Abstract
Purpose
We previously developed magnetic resonance (MR)-trackable magnetocapsules (MCs) that can simultaneously immunoprotect human islet cells and non-invasively monitor in real-time portal delivery and engraftment with magnetic resonance imaging (MRI). This study was designed to assess the physiological effects of the delivery of a clinical dose of MCs (140,000) into the portal vein in swine over a 1 month period.
Materials & Methods
MCs were formed using clinical-grade alginate mixed with a clinically applicable dosage of ferumoxide. Percutaneous access into the portal vein was obtained using a custom-built, MR-trackable needle, and 140,000 MCs were delivered under MR guidance in five swine. Portal pressures and liver function data were obtained over a 4-week period.
Results
A transient rise in portal pressure occurred immediately following MC delivery that returned to normal levels by four weeks following MC delivery. Liver function tests were normal during the entire period, and the appearance of the MCs on MRI did not change.
Conclusion
A clinically applicable dose of 140,000 MCs has no adverse effects on portal pressures or liver function in this normal swine model during the first month after delivery.
INTRODUCTION
Islet cell transplantation is an attractive treatment for patients with type 1 diabetes mellitus (T1DM) but success has been limited, partially due to cytotoxic immunosuppressive regimens (1-4). To avoid immunosuppressive therapy, microencapsulation has been proposed as a method of protecting transplanted cells from the immune system. By surrounding individual islets with thin semi-porous alginate membranes that are permeable to insulin and metabolites but impermeable to antibodies, microencapsulation provides a way to immuno-isolate cells while preserving cell function and integrity.
Delivery and placement of these microcapsules remains an important unresolved issue in their use for cell therapy. Currently, injection under fluoroscopic guidance into the liver via the portal vein is thought to be the optimal means for transplantation due to a high nutrient and oxygen supply (5, 6). While delivery into the liver through portal vein access has shown some success in sustaining cell function (5, 6), further improvement is needed. Specifically, assessment of the accuracy of delivery and extent of engraftment of microencapsulated cells is needed to correlate islet long-term function with anatomical location and route of delivery (7). This assessment would be possible if the microencapsulated cells could be visualized during and after delivery.
MRI can provide this visualization sensitivity due to excellent soft-tissue contrast, high resolution, and whole-body imaging capability; moreover, MRI has been used to reliably track magnetically labeled cells in animal models as well as in patients (8). We have previously developed magnetocapsules (MCs) that are trackable by MRI and capable of sustaining viable islets for at least one month (9). These trackable MCs provide a powerful method to both monitor the delivery of encapsulated islets and examine their stability over time. However, before MCs can be adopted for clinical use, the effect of MCs on portal pressure and liver function must be ascertained. In order to determine whether intraportal delivery of MCs is a viable option for treatment of T1DM patients, we evaluated portal pressures and performed several liver function tests over the course of 1 month following delivery.
MATERIALS AND METHODS
Synthesis of magnetocapsules
The synthesis of MCs was based on a one-step modification of the original alginate encapsulation method of Lim and Sun (10), and has been described in detail elsewhere (9, 11). Briefly, Feridex®, a commercial-brand superparamagnetic iron oxide nanoparticle, was mixed with ultrapure alginate to produce an alginate solution that is 20% Feridex® by volume. Using a high voltage power supply and a nanoinjector pump, gel droplets were formed and crosslinked with 0.05% poly-L-lysine, following which a second layer of alginate was added.
Animal Model
The study was approved by the Institutional Animal Care and Use Committee. Five healthy swine (40-45 kg) were sedated with an intramuscular (i.m.) injection of a mixture of ketamine (22 mg/kg), acepromazine (1.1 mg/kg), and atropine (0.05 mg/kg). An intravenous (i.v.) catheter was placed in the marginal ear vein and the animal was induced with sodium thiopental (20 mg/kg body weight), intubated, and mechanically ventilated with 1-2% isoflurane. A single dose of the intramuscular penicillin G (Dual-Cillin 300,000U/mL) was administered prior to vascular access.
MR-Guided Delivery of Magnetocapsules
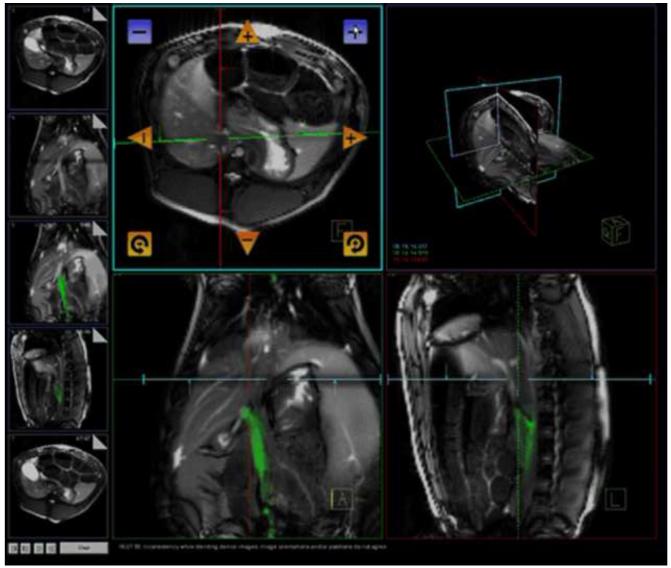
Percutaneous access into the right femoral vein was achieved with an 11-French sheath (St. Jude Medical, St. Paul, Minnesota, USA). All animals were then transferred to the MR suite for the remaining portion of the procedure. All procedures were performed in a 1.5 T MR scanner (MAGNETOM Espree, Siemens Healthcare, Erlangen, Germany). Imaging was acquired using a combination of external phased array coils and a custom-made active intravascular needle, as described previously (9, 13-14). The needle was introduced into the venous system via the right femoral vein through the previously placed 11-French sheath. Using an interactive real-time TrueFISP sequence (BEAT_IRTTT, Siemens Corporate Research) in combination with Interactive Front End (IFE, Siemens Corporate Research) graphical interface which enables interactive scan plane manipulation, three simultaneous imaging planes (axial, sagittal and oblique axial) were imaged to track the needle and identify the proper trajectory for portal vein puncture from the inferior vena cava (Figure 1). The imaging parameters were 1.7 ms echo time (TE); 3.4 ms repetition time (TR); 45° flip angle; bandwidth=977 Hz/pixel; 7 mm slice thickness; 300 mm field of view (FOV) ; and 128×128 image matrix. Prior to and following the puncture procedure, a contrast-enhanced MR angiogram (MRA) of the mesenteric venous system was obtained after injection of 30 ml gadopentetate dimeglumine (Magnevist, Bayer Healthcare AG, Leverkusen, Germany) in the marginal ear vein. After directed puncture into the portal vein, the needle system was advanced over a .035″ Rosen wire (Cook Medical Inc., Bloomington, IN, USA) further into the portal system for stability during MC delivery. A total of 140,000 MCs in a volume of about 100 ml were infused into the portal vein over about 10 minutes using this transcaval approach. With 1 islet per MC, 140,000 MCs represents a “clinically relevant dose” for an animal of this weight, according to the Edmonton protocol (2).
Figure 1.
Interactive Front End (IFE, Siemens) graphical interface in combination with an interactive real-time TrueFISP sequence (BEAT_IRTTT, Siemens) to guide transcaval punctures of the portal vein for magnetocapsule delivery.
Follow-up Evaluation
MR imaging of swine both at baseline and at follow-up was performed using a 6-element body matrix phased-array coil in combination with 12 elements of the integrated spine array and a breath held, 3D gradient echo (GRE) sequence. The imaging parameters were TR: 4.99 ms, TE: 2.32 ms, 72 slices, 320 mm FOV, flip angle: 10°, matrix: 256×256, receiver bandwith: 360 Hz/pixel, time of acquisition: 29 sec, 3D voxel size: 1.3 × 1.3 × 2.5 mm. MR data were analyzed using Amira 3.1 software (Mercury Computer Systems). Portal venograms were obtained before and after delivery of MCs as well as at the 4 week follow-up. Direct portal pressures were measured using a pressure transducer attached to a 5 F pigtail catheter (Cook Medical Inc., Bloomington, IN, USA) at baseline and at 1 minute, 30 minutes and four weeks after MC delivery. In addition, hematologic liver function markers such as bilirubin, albumin, alkaline phosphatase, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and platelet counts, were obtained at baseline and every week up to four weeks. At the end of four weeks, repeat MRI/MRA of the liver was performed as well as direct portal venograms and portal pressures. Assessment of MC signal intensity was done qualitatively by observing the appearance of the MCs on MRI and comparing them to the images taken immediately post-delivery.
Statistical Analysis
Data for portal pressures are given as the mean of each animal plus/minus standard deviation. The paired, two-tailed Student’s T test was performed to determine whether the differences at different time points were statistically significant (p < 0.05), specifically pre-procedure vs. 1 minute post-delivery, pre-procedure vs. 30 minutes post-delivery, and pre-procedure vs. 4 weeks post-delivery. Data for liver function tests are presented as the mean of each animal plus/minus standard deviation, and the normal values are given for reference.
RESULTS
MC Delivery and MR Properties
MCs were successfully infused into the portal venous system of all pigs and followed for 4 weeks after delivery. There were no significant procedural complications. Portal venograms showed no evidence of portal vein thrombosis up to the distal branches. Following delivery, MCs were clearly visualized as magnetic susceptibility-induced hypointensities that represented the distribution of the capsules within the entire liver (Figure 2). When administered in the main portal vein, the MC distribution was predominantly in the periphery of the liver with central sparing, which correlated with normal vascular flow patterns in the portal vein. As shown previously (9), capsule rupture is correlated with a change in signal intensity on MRI. Follow-up MR imaging performed at 4 weeks post-delivery demonstrated no changes in MR appearance of the capsules. Magnetocapsules appeared intact at four-week follow-up without disruption or change in distribution.
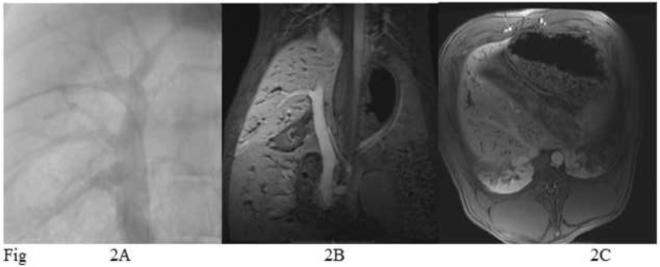
Figure 2.
A. Direct portal venogram performed from a transhepatic approach. B. Magnetic resonance venogram (MRV) with a coronal view that corresponds to the portal vein in figure 2A. C. Axial view shows capsules in the liver parenchyma.
Portal Pressures
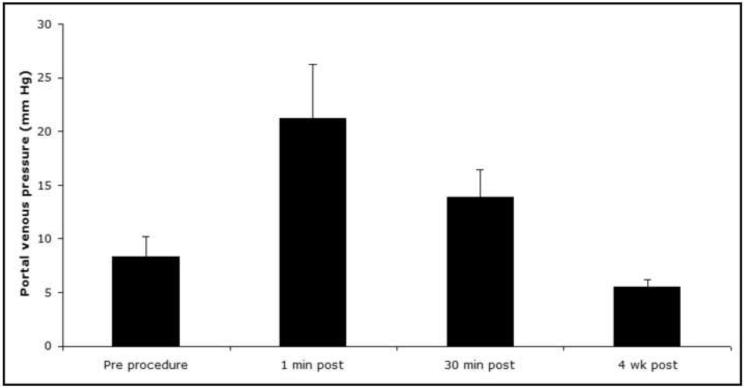
Introduction of 140,000 MCs into the liver resulted in a transient rise in portal pressure over baseline (7.7±1.4 mmHg) at 1 minute (21.5±4.2 mmHg) and 30 minutes (14.3±2.5 mmHg) post procedure (Figure 3). Portal pressure returned to normal values (5.5±0.6 mmHg) by the 4 week follow up. The difference in portal pressure compared to baseline was statistically significant at 1 minute (p = 0.007) and 30 minutes (p = 0.006) post-procedure, but not at the 4 week follow up (p = 0.9).
Figure 3.
Mean portal venous pressure: pre-procedure (7.7±1.4 mmHg), 1 minute (21.5±4.2 mmHg), and 30 minutes (14.3±2.5 mmHg) post-procedure, and at a four week follow-up (5.5±0.6 mmHg).
Liver Function
Blood serum analysis demonstrated values for total bilirubin, alkaline phosphate, ALT, AST that were in the normal range at baseline and throughout all four weeks (Table 1).
TABLE 1.
Mean values and standard deviation for liver function tests (total bilirubin [mg/dL], alkaline phosphate [IU/L], ALT [IU/L], AST [IU/L]) at baseline and 7, 14, 21, and 28 days follow-up. Normal values are listed also for reference.
| Total Bilirubin |
Alkaline Phosphate |
ALT | AST | |
|---|---|---|---|---|
| Day 0 | 0.2 ± 0.1 | 145.6 ± 37 | 28.8 ± 14 | 43.2 ± 25 |
| Day 7 | 0.2 ± 0.2 | 104.2 ± 21 | 41.2 ± 16 | 43.2 ± 8 |
| Day 14 | 0.1 ± 0 | 88.0 ± 24 | 38.2 ± 15 | 33.8 ± 15 |
| Day 21 | 0.1 ± 0.1 | 134.0 ± 36 | 50.2 ± 14 | 60.4 ± 34 |
| Day 28 | 0.2 ± 0.1 | 114.6 ± 48 | 52.0 ± 13 | 43.8 ± 19 |
| Normal | 0-1.0 | 118-395 | 31-58 | 32-84 |
DISCUSSION
In this study, 140,000 empty magnetocapsules (MCs) were delivered to the liver of five swine via portal injection. Transient portal hypertension was induced but was reduced by one-third 30 minutes after injection, and portal pressures returned to normal by four weeks, indicating that this dosage of MCs does not adversely affect portal pressure. A gradual return of portal pressure to normal values suggests that the vasculature of the liver may compensate for the capsule load by redirecting blood flow in case normal blood flow is impeded by the presence of MCs. Total bilirubin, alkaline phosphate, ALT and AST levels were in the normal range for at least four weeks after the procedure. These results suggest that the effect on liver function of a clinical dose of MCs is tolerable, and normal liver function can be maintained.
Immediately after delivery, the MCs were seen distributed throughout the liver as magnetic susceptibility-induced hypointensities. This suggests that injection into the portal vein is an efficient method of distributing MCs throughout the entire liver. Being able to determine where the MCs engraft helps to provide feedback on the success of the procedure. Four weeks after the procedure, there was no change in MR appearance of the MCs. This indicates that the MCs remained intact and did not redistribute, as capsule rupture would lead to a decrease in hypointensity as a result of Feridex® leakage. MR-visible capsules may thus present an effective way to study the fate of injected material post-delivery.
This advantage stems from the intrinsic abilities of MRI. MRI has excellent soft-tissue contrast, high resolution, whole-body imaging capability, and the ability to track magnetically labeled cells in vivo (8, 12). As a result of the improved speed of MRI acquisition and available magnetic resonance catheter-tracking technology, MRI is being explored as an alternative for various vascular (13) and cell delivery procedures (14). Due to the anatomic variability in the relationship of extrahepatic portal and superior mesenteric veins to the inferior vena cava and the close proximity of adjacent vessels and organs (eg, aorta, superior mesenteric artery, and pancreas), direct transcaval puncture currently cannot be performed safely with conventional guidance. Recent work from our group has demonstrated the ability to use real time MR imaging and an active MR needle system to safely and rapidly puncture the extrahepatic, retroperitoneal portal/mesenteric venous system from the inferior vena cava (13). MR-directed, transcaval portal access has the potential to be a safe and accurate technique for accessing the portal vein without damage to the liver parenchyma and with minimal risk of bleeding or surrounding organ damage. Thus, to make our microcapsules MR-visible, we incorporated an FDA-approved ferumoxide formulation. The magnetocapsules (MCs) contain a pharmaceutical grade alginate and a Feridex® dosage that is below the total current FDA-approved intravenous dose given to humans as a liver agent (9).
One limitation to this study is the lack of histological data to investigate the host’s reaction to the magnetocapsules. Various studies have shown inflammation, fibrosis, and foreign body reaction to alginate-encapsulated islets in the liver (5, 15). Pericapsular cellular infiltration has been found to be comprised mainly of macrophages and myofibroblasts (16). Whether or not this occurred in our study, these findings place importance on the evaluation of liver function tests after delivery of MCs, to determine whether fibrotic overgrowth comprises a significant portion of the liver. Our results may suggest that the liver is able to tolerate the presence of engrafted magnetocapsules without any adverse effect on physiological function.
Other limitations include the small sample size and the extent of liver function testing. Future experiments may include additional markers of liver function in order to more rigorously evaluate the effect on liver function. Furthermore, assessment of signal intensity was done qualitatively, as subjective comparison of MRIs taken immediately after injection and at the 4 week follow-up. Although quantitative analysis of signal hypointensities would have been preferable, current technology does not exist to adequately assess the high volume of capsules infused (>140,000). Distinguishing artificially induced image hypointensity from intrinsic contrast is a common and difficult challenge and thus multiple approaches to quantify these capsules are currently being explored. One approach is to generate positive contrast images to highlight the capsules and distinguish them from underlying potential false positive areas such as from tissue interfaces, blood vessels, necrosis and or hemorrhage. These techniques generate positive contrast images that complement anatomical magnitude images, but also carry limitations such as customized hardware, prolonged acquisition time and decreased SNR signal. Other methods are being explored to computationally estimate the SPIO deposits using magnetically susceptibility and iron concentration. Thus, multiple approaches are currently under investigation to quantify the capsules in an in vivo setting. It is also important to note that these experiments were done in healthy swine, and it has been shown that even large embolizations of the portal vein at weekly intervals would not create permanent portal hypertension in swine (17). Thus, extrapolation of these results to humans may be limited.
Using a non-invasive imaging technique like MRI to visualize the injected treatment post-infusion helps address uncertainties, such as ideal delivery site and success of engraftment, and provides information about the fate of the injected microcapsules over time. These questions need to be answered in order for islet transplantation with microcapsules to be clinically relevant. Furthermore, the long-term effects of a large quantity of MCs on physiology need to be assessed. This paper is a first step, showing that portal pressures and liver function tests were normal in all five swine for up to four weeks.
Acknowledgments
Grant Support: NIH (R01EB007825)
Footnotes
This material was not presented at an SIR annual meeting.
Author Disclosures: Dr. J.W.M. Bulte is a paid consultant for Surgivision, Inc. This arrangement has been approved by The Johns Hopkins University in accordance with its Conflict of Interest policies.
W.D. Gilson and L. Pan are employees of Siemens Corporate Research, a division of Siemens Corporation.
Dr. A. Arepally is a co-founder of Surefire Medical and is a paid consultant for Surgivision, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.David M. Nathan. Long-term complications of Diabetes Mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Ault A. Edmonton’s islet success tough to duplicate elsewhere. Lancet. 2003;361:2054. doi: 10.1016/S0140-6736(03)13680-X. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 5.Toso C, et al. Intra-portal injection of 400-micron microcapsules in a large-animal model. Transpl. Int. 2003;16:405–410. doi: 10.1007/s00147-003-0555-9. [DOI] [PubMed] [Google Scholar]
- 6.Toso C, et al. Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplant. 2005;14:159–167. doi: 10.3727/000000005783983223. [DOI] [PubMed] [Google Scholar]
- 7.Orive G, et al. Cell encapsulation: promise and progress. Nat. Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 8.Bulte JWM. In vivo MRI cell tracking: clinical studies. American Journal of Roentgenology. 2009;193:314–325. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett B, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islets. Nat. Med. 2007;13:986–991. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 10.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 11.Barnett BP, Arepally A, Kraitchman DL, Bulte JWM. Synthesis of MR-, X-ray- and US-visible alginate microcapsules for immunoisolation and non-invasive imaging of cellular therapeutics. Nat. Protocols. doi: 10.1038/nprot.2011.352. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulte JW, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 13.Arepally A, Karmarkar PV, Weiss C, Atalar E. Percutaneous MR imaging-guided transvascular access of mesenteric venous system: study in swine model. Radiology. 2006;238:113–118. doi: 10.1148/radiol.2381041533. [DOI] [PubMed] [Google Scholar]
- 14.Karmarkar PV, et al. MR-trackable intramyocardial injection catheter. Magn. Reson. Med. 2004;51:1163–1172. doi: 10.1002/mrm.20086. [DOI] [PubMed] [Google Scholar]
- 15.Zekorn TD, et al. Biocompatibility and immunology in the encapsulation of islets of Langerhans (bioartificial pancreas) Int J Artif Organs. 1996;4:251–257. [PubMed] [Google Scholar]
- 16.Mathe Z, et al. Short-term immunosuppression reduces fibrotic cellular infiltration around Barium-M-alginate microbeads injected intraportally. Transplantation Proceedings. 2004;36:1199–1200. doi: 10.1016/j.transproceed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Pavcnik D, et al. Attempted induction of chronic portal venous hypertension with polyvinyl alcohol particles in swine. J Vasc Interv Radiol. 1997 Jan-Feb;8:123–128. doi: 10.1016/s1051-0443(97)70527-x. [DOI] [PubMed] [Google Scholar]