Abstract
Sepsis is the leading cause of death in most ICU’s, and patients who survive the hyper-inflammation that develops early during sepsis later display severely compromised immunity. Not only is there apoptosis of lymphoid and myeloid cells during sepsis that depletes these critical cellular components of the immune system, but the remaining immune cells also show decreased function. Using a cecal-ligation and puncture (CLP) model to induce intra-abdominal polymicrobial peritonitis, we recently established a link between the apoptotic cells generated during sepsis and induction of sepsis-induced suppression of delayed-type hypersensitivity. The present study extends this earlier work to include a secondary heterologous bacterial infection (OVA-expressing Listeria monocytogenes; LM-OVA) subsequent to sepsis initiation to investigate sepsis-induced alterations in the control of this secondary infection and the associated naïve Ag-specific CD8 T cell response. We found that CLP-treated WT mice had a reduced ability to control the LM-OVA infection, which was paralleled by suppressed T cell responses, versus sham-treated WT mice. In contrast, CLP-treated Trail−/− and Dr5−/− mice were better able to control the secondary bacterial infection and the Ag-specific CD8 T cell response was similar to that seen in sham-treated mice. Importantly, administration of a blocking anti-TRAIL mAb to CLP-treated WT mice was able to restore the ability to control the LM-OVA infection and generate Ag-specific CD8 T cell responses like those seen in sham-treated mice. These data further implicate TRAIL-dependent immune suppression during sepsis, and suggest TRAIL neutralization may be a potential therapeutic target to restore cellular immunity in septic patients.
INTRODUCTION
Sepsis is the leading cause of death in ICU patients – taking >215,000 lives per year in the U.S. (1) – and is classically defined as the body’s massive hyper-inflammatory response to systemic infection. Not surprisingly, the majority of early studies on sepsis in humans and experimental animals focused on understanding the early hyper-inflammatory phase, which is characterized by the increased production of a number of pro-inflammatory cytokines, including IFN-γ, IL-1β, IL-6, and TNF (2–4), and other pro-inflammatory mediators, such as HMGB1, MIF, TREM-1, MRP8/14 (5–9). While the neutralization of IL-1β, TNF, or HMGB1 improved survival of septic mice and baboons (10–14), similar clinical attempts designed to neutralize IL-1β and/or TNF in septic patients failed to improve survival (15). Other anti-inflammatory based therapeutic trials, such as anti-endotoxin, steroids, anti-platelet-activating factor, also failed to produce a positive effect on the pathogenesis of sepsis (12, 15, 16).
The inability to decrease sepsis-induced mortality by dampening the hyper-inflammation led many to question the idea that the early, massive inflammation in sepsis was the sole cause of mortality. It has since been shown that the initial hyper-inflammation during sepsis is followed by a prolonged immunosuppressive phase that significantly increases morbidity and the chance of mortality caused by secondary (often nosocomial) infections (17). The increased susceptibility to secondary infection can be attributed to many things, but one hallmark of sepsis is the widespread apoptotic death of immune cells that provide protection. Adoptive transfer of apoptotic cells during sepsis increases mortality (18), whereas prevention of lymphocyte cell death using pharmaceutical agents (such as zVAD) or modified expression of pro- or anti-apoptotic proteins (such as Bim−/− or Bcl-2 overexpressing mice) improves survival following sepsis (19–21). It is important to keep in mind that the sepsis-induced lymphopenia is not the only means by which the immune system is altered, since the function of the remaining immune cells is also significantly decreased. Using the well-defined cecal-ligation and puncture (CLP) mouse model (19, 20, 22), we recently found a clear relationship between sepsis-induced immune cell apoptosis and the ability to mount delayed-type hypersensitivity (DTH) (23). Moreover, this previous study showed that the lack of DTH responses during sepsis was dependent on TNF-related apoptosis-inducing ligand (TRAIL)-mediated active immune suppression. Thus, the sepsis-induced hypoimmune phase is likely the result of multiple mechanisms that together lead to the compromised overall health of the septic individual.
The present study continues our analysis of the role of TRAIL-mediated immune suppression during sepsis by modifying the CLP model to include a secondary (heterologous) bacterial infection with the Gram-positive, facultative intracellular bacterium Listeria monocytogenes (LM). Murine infection with LM is a widely used model for the analysis of cell-mediated immunity to an intracellular bacterial pathogen (24–26). While both CD4 and CD8 T cells are activated in an Ag-specific manner after LM infection (27), CD8 T cells are the most effective mediators of anti-LM immunity (28–30). Thus, incorporation of secondary LM infection into the CLP model serves as a tool to specifically evaluate sepsis-induced alterations in CD8 T cell immunity to this heterlogous bacterial infection. The decision to use LM as the secondary infectious agent in this model was further buttressed by the fact that CD8 T cell-mediated immunity against LM is TRAIL-independent (31), so any changes in the CD8 T cell response to LM were predicted to be due to the sepsis-induced immune suppression. The data presented herein further implicate the prominent role of TRAIL during sepsis-induced immune suppression, and suggest the TRAIL/TRAIL receptor pathway as a therapeutic target for restoring T cell-mediated immunity in septic patients.
MATERIALS and METHODS
Mice
C57Bl/6 (B6) and BALB/c mice were purchased from The National Cancer Institute. Trail−/− and Dr5−/− B6 mice were obtained from Amgen (Seattle, WA)(32) and Dr. W. El-Diery (University of Pennsylvania, Philadelphia, PA) (33), respectively. Trail−/− BALB/c mice were obtained from Dr. Thomas Sayers (NCI Frederick). All mice were housed in the same facility for at least 4 weeks, regardless of their source, before use and animal procedures were performed according to National Institute of Health guidelines and approved by the University of Iowa IACUC. In all in vivo experiments, groups consisted of 4 or more animals, and experiments were repeated at least 2 times with similar results before reporting.
Cecal-ligation and puncture
Sepsis was induced by cecal-ligation and puncture, as described previously with slight modifications (34). Briefly, mice were anesthetized, and an abdominal incision was made to identify the cecum. The distal one-third of the cecum was ligated with 4-0 silk suture, and punctured once using a 25-gauge needle. A small amount of cecal contents was extruded through the perforation. This level of injury was used to create a chronic septic state characterized by the loss of appetite and body weight, ruffled hair, shivering, diarrhea, and/or periorbital exudates, and with <5% mortality. The peritoneum was closed with a continuous suture after returning the cecum into the abdomen. The skin was then glued shut with 3M Vetbond (3M Animal Care Products, St. Paul, MN), and 1 ml saline was injected i.p. for resuscitation. For sham-treated mice, all of the same steps were performed, except for ligation and puncture of the cecum. In some cases, the CLP-treated mice received 300 μg of the anti-TRAIL blocking mAb N2B2 (eBioscience) the day after surgery.
Bacterial preparation, infection, and titer
The attenuated Listeria monocytogenes strains DP-L1942 (attenuated LM) and OVA257-expressing (attenuated LM-OVA), which are ActA deficient, were grown, injected intravenously (i.v.), and quantified as previously described (35). Briefly, the bacteria were grown to log phase (O.D.600: 0.06–0.1) in Tryptic Soy Broth (TSB) Media. Bacteria were diluted to 107 colony forming units (CFU) in 0.2 ml sterile saline (Hospira Inc., Lake forest, IL) and injected i.v.. Serial dilutions of the same culture used for injections were plated and grown overnight at 37°C, and colonies counted to independently determine the actual number of injected bacteria. Spleens and livers were harvested from infected mice on d 3 post-infection, and homogenized in 0.2% IGEPAL buffer (Sigma, St. Louis, MO). The homogenized suspension was incubated at room temperature for 30 min, and serial dilutions were plated in streptomycin agar plates. Plates were cultured overnight at 37°C and bacterial counts were determined.
Flow cytometry
For staining of surface markers, cells were incubated with fluorochrome-conjugated mAb at 4°C for 30 min. The cells were then washed with FACS buffer (PBS containing 2% BCS and 0.2% NaN3) and then fixed with 2% paraformaldehyde in PBS. mAb used for surface staining were FITC-CD11a (Clone M17/4; Biolegend), PerCP- or APC-CD8 (Clone 53-6.7; Biolegend), PE- or PerCP/Cy5.5-CD3 (Clone 17A2 for PE and Clone 145-C211 for PerCP/Cy5.5; Biolegend), PerCP- or APC-CD4 (Clone GK1.5, Biolegend), and PE-CD19 (Clone 6D5; Biolegend).
For intracellular staining, 106 splenocytes were cultured at 37°C in the presence of OVA257 and Brefeldin A (BD Biosciences/Pharmingen) for 6 h. The cells were then washed and stained for surface markers as described above. The cells were then washed once with FACS buffer and fixed with 2% paraformaldehyde in PBS for 20 min at room temperature. After fixing, cells were washed and resuspended in Saponin buffer for 30 min at 4°C. PE- or APC-IFN-γ (Clone XMG1.2; Biolegend) was added to the cells and incubated for 20 min. Cells were washed and resuspended in FACS buffer and analyzed using a Becton Dickinson FacsCalibur.
Statistical analysis
Significant differences between 2 groups were evaluated using a two-tailed Student’s t test (p < 0.05). When more than 2 groups were compared, one-way ANOVA with a Dunnett’s post-test was used (p < 0.05). All statistical tests were performed using GraphPad Prism 5 for Mac OS X (GraphPad, La Jolla, CA).
RESULTS
Reduced bacterial clearance and Ag-specific CD8 T cell responses in CLP mice
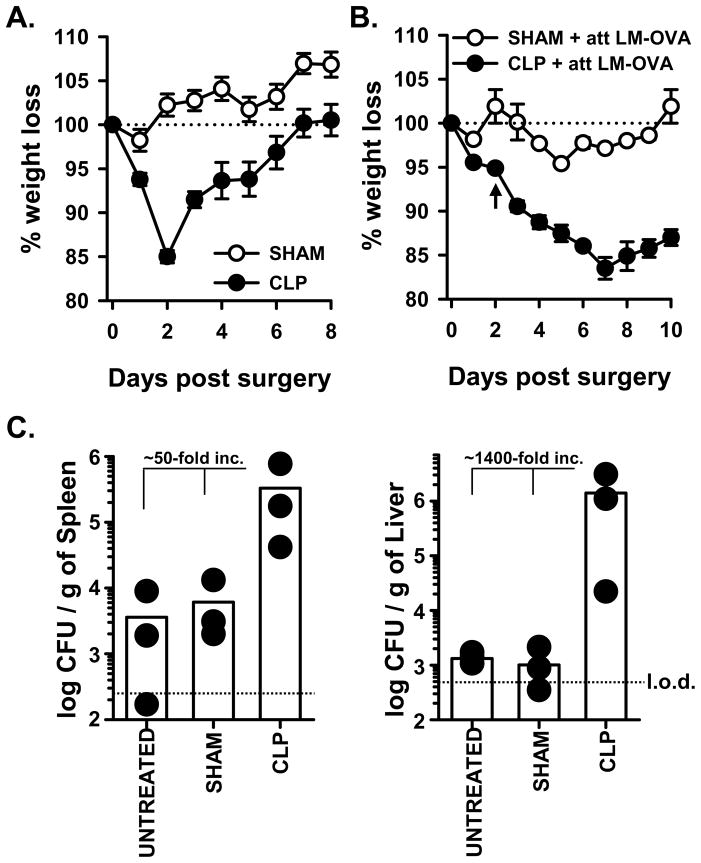
The initial hyper-inflammatory phase of sepsis is quickly followed by a sustained immunosuppressive state in patients and clinically-relevant animal models of sepsis (36, 37), and it has been suggested that this immune suppression is critical to the pathogenesis and subsequent morbidity and mortality in septic patients that acquire secondary infections. Thus, the current study examined the impact of sepsis-induced immunosuppression on the naïve CD8 T cell response to the Gram-positive, facultative intracellular bacterium Listeria monocytogenes (LM). Our version of the CLP model of sepsis involves ligation of the distal one-third of the cecum and a single puncture to permit release of the cecal contents into the peritoneum. This level of injury reproducibly resulted in the development of many clinical symptoms of sepsis, including a significant loss of weight compared to sham-treated WT B6 mice (Figure 1A). Importantly, these CLP-treated mice regained all of the lost weight by 7 d after surgery. When we added the secondary infection with attenuated LM-OVA, the sham or untreated WT B6 mice displayed minimal morbidity (as measured by weight loss; Figure 1B) and were able to control the LM-OVA infection (Figure 1C). In contrast, CLP-treated WT B6 mice that received the subsequent attenuated LM-OVA infection demonstrated sustained weight loss compared to sham-treated mice that was not regained, and these mice were less able to control the attenuated LM-OVA infection (50- and 1400-fold increase in bacterial titer in the spleen and liver, respectively, compared to untreated or sham-treated mice). These results suggest that the induction of sepsis compromises overall health such that even a secondary heterologous infection with an attenuated bacterial strain cannot be adequately controlled.
Figure 1.
CLP-treated WT B6 mice demonstrate increased morbidity and susceptibility to LM-OVA infection. A. WT B6 mice underwent sham or CLP surgery. Animal weight was measured daily beginning the day of surgery, and the percent weight loss with respect to the starting weight is shown. B. Sham or CLP surgery was performed on WT B6 mice. On d 2 post-surgery, the mice were infected with 107 CFU attenuated LM-OVA. Animal weight was measured daily beginning the day of surgery, and the percent weight loss with respect to the starting weight is shown. In A. and B., the dotted line represents the starting weight of the mice, normalized to 100%. In B., the arrow indicates the time of infection. C. Sham or CLP surgery was performed on WT B6 mice, and the mice were infected with attenuated LM-OVA on d 2 post-surgery as in B. In addition, naïve mice that did not undergo any surgery (“untreated”) were also infected with attenuated LM-OVA infection. The bacterial titer in the spleen and liver were then determined on d 3 post-infection. The dotted line represents the limit of detection (l.o.d.).
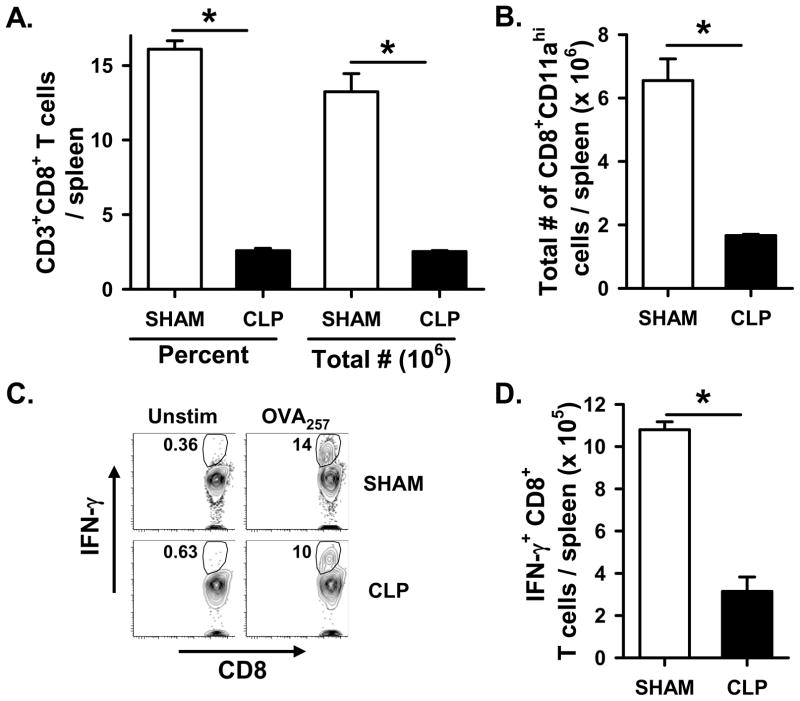
Sepsis induces widespread immune cell apoptosis, and consistent with previous studies (23), we observed a significant reduction in the number of CD8 T cells in WT B6 mice 2 d post CLP compared to sham-treated mice (Figure 2A). Similar significant reductions were also seen for CD3+CD4+, CD11c+, and CD19+ cells (Supplemental Figure 1). We then specifically examined the primary CD8 T cell response 7 d after attenuated LM-OVA infection, and found a significant decrease in total number of Ag-experienced (based on CD8α downregulation and CD11a upregulation (38); Figure 2B) and OVA257-specific CD8 T cells (based on IFN-γ production after in vitro OVA257 restimulation; Figure 2C & D) in the spleens of CLP-treated WT B6 mice compared to sham-treated mice. Since CD8 T cells are essential for the control of many bacterial infections, including LM, we have concentrated our analyses on the Ag-specific CD8 T cell response in sham and CLP-treated mice. However, a vigorous Ag-specific CD4 T cell response is generated in B6 mice to residues 190-201 of the L. monocytogenes protein listerolysin O (LLO190–201) presented on I-Ab (39, 40). Much like that for the Ag-specific CD8 T cell response, there was a significant decrease in number of LLO190-specific CD4 T cells in the spleens of LM-OVA-infected, CLP-treated WT B6 mice compared to sham-treated WT B6 mice (Supplemental Figure 2). Together, these data suggest that sepsis significantly decreases the ability of WT B6 mice to control an attenuated LM-OVA infection and generate pathogen-specific T cell responses.
Figure 2.
Ag-specific CD8 T cell responses to attenuated LM-OVA are reduced in CLP-treated WT B6 mice. WT B6 mice underwent sham or CLP surgery, and were then infected with 107 CFU attenuated LM-OVA on d 2 post-surgery. A. Total CD8 T cell numbers in the spleen were determined on d 2 post-surgery (i.e. day of infection). B–D. On d 7 after infection, the number of Ag-experienced (based on CD11a upregulation) and OVA257-specific CD8 T cells in the spleen were determined. The total number of Ag-experienced CD11ahi CD8 T cells in spleen was determined directly ex vivo by flow cytometry, while the number of OVA257-specific CD8 T cells in the spleen were determined by intracellular cytokine staining for IFN-γ afterin vitro restimulation with OVA257. Panel C shows representative flow plots measuring the frequency of IFN-γ-producing CD8 T cells with and without OVA257 restimulation in vitro. *p < 0.05 (Student’s t-test).
CLP-treated Trail−/− or Dr5−/− mice retain the ability to control a secondary LM infection and mount CD8 T cell immunity
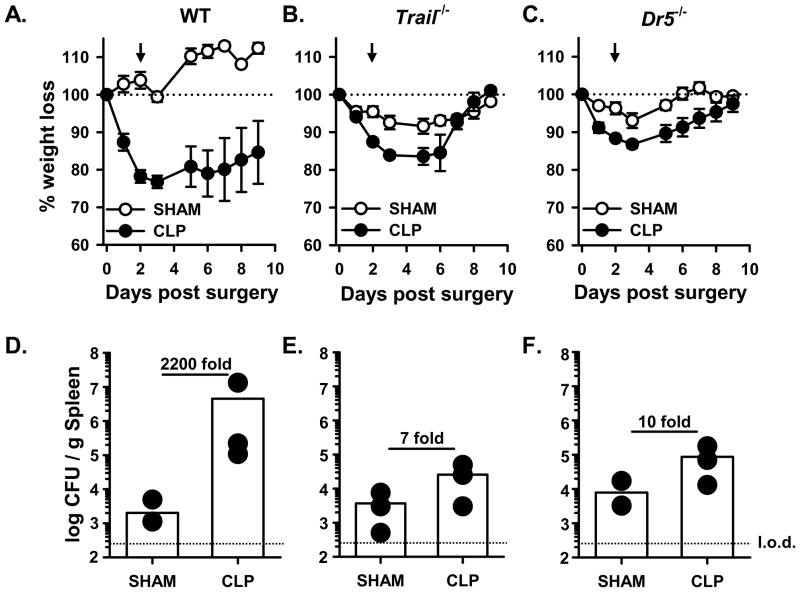
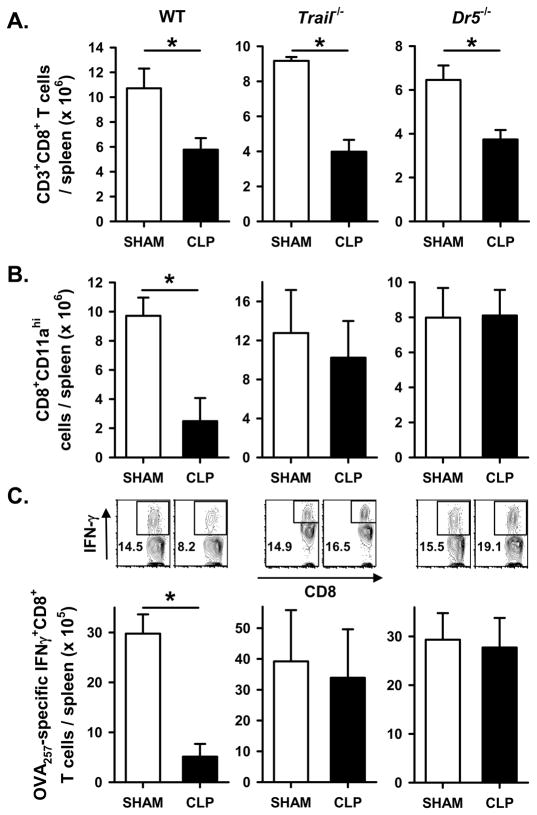
We recently showed that sepsis-induced apoptosis resulted in the TRAIL-dependent suppression of DTH (23). To determine the extent to which the TRAIL-dependent immune suppression contributed to the sepsis-induced morbidity and inability to control the secondary LM infection, we first examined weight loss in sham- and CLP-treated WT, Trail−/−, and Dr5−/− B6 mice given a secondary infection with attenuated LM-OVA. As in Figure 1, CLP-treated WT B6 mice showed sustained weight loss compared to sham-treated mice and were unable to control the attenuated LM-OVA infection as well as sham-treated mice (2200-fold increase in LM CFU in the spleen of CLP-treated mice; Figure 3A & D). By comparison, the CLP-treated Trail−/− and Dr5−/− B6 mice were able to recover their body weight back to that of the sham-treated mice (Figure 3B & C) after infection. Moreover, the splenic LM-OVA burden in CLP-treated Trail−/− and Dr5−/− B6 mice were only slightly increased over sham-treated Trail−/− and Dr5−/− B6 mice (7- and 10-fold, respectively; Figure 3E & F). Data presented in our previous report showed sepsis-induced lymphopenia was TRAIL-independent (23), and similar findings were seen in the present study (Figure 4A). However, there was no decrease in the total number of Ag-experienced CD11ahi (38) or OVA257-specific CD8 T cells in the spleens of CLP-treated Trail−/− and Dr5−/− mice compared to sham-treated mice (Figure 4B & C). Thus, these data suggest that sepsis-induced alterations in morbidity, bacterial control, and CD8 T cell responses following a secondary infection with attenuated LM-OVA requires an intact TRAIL/DR5 signaling pathway.
Figure 3.
CLP-treated Trail−/− and Dr5−/− mice have reduced morbidity after LM-OVA infection and retain the ability to control the LM-OVA infection. Sham or CLP-treated B6 WT, Trail−/−and Dr5−/− mice were infected with 107 CFU attenuated LM-OVA 2 d post-surgery. A–C. Animal weight was measured daily beginning the day of surgery, and the percent weight loss with respect to the starting weight is shown. The dotted line represents the starting weight of the mice, normalized to 100%, and the arrow indicates the time of infection. Arrowhead points to the time mice were infected with att. LM-OVA. D–F. Sham or CLP surgery was performed on WT, Trail−/−, and Dr5−/− B6 mice, and the mice were infected with attenuated LM-OVA on d 2 post-surgery as in A. The bacterial titer in the spleen was then determined on d 3 post-infection. The dotted line represents the limit of detection (l.o.d.).
Figure 4.
Maintenance of CD8 T cell responses to LM-OVA in CLP-treated Trail−/− and Dr5−/−B6 mice. Sham or CLP-treated WT, Trail−/− and Dr5−/− B6 mice were infected with 107 CFU attenuated LM-OVA 2 d post-surgery. A. Total CD8 T cell numbers in the spleen were determined 2 d post-surgery. On d 7 after infection, the number of Ag-experienced (B) and OVA257-specific (C) CD8 T cells in the spleen was determined. Panel C also shows representative flow plots measuring the frequency of IFN-γ-producing CD8 T cells with and without OVA257 restimulation in vitro. *p < 0.05 (Student’s t-test).
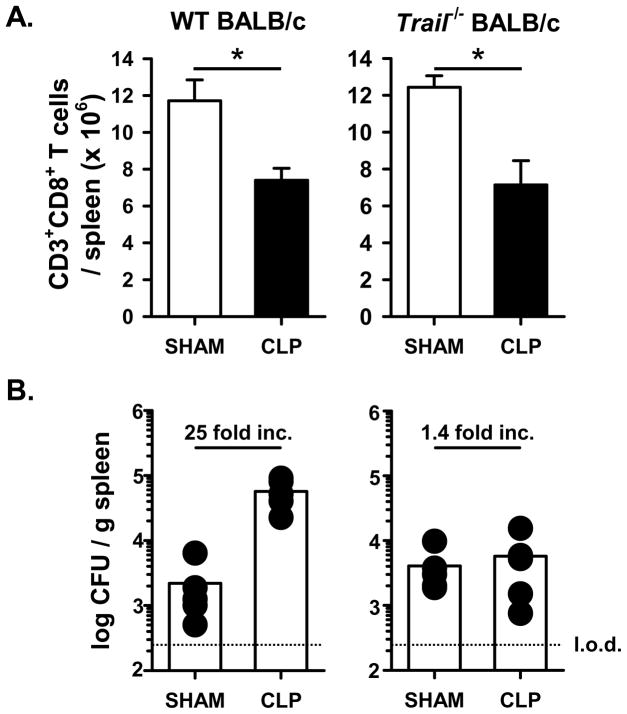
To investigate the extent to which the TRAIL-dependent decrease in ability to control a secondary bacterial infection after sepsis was mouse strain sensitive, we examined the ability of sham- and CLP-treated WT and Trail−/− BALB/c mice to control the secondary LM infection. There was a similar reduction of CD8 and CD4 T cells in CLP-treated WT and Trail−/− BALB/c mice compared to the sham-treated mice (Figure 5A). Moreover, the CLP-treated WT BALB/c mice had increased splenic LM-OVA titers compared to sham-treated mice, but there were nearly identical LM-OVA titers in the spleens of the sham- and CLP-treated BALB/c Trail−/−CLP mice (Figure 5B). Thus, these data suggest that there is no strain specificity for the observed TRAIL-dependent alterations in the ability to control a secondary bacterial infection during sepsis.
Figure 5.
CLP-treated Trail−/− BALB/c mice are able to control a secondary LM-OVA infection to the same degree as sham-treated mice. Sham or CLP-treated WT, Trail−/− and Dr5−/− BALB/c mice were infected with 107 CFU attenuated LM-OVA 2 d post-surgery. A. Total CD8 T cell numbers in the spleen were determined 2 d post-surgery. *p < 0.05 (Student’s t-test). B. The bacterial titer in the spleen was then determined on d 3 post-infection. The dotted line represents the limit of detection (l.o.d.).
Therapeutic use of a blocking anti-TRAIL mAb in septic mice decreases weight loss, improves the control of secondary bacterial infection, and restores CD8 T cell responses
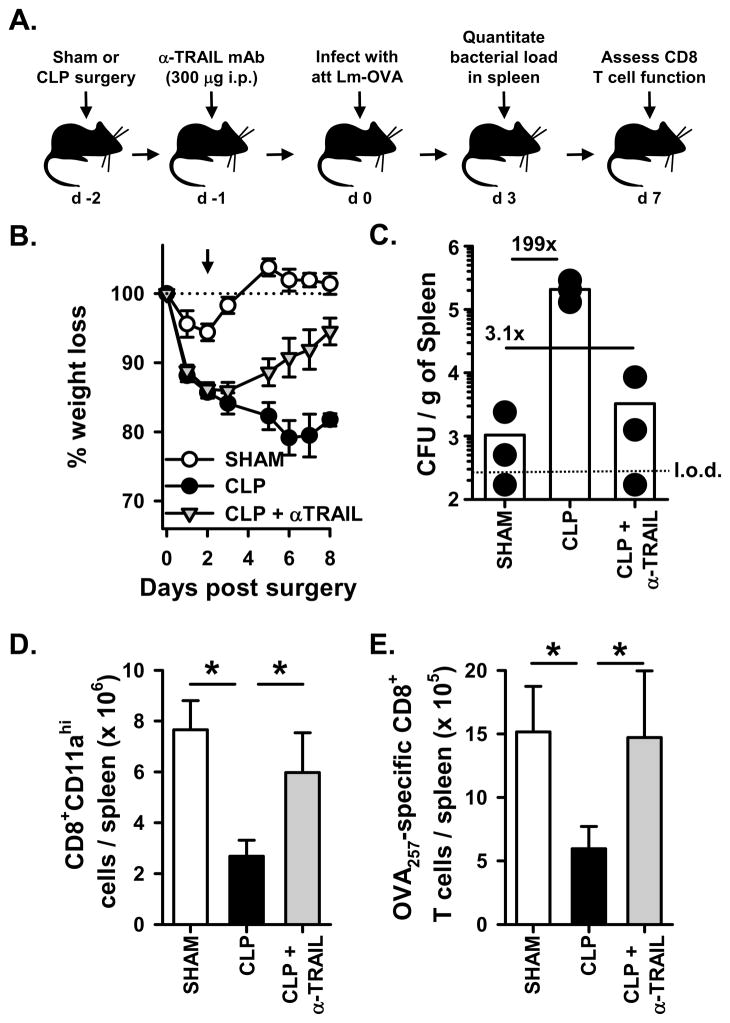
The data presented thus far suggests that an intact TRAIL/DR5 pathway correlates with increased morbidity, reduced control of a secondary bacterial infection, and decreased CD8 T cell response in CLP-treated mice. Since the genetic elimination of either TRAIL or DR5 in CLP-treated mice resulted in them behaving similar to sham-treated mice, we determined the extent to which the response of CLP-treated mice to secondary LM infection could be improved through the therapeutic disruption of the TRAIL/DR5 pathway. Thus, CLP-treated WT B6 mice were treated with a blocking anti-TRAIL mAb after the induction of sepsis, but before LM-OVA infection (Figure 6). Using this approach, the anti-TRAIL mAb-treated mice lost more weight compared to the sham-treated mice, but not as much as the CLP-treated mice (Figure 6A). However, there was a dramatic reduction in the splenic bacterial burden in the anti-TRAIL mAb-treated mice compared to the CLP-treated mice that did not receive the anti-TRAIL mAb. (Figure 6B). Similarly, the CD8 T cell response was restored in the anti-TRAIL mAb-treated mice (Figure 6C & D). The magnitude of the Ag-specific CD8 T cell response in CLP-treated mice was similar to that seen in CLP-treated mice given an isotype control IgG (data not shown), which is consistent with other reports describing the in vivo use of the anti-TRAIL blocking mAb N2B2 and isotype control Ab (41–43). Together, these results suggest that mAb-mediated disruption of the TRAIL/DR5 pathway had a therapeutic benefit in the septic mice with regard to morbidity, bacterial control, and the CD8 T cell response after a secondary bacterial infection.
Figure 6.
Administration of an anti-TRAIL blocking mAb to WT B6 mice after CLP treatment lessens morbidity, increases control of the bacterial infection, and restores CD8 T cell responses. A. Experimental design. Sham or CLP surgery was performed on WT B6 mice, and some of the CLP-treated mice received the blocking anti-TRAIL mAb N2B2 (300 μg i.p.) on d 1 post-surgery. On d 2 post-surgery, the mice were infected with 107 CFU attenuated LM-OVA. B. Animal weight was measured daily beginning the day of surgery, and the percent weight loss with respect to the starting weight is shown. The dotted line represents the starting weight of the mice, normalized to 100%, and the arrow indicates the time of infection. C. The bacterial titer in the spleen was determined on d 3 post-infection. The dotted line represents the limit of detection (l.o.d.) for bacterial count in the spleen. On d 7 after infection, the number of Ag-experienced (D) and OVA257-specific (E) CD8 T cells in the spleen was determined. *p < 0.05 (ANOVA). There was no significant difference between the Sham and CLP + anti-TRAIL mAb groups.
DISCUSSION
Sepsis induces widespread immune cell apoptosis that is required for the subsequent immune suppression (23). Consequently, septic patients and animals become very susceptible to subsequent infection that can often result in death (44). In the present study, we used a modified version of the clinically-relevant CLP sepsis model to include a secondary heterologous bacterial infection to study the role of TRAIL in sepsis-induced alterations in bacterial clearance and CD8 T cell immunity. Our results provide further evidence to suggest that TRAIL is a key molecular component in the establishment of sepsis-induced immune suppression. In this particular case, sepsis induced TRAIL-dependent immune suppression that limited the host’s ability to control a secondary bacterial infection and reduced the bacterial Ag-specific CD8 T cell response. Our data also suggest that TRAIL may be a potential therapeutic target during sepsis, since neutralization of TRAIL in septic mice restored the ability of these mice to clear the bacterial infection and mount a normal bacterial Ag-specific CD8 T cell response.
The CLP mouse model is considered the “gold standard” of sepsis models, since it reproduces the pathophysiology of polymicrobial sepsis and intra-abdominal peritonitis seen in humans (34). Early after CLP, mice show many of the symptoms of sepsis that are seen in septic humans, such as hypothermia, tachycardia, and proinflammatory cytokine production. Depending on the severity of the sepsis induced (and the experimental interest of the investigator), death may occur within the first 48 h or the mice will survive and develop immune suppression. This flexibility in disease severity and ability to study acute or chronic sepsis is simultaneously one of the biggest advantages and disadvantages of the CLP model. We reproducibly have a >95% animal survival rate in CLP-treated mice, which is similar to that reported by others examining the chronic effects of sepsis on the responsiveness of the immune system. A number of reports have combined secondary infection with CLP, and it is clear that sepsis alters the immune response to the infectious pathogen in the preclinical models much like that seen in the clinical situation. For example, CLP-treated mice are highly susceptible to pulmonary Pseudomonas aeruginosa infection and demonstrate increased mortality compared to sham-treated mice (2, 45, 46). One weakness in using P. aeruginosa as the secondary infections agent is that specific T cell responses are not as easily followed as they are when LM is used. The vast amount of information gathered over recent years regarding the T cell response to LM is one reason why we chose to use this bacterial pathogen in our system. LM normally induces a robust CD8 T cell response (25), so the observation that CLP-treated WT mice had a reduced ability to control a secondary attenuated LM infection and reduced CD8 T cell responses compared to sham-treated WT mice or CLP-treated Trail−/− or Dr5−/− mice was very intriguing. Interestingly, a recent report by Delano et al. showed that CLP mice were more resistant to virulent LM infection when compared to sham-treated mice, resulting in increased survival (46). These data are in direct contrast with the data we show in this report. Possible explanations for these differences, compared to our data, include (but are not limited to): 1) use of attenuated versus virulent LM; 2) day of infection (d 2 versus d 3 post-surgery); 3) infectious dose (107 CFU attenuated LM versus 104 CFU virulent LM); 4) day of assessment of bacterial titer (d 3 versus d 5 post-surgery); and 5) severity of sepsis induction (<5% versus ~15% mortality). Attenuated and virulent LM elicits very distinct immune responses (47). Attenuated LM is replication deficient such that it enters the cell and does not propagate; however, virulent LM replicates and propagates from one cell to another (48). The kinetics of bacterial burden and CD8 T cell responses elicited by attenuated and virulent LM are also considerably different (47) – note that LM-specific CD8 T cell were not directly measured in the Delano et al. study. Given these multiple differences, we are presently unable to reconcile the differences between the data in Delano et al. and our data. Regardless, our study adds the important observation that bacterial Ag-specific CD8 T cell responses are suppressed in septic mice in a TRAIL-dependent manner.
We previously showed that sepsis-induced apoptosis led to suppression of DTH through a TRAIL-dependent mechanism (23), but the similar importance of TRAIL-dependent suppression of CD8 T cell responses during sepsis to a secondary heterologous bacterial infection had not been demonstrated until now. In our previous study, the suppression of DTH in septic mice resulted from the activity of TRAIL-expressing CD8 Treg cells (23). We have had a long-standing interest in the tolerogenic nature of apoptotic cells, especially the ability of apoptotic cells to influence the induction of T cell-mediated immunity, and the induction of Ag-specific unresponsiveness (or tolerance) in an adult can be achieved experimentally through a variety of approaches (49–53). The use of several different experimental “tolerance” models has helped us demonstrate that one way the immune system reacts to the plethora of self Ag derived from a large wave of apoptotic cells is the generation of a CD8 Treg population that can suppress subsequent immune responses in TRAIL-dependent manner (51, 53, 54).
Despite all the studies that have been performed to understand the pathogenesis of sepsis, therapeutic targets still remain ill-defined, especially for immunosuppressed sepsis patients that are susceptible to secondary infections. Recent studies have shown that therapies that block apoptosis of lymphocyte populations, either through administration of IL-7, IL-15, or anti-PD1 mAb, result in increased survival after sepsis induction in mice (55–57). While these findings are exciting, these reports did not investigate the health of the immune system in regard to the ability to control subsequent heterologous infections. We included the use of a blocking anti-TRAIL mAb in the present series of experiments, not only as a complementary way to demonstrate the importance of TRAIL in the sepsis-induced unresponsiveness to a secondary heterologous bacterial infection, but to also test the extent to which therapeutic disruption of the TRAIL/DR5 pathway would have any benefits during sepsis. We hypothesized the anti-TRAIL mAb would inhibit the function of the immunosuppressive TRAIL-expressing CD8 Treg cells generated during sepsis (23), and the data showing the restored ability to control the LM-OVA infection and CD8 T cell responses would suggest this occurred. It is also possible that the anti-TRAIL mAb was having an effect outside of the immune system. For example, apoptosis of epithelial cells in the small and large intestine is increase in animals and human suffering from sepsis, and is related to a poor prognosis (37, 58–60). While the data presented in Figure 6 investigated the potential impact of the anti-TRAIL mAb on immune system responses to a secondary heterologous bacterial infection after sepsis, we cannot exclude the possibility that the anti-TRAIL mAb was also inhibiting the death of cells outside of the immune system. Thus, anti-TRAIL mAb blockade of intestinal epithelial cell apoptosis may have contributed to the increased weight gain in this group of CLP-treated mice. Further investigation, which would also include analyses of CLP-treated Trail−/− and Dr5−/− mice, is needed to determine the likelihood of this. Regardless, the data presented herein suggest TRAIL could be a potential therapeutic target in sepsis to help decrease the susceptibility to potentially life-threatening secondary heterologous infections.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants AI 077565 (TSG), CA109446 (TSG), AI83286 (VPB), and a University of Iowa Carver College of Medicine Medical Research Initiative Grant (TSG).
References
- 1.Angus DC, Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. 2006;6:207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- 2.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 78:1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 5.van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, van der Poll T. Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med. 2007;35:2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- 6.Emonts M, Sweep FC, Grebenchtchikov N, Geurts-Moespot A, Knaup M, Chanson AL, Erard V, Renner P, Hermans PW, Hazelzet JA, Calandra T. Association between high levels of blood macrophage migration inhibitory factor, inappropriate adrenal response, and early death in patients with severe sepsis. Clin Infect Dis. 2007;44:1321–1328. doi: 10.1086/514344. [DOI] [PubMed] [Google Scholar]
- 7.van Zoelen MA, Vogl T, Foell D, Van Veen SQ, van Till JW, Florquin S, Tanck MW, Wittebole X, Laterre PF, Boermeester MA, Roth J, van der Poll T. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med. 2009;180:1098–1106. doi: 10.1164/rccm.200810-1552OC. [DOI] [PubMed] [Google Scholar]
- 8.Klesney-Tait J, I, Turnbull R, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454–1461. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 11.Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24(Suppl 1):107–119. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 12.Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, DeForge L, Kenney JS, Remick DG, Bloedow DC, et al. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992;89:1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 14.Yang QH, Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004;279:16963–16970. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- 15.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- 16.Nasraway SA., Jr Sepsis research: we must change course. Crit Care Med. 1999;27:427–430. doi: 10.1097/00003246-199902000-00054. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 363:87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci U S A. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 21.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J Immunol. 2010;184:6766–6772. doi: 10.4049/jimmunol.0904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 25.Harty JT, V, Badovinac P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Brunt LM, Portnoy DA, Unanue ER. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 28.Mielke ME, Niedobitek G, Stein H, Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989;170:589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladel CH, I, Flesch E, Arnoldi J, Kaufmann SH. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- 30.Kaufmann SH, Ladel CH. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 31.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, Burns TF, Ajuha H, Page R, Wu GS, Chen Y, McKenna WG, Bernhard E, Lowe S, Mak T, El-Deiry WS. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 38.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbin GA, Harty JT. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J Immunol. 2004;173:5679–5687. doi: 10.4049/jimmunol.173.9.5679. [DOI] [PubMed] [Google Scholar]
- 40.Geginat G, Schenk S, Skoberne M, Goebel W, Hof H. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J Immunol. 2001;166:1877–1884. doi: 10.4049/jimmunol.166.3.1877. [DOI] [PubMed] [Google Scholar]
- 41.Tasew G, Nylen S, Lieke T, Lemu B, Meless H, Ruffin N, Wolday D, Asseffa A, Yagita H, Britton S, Akuffo H, Chiodi F, Eidsmo L. Systemic FasL and TRAIL neutralisation reduce leishmaniasis induced skin ulceration. PLoS Negl Trop Dis. 4:e844. doi: 10.1371/journal.pntd.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–213. [PubMed] [Google Scholar]
- 43.Fang Y, Sharp GC, Yagita H, Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216:505–513. doi: 10.1002/path.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J Leukoc Biol. 2009;85:382–390. doi: 10.1189/jlb.0808491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 46.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O’Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter BB, Harty JT. The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display. Infect Immun. 2006;74:1528–1536. doi: 10.1128/IAI.74.3.1528-1536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darji A, Bruder D, zur Lage S, Gerstel B, Chakraborty T, Wehland J, Weiss S. The role of the bacterial membrane protein ActA in immunity and protection against Listeria monocytogenes. J Immunol. 1998;161:2414–2420. [PubMed] [Google Scholar]
- 49.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 51.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 52.Griffith TS, Brincks EL, Gurung P, Kucaba TA, Ferguson TA. Systemic immunological tolerance to ocular antigens is mediated by TRAIL-expressing CD8+ T cells. J Immunol. 2011;186:791–798. doi: 10.4049/jimmunol.1002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurung P, Kucaba TA, Schoenberger SP, Ferguson TA, Griffith TS. TRAIL-expressing CD8+ T cells mediate tolerance following soluble peptide-induced peripheral T cell deletion. J Leukoc Biol. 2010;88:1217–1225. doi: 10.1189/jlb.0610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurung P, Kucaba TA, Ferguson TA, Griffith TS. Activation-induced CD154 expression abrogates tolerance induced by apoptotic cells. J Immunol. 2009;183:6114–6123. doi: 10.4049/jimmunol.0901676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husain KD, Coopersmith CM. Role of intestinal epithelial apoptosis in survival. Curr Opin Crit Care. 2003;9:159–163. doi: 10.1097/00075198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–592. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 60.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.