. Real-time quantitative PCR (Q-PCR)
Three hippocampal sectors (CA1, 2/3 and 4) were dissected from a 2.5 mm slab of fixed, frozen tissue, collected from the middle body of the hippocampus, and RNA was extracted using the Recoverall Total Nucleic Acid Isolation kit (Applied Biosystems, Foster City, CA, USA). Cornu ammonis borders were determined on an adjacent, cresyl violet-stained slice. The details of the Q-PCR method are described in the supplementary material.
2.9. Statistical analysis
We analyzed all histological data in pairs matched for age, gender and postmortem interval (n=14 per diagnostic group), and in diagnostic groups in a larger data set (n=14 schizophrenia, n=20 controls; table 1). Since we did not observe any significant difference between these two analyses, we are reporting the analysis of the larger data set. In the histological studies, we treated hippocampal sector as repeated measure. To reduce the weight of outliers, all histological data were log2 transformed for analysis. Initially, repeated measures ANOVA was performed with ‘diagnosis’ as the between-subject effect and ‘CA sector’ as the within-subject effect. Individual CA sectors were analyzed with Analyses of Covariance (ANCOVA), including gender, age, PMI and brain hemisphere as covariates. Due to the restriction to 96 wells on Q-PCR plates, Q-PCR data were collected and analyzed individually for each sector. The JMP program (v 9.0) was used for all analyses.
3. Results
The cellular and laminar organization of whole hippocampi was examined in systematically sampled coronal sections of control and schizophrenia subjects (table 1). In each section, sectors CA1, 2/3 and 4 were delineated, and the stratum oriens, stratum pyramidale, and stratum radiatum / lacunosum / moleculare (RLM) were delineated in CA1 and CA2/3 (Figure 1A). CA4 consists of stratum pyramidale only.
3.1. Cells in the stratum pyramidale
The total neuron number in stratum pyramidale did not differ significantly between the two groups (main effect of diagnosis F [1,31] = 1.33, p<=0.258; repeated measures ANOVA) (Figure 1B). The total neuron number was 10.02 ± 0.70 (× 10ˆ6, average ± SEM) in schizophrenia subjects and 11.40 ± 0.58 in control subjects, with the largest number in CA1 (7.32 ± 0.57 and 8.57 ± 0.44) and significantly fewer numbers in CA 2/3 (1.77 ± 0.16 and 1.76 ± 0.13) and CA4 (0.93 ± 0.04 and 1.07 ± 0.06), (main effect of sector: F [2,30]=1092.4, p<=0.0001; repeated measures ANOVA).
Volumes of the pyramidal areas (F [1,31]=0.17, p=0.735) and non-pyramidal areas (F [1,31]=0.19, p<=0.667) did not differ significantly between the two groups (Figure 1C).
The average volume of neuronal somata did not differ significantly between the two groups (main effect of diagnosis: F [1,31]=0.99, p<=0.328), but was significantly different between sectors (main effect of sector: F [2,30]=155.3, p<=0.0001; Figure 1D), with the following gradient CA4>CA2/3>CA1.
3.2. Somatostatin- and parvalbumin positive interneurons
Somatostatin-positive neurons were small neurons with sparse labeling of the axon and dendritic tree. Their cell bodies were located predominantly in stratum pyramidale and stratum oriens, while neuronal processes were particularly evident in stratum lacunosum / moleculare (Figures 2, 3A).
Parvalbumin-positive neurons were larger neurons with extensive labeling of axons and dendritic trees (Figure 2). Cell bodies of parvalbumin-positive neurons were located predominantly in the pyramidal cell layer, and to a lesser extent in the stratum oriens (Figures 2, 4A). Intense staining of neuronal processes was observed in stratum pyramidale and stratum radiatum (Figure 2).
The total number of somatostatin-positive neurons was largest in CA1 and smallest in CA2/3. This pattern was similar in both groups, but the total number of somatostatin-positive neurons was significantly reduced in schizophrenia (figure 3B), (main effect of diagnosis: F [1,30]=12.7, p<=0.001; main effect of sector: F [2,29]=121.9, p<=0.0001). These differences reached significance in a post-hoc ANCOVA for each of the three sectors: CA1 (F [5,26]=16.1, p<=0.0001), CA2/3 (F [5,26]=11.4, p<=0.002) and CA4 (F [5,26]=10.0, p<=0.004; age, gender, hemisphere and PMI as covariates).
The total number of parvalbumin-positive neurons was largest in CA1 and smallest in CA4. This pattern was similar in both groups, but the total number of parvalbumin-positive neurons was significantly reduced in schizophrenia (main effect of diagnosis: F [1,27]=4.3, p<=0.048; main effect of sector: F [2,26]=353.0, p<= 0.0001). These differences approached significance in a post-hoc ANCOVA for sectors CA1 (F [5,23]=3.9, p<=0.059) and CA4 (F [5,23]=3.5, p<=0.075), but not for sector CA2/3 (F [5,23]=2.7, p<=0.116; age, gender, hemisphere and PMI as covariates), (figure 4B).
3.3. Gene expression of hippocampal interneurons
To further explore abnormalities of hippocampal interneurons we examined mRNA expression levels of somatostatin, parvalbumin and GAD1 in the three sectors of the hippocampus (figure 5). When compared to the control group, the somatostatin mRNA levels were significantly lower in schizophrenia in sector CA2/3 (F [5, 18]=6.4, p<=0.021) and sector CA4 (F [5, 16]=5.7, p<=0.029), and approached significance in sector CA1 (F [5, 16]=4.0, p<=0.063). Parvalbumin mRNA levels were significantly lower in sector CA2/3 (F [5, 17]=8.9, p<=0.008), and GAD1 mRNA levels were significantly lower in sector CA2/3 (F [5,17]=6.5, p<=0.020) and approached significance in sector CA1 (F [5, 17]=4.1, p<=0.058) in the schizophrenia subjects (all analyses with age, gender, hemisphere and PMI as covariates).
Figure 5. Real-time quantitative PCR analysis of somatostatin, parvalbumin and GAD1.
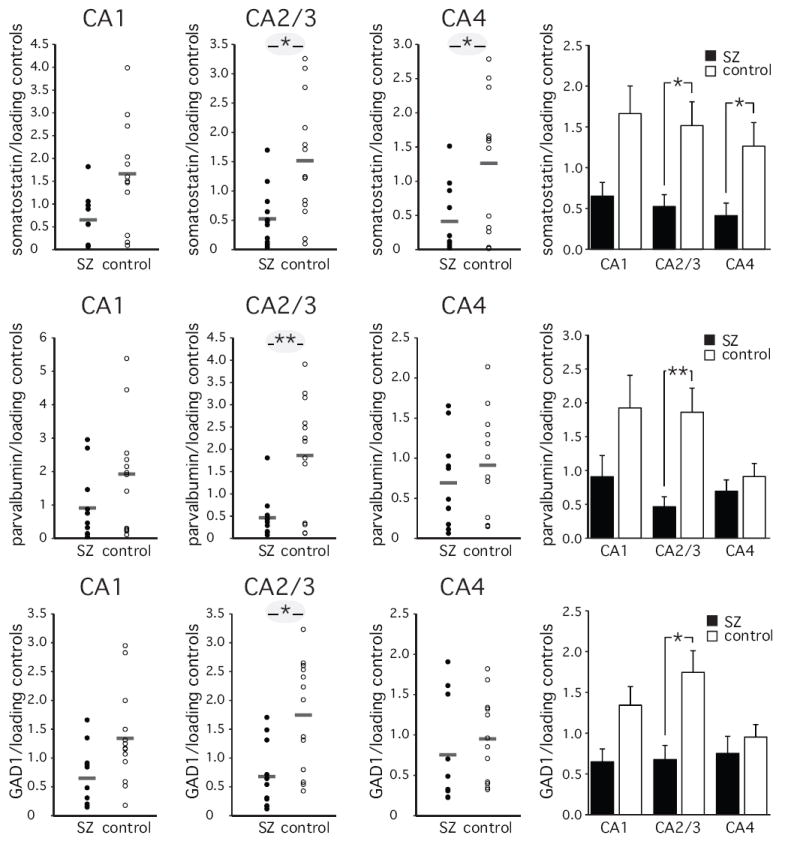
Bar indicates average of 11 schizophrenia subjects and 13 control subjects. (average ± SEM) in sectors CA1-4. * p<=0.05, ** p<=0.01
3.4. Effect of antipsychotic drugs
We did not find any significant correlation between chlorpromazine equivalents and cell numbers or gene expression levels in the subjects with schizophrenia. We also did not see an effect of 24 day treatment with one of three dosages of clozapine (4 mg/kg/day, 8 mg/kg/day or 20 mg/kg/day) or one of three dosages of haloperidol (0.05 mg/kg/day, 0.2 mg/kg/day or 0.5 mg/kg/day) on mRNA levels of somatostatin, parvalbumin, and GAD1 in rat hippocampi (supplemental table 1, supplemental methods). This replicated our previous study (Konradi et al., 2010).
4. Discussion
Our study provides strong evidence that hippocampal interneurons are abnormal in schizophrenia. We used a comprehensive design to study total neuron number, immunoreactivity and mRNA expression within the same specimens. This combines the strength of unbiased stereology (Dorph-Petersen and Lewis, 2011) with the functional assessment of protein and gene expression in subsets of neurons. Our findings confirm and considerably extend the emerging evidence for hippocampal interneuron dysfunction in schizophrenia (Heckers and Konradi, 2010). Here we will discuss the implications of our two main findings in schizophrenia: normal total neuron number in the pyramidal cell layer of the hippocampus, yet decreased protein and gene expression in hippocampal interneurons.
The volume of a brain region is often viewed as a predictor of total cell number (Carlo et al., 2010). Smaller hippocampal volume is a robust finding of many neuroimaging studies (effect size of 0.8, see Wright et al., 2000), and the initial postmortem study of the hippocampus reported cell loss in schizophrenia (Falkai and Bogerts, 1986). However, three stereological studies did not find any evidence for a loss of neurons in schizophrenia (Heckers et al., 1991; Schmitt et al., 2009; Walker et al., 2002) and the current study confirms these negative findings. Because studies of total hippocampal neuron number sample only the pyramidal cell layer, which makes up less than 50% of total hippocampal volume, we also estimated the non-pyramidal cell layer volume, which was reduced in schizophrenia in a previous study (Heckers et al., 1991). The present study could not confirm a reduction in the non-pyramidal cell layer, though markers for the selected interneuron populations, many of which are located in the non-pyramidal cell layer, were reduced. Taken together, there is now strong evidence that total hippocampal neuron number in the pyramidal layer is normal in schizophrenia. This is in contrast to disorders that are routinely diagnosed and staged using hippocampal volume measurements (e.g., epilepsy and dementia) and which are invariably associated with significant hippocampal neuron loss, both in patients and in animal models (Heckers and Konradi, 2010). The fact that smaller hippocampal volume in schizophrenia is not a predictor of overall neuron number loss has implications for the validity of those animal models that induce significant cell loss in the hippocampus (Young et al., 2010).
In the context of normal total hippocampal neuron number, we found strong evidence for abnormalities of two types of hippocampal interneuron populations in schizophrenia. Our results confirm earlier studies of reduced parvalbumin-positive neurons (Eyles et al., 2002; Torrey et al., 2005; Zhang and Reynolds, 2000) and further clarify the literature on GABAergic gene expression in the hippocampus in schizophrenia (Benes et al., 2007; Heckers et al., 2002; Konradi et al., 2004a; Straub et al., 2007).
First, the number of somatostatin-positive neurons and the level of somatostatin mRNA were significantly reduced in all three hippocampal sectors. These neurons constitute about 30-50% of all hippocampal interneurons and regulate the efficacy and plasticity of excitatory inputs to hippocampal pyramidal cells (Freund and Buzsaki, 1996; Viollet et al., 2008). Perturbations of the inhibitory role of somatostatin-positive neurons can lead to abnormal pyramidal cell firing, including seizure activity. Abnormal firing of hippocampal neurons could give rise to the psychotic symptoms seen in both temporal lobe epilepsy and schizophrenia (Lisman et al., 2008; Roberts et al., 1990; Stevens, 1988).
Second, the number of parvalbumin-positive interneurons was significantly reduced in sectors CA1 and CA4 and the level of parvalbumin mRNA was significantly reduced in sector CA2. Parvalbumin-positive interneurons in the hippocampus are crucial for organized temporal encoding and retrieval of information, by synchronizing the firing pattern of pyramidal cells in the 30-100 Hz range (i.e. gamma oscillations), (Bartos et al., 2007; Lewis et al., 2005). A loss of parvalbumin-containing interneurons was associated with diminished oscillatory activity in an animal model of schizophrenia (Lodge et al., 2009). Our data indicate that the firing pattern of hippocampal neurons is abnormal in schizophrenia, likely resulting in abnormalities of memory and other hippocampal function.
We cannot unequivocally answer the question whether hippocampal interneurons are lost in schizophrenia or whether they simply express less mRNA and protein. The 55% loss of somatostatin-positive neurons and a 38% loss of parvalbumin-positive neurons we found are well within the non-significant difference of 1.3 Million neurons between schizophrenia patients and normal controls in the total cell count of the pyramidal cell layers. However, our results indicate an imbalance between GABA-ergic inhibition and glutamatergic excitation in the hippocampus in schizophrenia. This provides a compelling target for the development of novel drug treatments for schizophrenia (Gonzalez-Burgos et al., 2010; Lewis and Sweet, 2009; Lisman et al., 2008). Interestingly, several studies have provided evidence that genes that increase the risk for schizophrenia cause an abnormal development of hippocampal interneurons. For example, DISC1 is expressed in a subset of hippocampal interneurons and affects neuronal migration and neuron number (Duan et al., 2007; Jaaro-Peled et al., 2010; Meyer and Morris, 2008).
The finding of abnormal hippocampal interneuron function in schizophrenia resembles previous reports of abnormal cortical interneuron function (Gonzalez-Burgos et al., 2010; Hashimoto et al., 2008). The reduction of parvalbumin-positive interneurons in layer five of the prefrontal cortex has been interpreted as the cellular substrate for impaired working memory function in schizophrenia (Volk and Lewis, 2010). More recently, a similar reduction of parvalbumin mRNA expression was reported for a large number of cortical areas, including primary sensory and cortico-limbic areas (Gonzalez-Burgos et al., 2010; Hashimoto et al., 2008). Our findings of equally impressive differences in interneuron protein and gene expression in the hippocampus support the notion that interneuron pathology in schizophrenia is not regionally selective (Hashimoto et al., 2008).
Recently we reported a reduction of parvalbumin-positive and somatostatin-positive interneurons in bipolar disorder (Konradi et al., 2010). In addition, a previous in-situ hybridization study (Heckers et al., 2002) and a microarray profiling study (Konradi et al., 2004b) provided strong evidence for decreased expression of GABA-ergic genes in bipolar disorder. This puts hippocampal interneurons in a central position for a mechanistic model of the continuum of psychosis, including schizophrenia, schizoaffective disorder and psychotic bipolar disorder (Benes, 2010; Heckers and Konradi, 2010; Lisman et al., 2008; Nakazawa et al., 2011).
Our study has several limitations. First, the collection of whole hippocampal specimens is challenging, resulting in small sample sizes. However, the dramatic reduction of (especially) the somatostatin-positive neurons, in the context of an overall normal neuron number, provides compelling data for interneuron pathology in schizophrenia. Second, the cell counts were carried out in different reference regions (pyramidal cell layer versus whole hippocampus) and the immunopositive neurons were not studied with the fractionator or disector in order to have consistent and reliable criteria for counting. Third, the nucleator probe was used in sections without random rotation. Fourth, protein degradation during the processing of the hippocampal tissue is likely to result in an underestimation of the total number of the immunopositive neurons, but there is no evidence that this would differently affect the two study groups. Finally, we cannot rule out an effect of treatment. However, chronic haloperidol or clozapine treatment does not alter parvalbumin immunoreactivity in the rat frontal cortex or hippocampus (Cahir et al., 2005), nor does it change mRNA levels in the rat hippocampus, as we have shown previously (Konradi et al., 2010) and show again here.
In conclusion, we present novel evidence for abnormalities of hippocampal interneurons, in the context of overall normal neuron number, in schizophrenia. This extends the already compelling evidence for hippocampal pathology in schizophrenia and suggests, together with similar data in psychotic bipolar disorder, impaired GABA-ergic inhibition of hippocampal pyramidal cells as a mechanism of psychosis.
Supplementary Material
Acknowledgments
The authors thank Francine Benes and the staff of the Harvard Brain Tissue Resource Center at McLean Hospital, who provided all tissues. We are indebted to the study subjects and their next of kin.
Role of funding source This work was supported by National Institute of Mental Health grants MH67999 (SH) and MH74000 (CK), and by MH068855 (Francine Benes, Harvard Brain Tissue Resource Center). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors Authors S.H. and C.K. designed and supervised the study. Authors E.Z., K.Y., K.M.L., P.G., H.P. and S.B. collected all data. Authors C.K. and S.H. completed all data analysis and wrote the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldessarini RJ, Tarazi FI. Pharmacotherapy of Psychosis and Mania. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11/e. McGraw-Hill; New York: 2006. pp. 461–499. [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Benes FM. Relationship of GAD(67) regulation to cell cycle and DNA repair in GABA neurons in the adult hippocampus: bipolar disorder versus schizophrenia. Cell Cycle. 2010;9:625–627. doi: 10.4161/cc.9.4.10820. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Wickramasinghe R, Vincent SL, Khan Y, Todtenkopf M. Uncoupling of GABA(A) and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res. 1997;755:121–129. doi: 10.1016/s0006-8993(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Cahir M, Costello I, King DJ, Reynolds GP. Chronic haloperidol or clozapine treatment does not alter parvalbumin immunoreactivity in the rat frontal cortex or hippocampus. Neurosci Lett. 2005;373:57–60. doi: 10.1016/j.neulet.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Carlo CN, Stefanacci L, Semendeferi K, Stevens CF. Comparative analyses of the neuron numbers and volumes of the amygdaloid complex in old and new world primates. J Comp Neurol. 2010;518:1176–1198. doi: 10.1002/cne.22264. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Lewis DA. Stereological approaches to identifying neuropathology in psychosis. Biol Psychiatry. 2011;69:113–126. doi: 10.1016/j.biopsych.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles DW, McGrath JJ, Reynolds GP. Neuronal calcium-binding proteins and schizophrenia. Schizophr Res. 2002;57:27–34. doi: 10.1016/s0920-9964(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci. 1986;236:154–161. doi: 10.1007/BF00380943. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ. The nucleator. J Microsc. 1988;151:3–21. doi: 10.1111/j.1365-2818.1988.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry. 1991;48:1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull. 2010;36:301–313. doi: 10.1093/schbul/sbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004a;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004b;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal Interneurons in Bipolar Disorder. Arch Gen Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Morris JA. Immunohistochemical analysis of Disc1 expression in the developing and adult hippocampus. Gene Expr Patterns. 2008;8:494–501. doi: 10.1016/j.gep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich HG, Braak H. Ratio of pyramidal cells versus non-pyramidal cells in sector CA1 of the human Ammon’s horn. Anat Embryol (Berl) 1985;173:105–110. doi: 10.1007/BF00707308. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61:640–652. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Done DJ, Bruton C, Crow TJ. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry. 1990;28:127–143. doi: 10.1016/0006-3223(90)90630-k. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathologica. 2009;117:395–407. doi: 10.1007/s00401-008-0430-y. [DOI] [PubMed] [Google Scholar]
- Seress L, Gulyas AI, Ferrer I, Tunon T, Soriano E, Freund TF. Distribution, morphological features, and synaptic connections of parvalbumin- and calbindin D28k-immunoreactive neurons in the human hippocampal formation. Journal of Comparative Neurology. 1993;337:208–230. doi: 10.1002/cne.903370204. [DOI] [PubMed] [Google Scholar]
- Stevens JR. Epilepsy, psychosis and schizophrenia. Schizophr Res. 1988;1:79–89. doi: 10.1016/0920-9964(88)90044-8. [DOI] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, Crow TJ. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. American Journal of Psychiatry. 2002;159:821–828. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. Journal of Comparative Neurology. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Young JW, Zhou X, Geyer MA. Animal models of schizophrenia. Curr Top Behav Neurosci. 2010;4:391–433. doi: 10.1007/7854_2010_62. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective deficit in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia Research. 2000;49:65. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


