Abstract
Type I natural killer T (NKT) cells, or iNKT cells, express a semi-invariant T cell receptor characterized by its unique V α 14-Jα 18 usage (iV α 14TCR). Upon interaction with glycolipid/CD1d complexes, the iV α 14TCRs transduce signals that are essential for iNKT selection and maturation. However, it remains unclear how these signals are regulated and how important such regulations are during iNKT development. Diacylglycerol (DAG) is an essential second messenger downstream of the TCR that activates the PKCθ-IKKα/β-NFκB pathway, known to be crucial for iNKT development, as well as the RasGRP1-Ras-Erk1/2 pathway in T cells. DAG kinases (DGKs) play an important role in controlling intracellular DAG concentration and thereby negatively regulate DAG signaling. Here we report that simultaneous absence of DAG kinase α and ζ causes severe defects in iNKT development, coincident with enhanced IKK-NFκB and Ras-Erk1/2 activation. Moreover, constitutive IKKβ and Ras activities also result in iNKT developmental defects. Thus, DAG-mediated signaling is not only essential but also needs to be tightly regulated for proper iNKT cell development.
INTRODUCTION
Natural killer T (NKT) cells are a subset of rare T cells that bridge innate and adaptive immunity. Despite their rarity, NKT cells play a significant role in the modulation and/or pathogenesis of infectious diseases, allergy, autoimmunity and cancer, in part due to their ability to secrete a vast array of cytokines within minutes to hours of stimulation (1, 2). Accumulating data supports the notion that the NKT population is actually comprised of a number of developmentally and functionally distinct subsets (3). The majority of NKT cells express a semi-invariant T cell receptor (TCR) with a unique V α 14-Jα 18 chain and a limited Vβ repertoire. These cells, called Type I NKT cells or iNKT cells, recognize glycolipids presented by CD1d, and can be readily detected by α-galactosylceramide (α-Galcer)-loaded CD1d-tetramers. While iNKT cells also arise from double positive (DP) thymocytes, they differ from conventional αβ T (cαβT) cells in that they are selected on fellow CD1d-expressing cortical thymocytes (as opposed to MHC-bearing thymic epithelial cells) (4, 5). Positively selected iNKT thymocytes down-regulate CD24 expression and undergo further maturation marked by sequential upregulation of CD44 and NK1.1 on the cell surface (3). While terminally mature CD44+NK1.1+ iNKT thymocytes become long-term residents of the thymus, for reasons yet to be completely understood, CD44+NK1.1− iNKT thymocytes exit to the periphery, where they mature independently and acquire NK1.1 expression (6, 7).
Given the semi-fixed nature of the iVα14TCRs and the fact that iNKT cells are selected on cortical thymocytes instead of thymic epithelial cells, it is not surprising that iNKT and cαβT cells have differential signaling requirements for their proper development. For instance, homotypic interactions of cortical thymocyte surface receptors Slamf1 and Slamf6 (8), as well as their associated signaling components Fyn (9, 10) and SAP (11, 12), are critically required for iNKT selection, but largely dispensable for cαβT cell development. Moreover, while the PKCθ-Carma1/Bcl10-IKK-NFκB pathway plays a minimal role in cαβT cell development, deficiency of various components of this pathway has been shown to affect iNKT selection and maturation at multiple stages (13–18).
While much effort has been devoted to identifying signals required for iNKT development, mechanisms that regulate these signals and the importance of such regulation remain largely unexplored. DAG kinases (DGKs) are a family of ten enzymes that catalyze the phosphorylation of DAG to produce phosphatidic acid (PA); they thereby control intracellular concentrations of both these critical second messengers (19–21). Recent studies have revealed that DGKα and DGKζ, isoforms expressed in T cells, play a critical role in preventing T cells from hyper-activation following TCR stimulation by inhibiting the DAG-RasGRP1-Ras-Erk1/2-AP1 signal cascade (22–24). Deficiency of either DGK α or ζ in mice results in hyper-responsiveness to TCR stimulation and correlates with decreased conversion of DAG to PA and enhanced activation of the Ras-Erk1/2-AP1 pathway (25, 26). While deficiency of either DGKα or ζ does not obviously alter cαβT cell maturation, simultaneous loss of both DGKα and ζ results in a significant decrease of CD4 or CD8 single positive (SP) thymocytes (27). In this report, we investigated the role of DGKα and ζ and the importance of tight regulation of DAG-mediated signaling for iNKT cell development. We demonstrate that DGKα and ζ play a redundant and essential role in iNKT cell ontogeny. Although iNKT cell numbers are not obviously altered in DGKα or ζ deficient mice, they are dramatically reduced in DGKα and ζ double knockout mice (DGKαζDKO) mice at multiple stages during iNKT cell development. These developmental abnormalities are correlated with dysregulated signaling downstream of DAG, as manifested by enhanced activation of the IKK-NFκB and Ras-Erk1/2 pathways in DGKαζDKO thymocytes. Moreover, hyper-activating IKKβ causes severe decreases of iNKT cell numbers in multiple stages during iNKT cell development correlated with increased death of these cells and decreased expression of ICOS. In contrast, hyper-activating Ras results in incomplete terminal differentiation of iNKT cells. Together, our data demonstrate that tight control of DAG-mediated signaling is critical for proper iNKT cell development and that DGKα and ζ redundantly inhibit IKK and Ras signaling pathways to ensure normal iNKT cell maturation.
MATERIALS AND METHODS
Mice
DGKα−/−, DGKζ−/−, and DGKα−/− ζ−/− mice were previously described (25–27). The conditional constitutively active (CA)-KRas mice (28), CA-IKKβ mice (29), and TCRβ −/−δ−/− mice were purchased from the Jackson Laboratory. The CD4Cre transgenic mice were purchased from Taconic Farm. All mice were backcrossed onto B6 background for at least 9 generations. The experiments described in this study were reviewed and approved by the Duke University Institute Animal Care and Use Committee.
Isolation of liver mononuclear cells
Whole livers harvested from mice were thoroughly mashed in 20 ml of IMDM medium (10% fetal bovine serum). After the debris settled, the upper cell suspension was passed through nylon mesh and pelleted by centrifuging. The cell pellet was then resuspended in 12 ml of 35% percoll (GE Healthcare), carefully underlayed with 12 ml of 75% percoll, and centrifuged at 1000×g for 20 minutes at room temperature with no brake. Cells accumulated at the interface were collected, washed, pelleted, and again resuspended in 10 ml of IMDM medium (10% FBS). The cell suspension was then underlayed with 2ml of Lympholyte-M (Cedarlane Laboratories), and centrifuged at 1000×g for 15 minutes at room temperature with no brake. Cells accumulated at the interface (mononuclear cells) were collected, counted, and subjected to further analysis.
Antibodies and flow cytometry
PE-conjugated mouse CD1d tetramer loaded with α-GalCer was kindly provided by the NIH tetramer core facility. Live/Dead® Fixable Violet Dead Cell Stain was purchased from Invitrogen. Fluorescence-conjugated anti-mouse CD24, CD44, NK1.1, CD4, CD8, TCRβ, CD45.1, CD45.2, CD122, T-bet, CD1d, ICOS, CD127, CD150 (SLAM), and Ly108 (SLAM6) antibodies were all purchased from BioLegend. Anit-Nur77 was from ebioscience. After surface staining of related iNKT lineage markers, the intracellular staining of T-bet was performed with the eBioscience Foxp3 staining buffer set following the manufacturer’s manual. All flow cytometry data were collected on FACSCanto™ II (BD Biosciences), and analyzed using the Flowjo software.
Bone marrow reconstitution
Recipient TCRβ−/− δ −/− mice were sublethally irradiated (600 rad) one day before adoptive transfer. Bone marrow cells from age- and sex-matched CD45.1+ B6 and CD45.2+ DGKαζDKO or CA-IKKβ mice were mixed at a 1:1 ratio. Ten million mixed cells were then intravenously injected into each recipient mouse. The resulting chimeric mice were analyzed 7 to 8 weeks later.
Western blot
5–10 million total thymocytes from WT or DGKαζDKO mice were rested in 0.5 ml PBS at 37°C for 20 min. Cells were then either left untreated or stimulated with an anti-CD3 antibody (500A2, 5 μg/ml, BD Biosciences) for 2 min. Cells were lysed in 1% NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, pH 7.4) with protease and phosphatase inhibitors. Proteins in lysates were separated by SDS/PAGE and transferred onto nitrocellulose membrane. The blots were probed with anti-phospho-Erk1/2, anti-phospho-IκBα (Ser32), anti-total-IκBα, and anti-phospho-NFκB (Ser536), all of which were purchased from Cell Signaling. For loading control, the blots were stripped and reprobed with anti-β-actin (Sigma).
Real time PCR
Fifteen million viable CD4+CD8+ DP thymocytes from age- and sex-matched WT, DGKαζDKO, and CA-IKKβ mice were sorted on MoFlo Cell Sorter (Beckman Coulter), with post-sort purity>98%, and lysed in Trizol (Invitrogen). Total RNAs were extracted, and cDNAs were obtained using the Superscript III First-Strand Synthesis System (Invitrogen). Realtime PCR was prepared using the RealMasterMix (Eppendorf) and performed on the Mastercycler® ep realplex2 system (Eppendorf). Primers used for different genes are listed in supplemental Table 1.
Assessment of V α-J α recombination
Five million viable CD4+CD8+ thymocytes from age- and sex-matched WT, DGKαζDKO, and CA-IKKβ mice were sorted on MoFlo Cell Sorter (Beckman Coulter), with post-sort purity>98%, and genomic DNAs were extracted with phenol/chloroform, precipitated with 70% ethanol, and dissolved in TE buffer (10 mM Tris-0.5 mM EDTA, pH 8.0). For semi-quantitative PCR, decreasing amounts of DNA template (100 ng, 33 ng, 11 ng) from each sample were used. The forward primer for V α 14 segment was 5′-acactgccacctacatctgt-3′. The reverse primers for different Jα segments were: Jα2 5′-ggttgcaaatggtgccactt-3′; Jα 18 5′-gtagaaagaaacctactcacca-3′; Jα56 5′-tgtcatcaaaacgtacctggt-3′. Primers for CD14 PCR (loading control) were: forward 5′-gctcaaactttcagaatctaccgac-3′, reverse agtcagttcgtggaggccggaaatc-3′.
Statistics
For statistic analysis, two-tail Student t-test was performed. *, p<0.05. **, p<0.01, ***, p<0.001.
RESULTS
Deficiency of DGKα or ζ has minimal impact on iNKT development
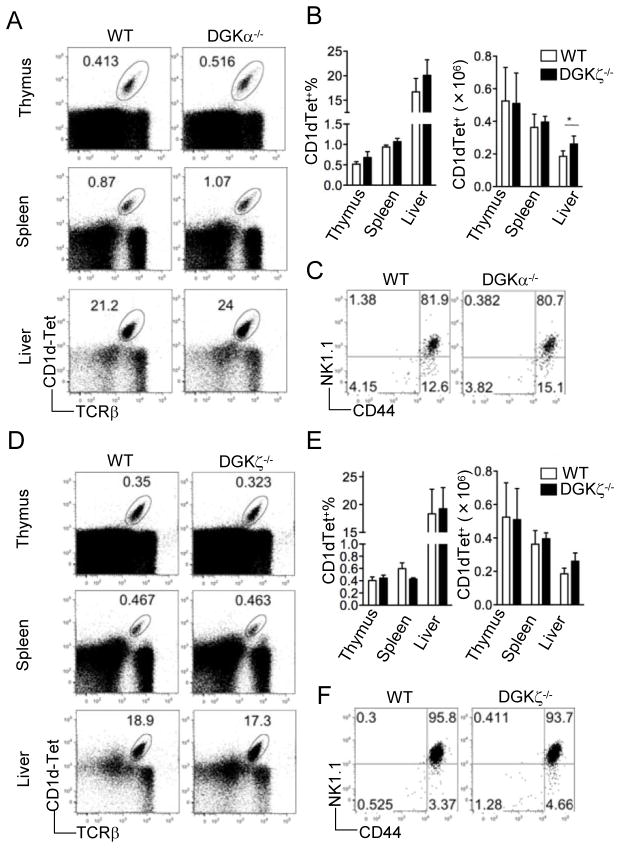
We first examined iNKT cell development in mice deficient of either DGKα or ζ. Total iNKT cells in the thymus, spleen, and liver were examined by dual surface staining of TCRβ and α-Galcer-loaded CD1d-Tetramer (CD1d-Tet). No significant defects in percentages or absolute numbers of total iNKT cells were found in DGKα−/− or DGKζ−/− mice except that liver iNKT cell number was slightly increased in DGKα−/− mice (Fig 1A–B and D–E). Individual developmental stages within the CD1dTet+CD24− thymic iNKT cells were further analyzed by their surface expression of CD44 and NK1.1, and no obvious differences were detected between DGKα−/− or DGKζ−/− mice and WT controls (Fig 1C and F). Thus, absence of either DGKα or ζ does not drastically affect iNKT cell development.
Figure 1.
iNKT cell developmental in mice deficient of either DGKα or ζ Thymocytes, splenocytes, and liver mononuclear cells from DGKα−/− (A–C) or DGKζ−/− (D–F) mice and age/sex-matched WT controls were isolated, counted, and subjected to flow cytometry analysis. FACS plots shown are representative of five mice per group. (A) and (D), Flow cytometry of total thymocytes, splenocytes, and liver mononuclear cells stained with CD1d-Tet and anti-TCRβ. (B) and (E), Percentage (left) and number (right) of live CD1d-Tet+TCRβ+ cells in thymus, spleen, and liver (mean, s.e.m.). *, p<0.05 (student t-test). (C) and (F), Expression of CD44 vs. NK1.1 on live CD1d-Tet+CD24− gated thymocytes.
Severe developmental defects of iNKT cells in DGKα−/− ζ−/− mice
To determine whether DGKα and ζ play a redundant role in iNKT cell development, we analyzed mice deficient in both DGKα and ζ. Drastic reduction of CD1dTet+TCRβ+ iNKT cells was observed in the thymus, spleen, and liver of DGKα −/−ζ−/− (DGKαζDKO) mice as compared to WT mice (Fig 2A–B). Further analysis of the few remaining iNKT cells revealed significant decreases in the percentage of stage 3 cells and corresponding increases in the percentage of stage 2 cells in DGKαζDKO mice (Figure 2C–2D). However, due to the drastic decrease of total iNKT cells in DGKαζDKO mice, the absolute numbers of CD44−NK1.1− (stage 1), CD44+NK1.1− (stage 2), and CD44+NK1.1+ (stage 3) DGKαζDKO iNKT cells were all decreased as compared to WT controls (Fig 2D). Furthermore, high death rates could be detected in all three stages of DGKαζDKO iNKT cells, suggesting that enhanced death may contribute to the decrease of iNKT cells in DGKαζDKO mice (Fig 2E). Together, these observations demonstrate that DGKα and ζ play redundant and crucial roles for normal iNKT development.
Figure 2.
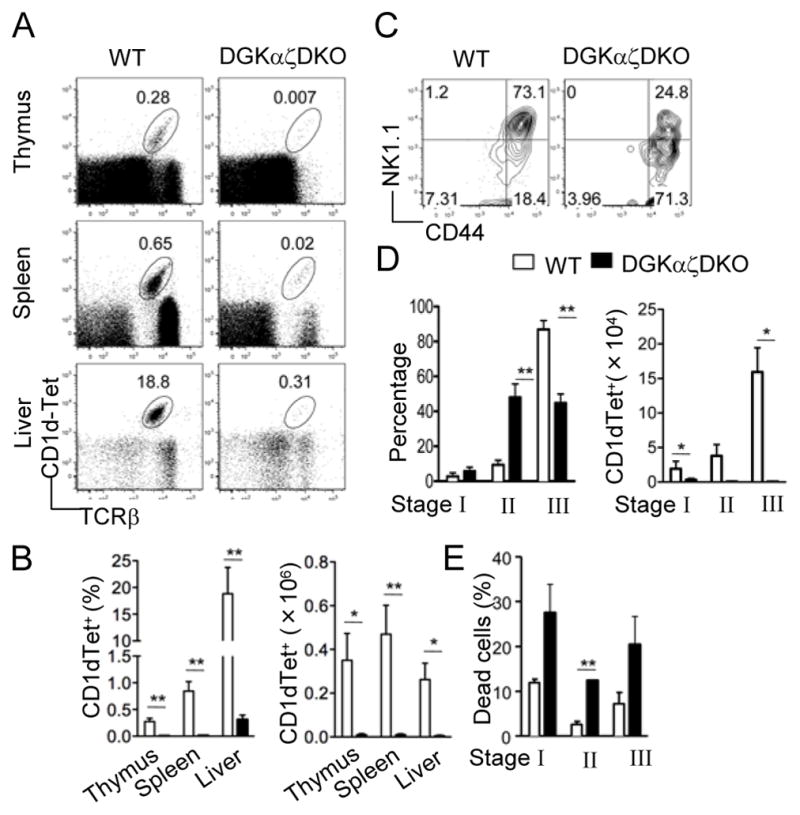
Severe iNKT cell developmental defects in DGKαζDKO mice Thymocytes, splenocytes, and liver mononuclear cells from age- and sex-matched DGKαζDKO mice and WT controls were subjected to flow cytometric analysis. Data shown are representative of five mice per group. (A) Flow cytometry of cells stained with CD1d-Tet and anti-TCRβ. (B) Percentage (left) and number (right) of live CD1d-Tet+ TCRβ+ cells (mean, s.e.m.). (C) Expression of CD44 vs. NK1.1 on live CD1d-Tet+CD24− gated thymocytes. (D) Percentage (left) and number (right) of CD1d-Tet+CD24− live thymocytes in different iNKT developmental compartments (mean, s.e.m.). (E) Percentage of cell death (defined by positive Live-Dead® staining) in different iNKT developmental compartments (mean, s.e.m.). *, p<0.05; **, p<0.01; ***, p<0.001.
Cell intrinsic defect of developing DGKαζDKO iNKT cells
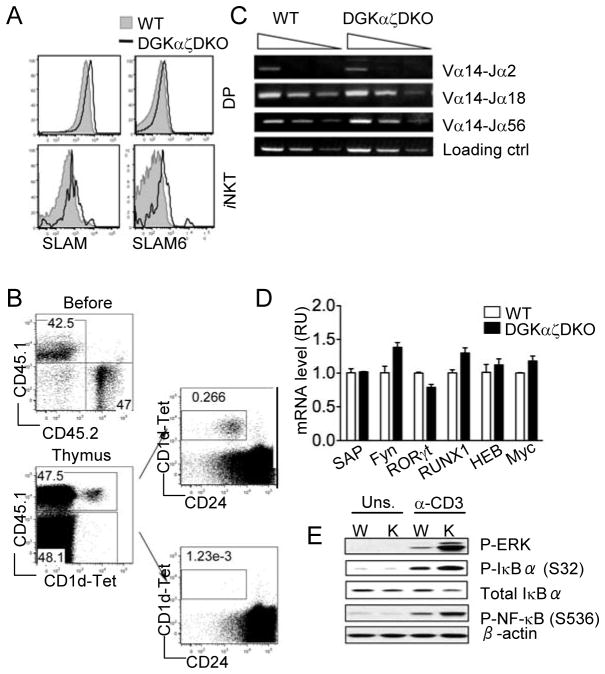
iNKT cells are positively selected when their iVα14TCRs interact with glycolipid-CD1d complexes presented by cortical thymocytes. Defects in CD1d expression itself (30, 31) or in the lipid antigen loading process (1, 32) will all lead to abolishment of the iNKT development. In addition, cortical thymocyte surface receptors Slamsf1 and Slamsf6 provide co-stimulatory signals that are also essential for iNKT selection and development (8, 33). First, we asked whether the combined deficiency of DGKα and ζ affects the expression of these cell surface molecules. No significant difference in the surface expression levels of CD1d were detected between WT and DGKαζDKO CD4+CD8+ DP thymocytes (data not shown). Slamsf1 (SLAM), and Slamsf6 (SLAM6) were slightly increased in DP thymocytes but obviously increased in iNKT cells from DGKαζDKO mice as compared to WT controls (Fig 3A), suggesting that the developmental defects of DGKαζDKO iNKT cells are unlikely caused by decreased SLAM or SLAM6 expression. At present, it is unclear whether elevated SLAM and SLAM6 expression may contribute to the developmental abnormalities of DGKαζDKO iNKT cells.
Figure 3. iNKT cell developmental defects in DGKαζDKO mice are cell-intrinsic and correlated with elevated DAG-mediated signaling.
(A) Expression of SLAM (CD150) and SLAM6 (Ly108) on iNKT cells and DP thymocytes and TCR from WT and DGKαζDKO mice. Data are representative of three mice per group. (B) Left top panel, expression of CD45.1 and CD45.2 on mixed WT and DGKαζDKO bone marrow cells before adoptive transfer. Left bottom panel, CD45.1 and CD1d-Tet staining on total live thymocytes from recipient mice 7–8 weeks after bone marrow reconstitution. Right panels, CD1d-Tet and CD24 staining on CD45.1+ WT (top) and CD45.1− DGKαζDKO (bottom) live thymocytes from recipient mice. Data are representative of three experiments. (C) Semi-quantitative PCR analysis of sorted CD4+CD8+ thymocytes from WT and DGKαζDKO mice with primers for V α 14-Jα2, V α 14-Jα 18, V α 14-Jα56, and CD14 (loading control). (D) Real-time PCR analysis of mRNA expression of various proteins in sorted CD4+CD8+ thymocytes from WT and DGKαζDKO mice. (E) Western blotting analysis with indicated antibodies of WT (W) and DGKαζDKO (K) thymocytes left unstimulated (Uns.) or stimulated with anti-CD3.
To further examine if the DGKαζDKO iNKT developmental defects are cell intrinsic, CD45.2+ DGKαζDKO bone marrow (BM) cells were mixed with an equal amount of CD45.1+ WT BM cells, and adoptively transferred into sublethally irradiated TCRβ−/−δ−/− hosts. iNKT cell development in recipient chimeric mice was analyzed 7–8 weeks after reconstitution. As shown in Figure 3B, equal reconstitution of total thymocytes from WT and DGKαζDKO BM cells was achieved in the recipient mice. However, CD1dTet+ iNKT cells could only be detected in the CD45.1+ WT thymocytes, but not in the CD45.2+ DGKαζDKO compartment. Similar observations were made in the spleen and liver as well (Fig S1). Thus, the aforementioned iNKT developmental defects in the DGKαζDKO mice are cell-intrinsic.
Intrinsically, the most important driving force for iNKT cell development is the generation of functional, CD1d-restricted iV α 14TCRs by the cortical DP thymocytes. Insufficient V α 14-Jα 18 recombination has been shown to cause severe early block in iNKT development (34, 35). We found that the DGKαζDKO DP thymocytes are equally capable of rearranging the V α 14 segment to Jα18, Jα2 or Jα56 segments as compared to the WT controls (Fig 3C), suggesting a normal frequency of iV α 14TCR-expressing iNKT precursors at the DP stage. In addition, many other intrinsic factors have been identified as critical for early iNKT cells development, including signaling proteins SAP and Fyn as well as transcription factors RORγt (35, 36), Runx1 (36), cMyc (37), and HEB (34). However, no obvious decreases in mRNA expression levels of these molecules were detected between WT and DGKαζDKO DP thymocytes (Fig 3D).
Altered signaling in DGKαζDKO thymocytes
Based on the data discussed above, we reasoned that the intrinsic developmental defects in DGKαζDKO iNKT cells are most likely caused by dysregulated intracellular signaling activities. Next, we investigated how DGKα and ζ deficiency may affect TCR-induced DAG-mediated signaling pathways in thymocytes. As shown in Figure 3E, TCR induced phosphorylation of IκBα at serine 32 and NFκB at serine 536, both IKK dependent events, were elevated in DGKαζDKO thymocytes as compared to WT thymocytes. IκBα phosphorylation leads to its ubiquitination and degradation, allowing for the nuclear translocation of NFκB. Indeed, total IκBα protein level was decreased in DGKαζDKO thymocytes following TCR engagement as compared with WT thymocytes. Similar to previous observations derived from studies performed with mice in 129/B6 mixed background, TCR-induced Erk1/2 phosphorylation was also elevated in DGKαζDKO thymocytes of C57B6/J background. Together, these data suggest that in DGKαζDKO thymocytes, DAG-mediated activation of both the Ras-Erk1/2 and PKCθ-IKK-NFκB pathways is enhanced.
Defective iNKT cell terminal maturation due to enhanced Ras signaling
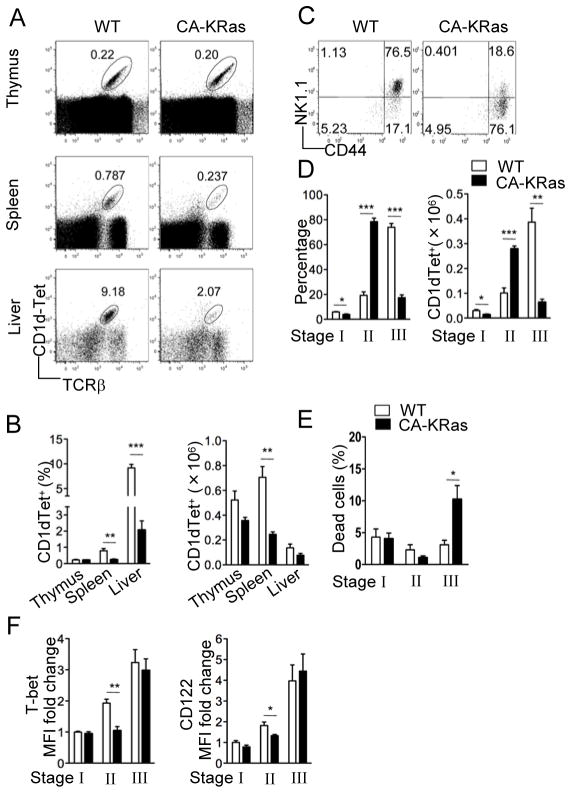
Since the Ras-Erk1/2 pathway is significantly enhanced in DGKαζDKO thymocytes, we asked how elevated Ras signaling might affect iNKT cell development. We generated mice expressing a T-cell specific CA-KRas by breeding mice carrying a conditional CA-KRas allele with CD4-Cre transgenic mice. These mice carry a point mutation (G12D) in the KRas gene whose expression is normally blocked by the presence of a loxP-flanked transcription stop cassette (28). CD4-Cre mediated deletion of the stop cassette turns on the CA-KRas expression before the DP stage, which is when iNKT development begins. In contrast to DGKαζDKO mice, total iNKT cell numbers are only moderately decreased in these CA-KRas mice (Fig 4A–B). Strikingly, the mature CD44+NK1.1+ subset, which generally dominates the iNKT cell pool in WT thymus, was dramatically decreased in the CA-KRas mice and correlated with a significant accumulation of the CD44+NK1.1− cells (Fig 4C–D). Such a maturation block, albeit less severe, was also observed in the periphery (Fig S2). Interestingly, an increase of death of stage 3 iNKT cells was observed in CA-KRas mice as compared with WT mice, which may contribute to the decrease of total stage 3 iNKT cells in CA-KRas mice. However, different from DGKαζDKO mice, the death rates of stage 1 and 2 iNKT cells in CA-KRas mice were not increased (Fig 4E).
Figure 4.
Impaired iNKT cell terminal maturation caused by enhanced Ras signaling Thymocytes, splenocytes, and liver mononuclear cells from age- and sex-matched CA-KRas mice and WT controls were subjected to flow cytometric analysis. Data shown are representative of five mice per group. (A) Flow cytometry of cells stained with CD1d-Tet and anti-TCRβ. (B) Percentage (left) and number (right) of live CD1d-Tet+TCRβ+ cells (mean, s.e.m.). (C) Expression of CD44 vs. NK1.1 on live CD1d-Tet+CD24− gated thymocytes. (D) Percentage (left) and number (right) of CD1d-Tet+CD24− live thymocytes in different iNKT developmental compartments (mean, s.e.m.). (E) Percentage of cell death in different iNKT developmental compartments (mean, s.e.m.). (F) Fold change in mean fluorescence intensity (MFI) of intracellular T-bet (left) and surface CD122 (right) staining in various subsets of CD1d-Tet+CD24− thymocytes (mean, s.e.m.). *, p<0.05; **, p<0.01, ***, p<0.001.
IL-15 and T-bet are two factors identified so far that specifically affect the terminal maturation of iNKT cells. The expression of both IL-15Rβ (CD122) and T-bet is progressively upregulated as the iNKT cells mature (38, 39). Mice with either IL-15 or IL-15 receptor deficiency lack the mature NK1.1+ iNKT cells (40, 41), and T-bet−/− mice exhibit a similar block in iNKT maturation as observed in the CA-KRas mice (38). We asked whether elevated Ras signaling affected T-bet and/or IL-15R expression during iNKT development. As shown in Figure 4F, we found both intracellular T-bet and surface IL-15Rβ expression to be moderately lower in the CA-KRas CD44+NK1.1− cells as compared to WT, but relatively normal in the CD44−NK1.1− cells and CD44+NK1.1+ cells. These data suggest that the impaired iNKT maturation from stage 2 to stage 3 in CA-KRas mice might have resulted from insufficient IL-15 signaling and/or defective T-bet-mediated transcription programming at stage 2.
Overall, CA-KRas causes a late maturation block of iNKT cells and increased death of stage 3 iNKT cells, suggesting that enhanced Ras activation in DGKαζDKO mice may have contributed to the inefficient stage 2 to stage 3 transition observed in these mice.
iNKT cell developmental defect in thymocytes expressing constitutively active IKKβ
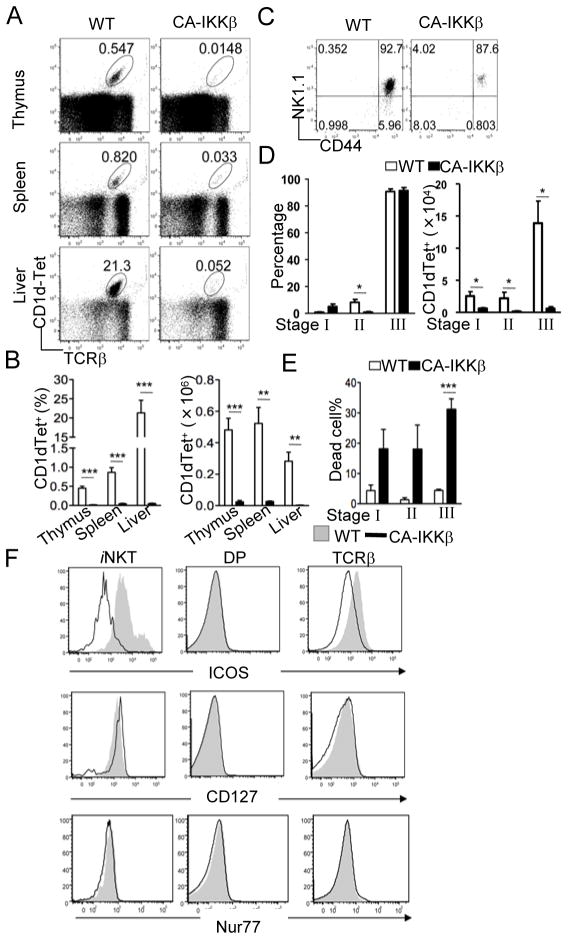
As shown above, enhanced Ras-Erk activation cannot account for the dramatic decrease of iNKT cells observed in the DGKαζDKO mice. Since DGKαζDKO thymocytes also manifest elevated IKK-NFκB signaling, we asked how enhanced signaling from this pathway might affect iNKT cell development. We generated mice expressing a CA-IKKβ in T cells by breeding mice carrying a conditional CA-IKKβ allele with CD4-Cre transgenic mice. In these CA-IKKβ mice, a floxed transcription stop cassette is located 5′ of the CA-IKKβ gene to prevent its expression until Cre-mediated deletion of this cassette occurs (29). Similar to DGKαζDKO mice, a severe decrease of iNKT cells was observed in thymus, spleen, and liver of the CA-IKKβ mice (Figure 5A–B). However, different from DGKαζDKO and CA-KRas mice, a majority of the rare iNKT cells found in the CA-IKKβ mice expressed high levels of NK1.1 (Fig 5C–D). Due to the severe decrease of iNKT cell numbers in CA-IKKβ mice, stages 1, 2, and 3 iNKT cell numbers were all decreased (Fig 5C). A potential cause of the developmental defect of CA-IKKβ iNKT cells could be caused by enhanced negative selection due to hyperactivation of IKKβ. However, no obvious difference of Nur77 expression between WT and CA-IKKβ DP thymoyctes or iNKT cells was observed following intracellular staining (Fig 5F), suggesting that CA-IKKβ may not enhance negative selection of iNKT cells. In contrast, expression of ICOS, a costimulatory molecule known to be important for iNKT cell homeostasis (21, 24, 42), was decreased in iNKT cells as well as in cαβT cells but not in DP thymocytes from CA-IKKβ mice. Although it has been proposed that ICOS promotes IL7α (CD127) expression, there was no obvious decrease of CD127 expression in CA-IKKβ iNKT cells. However, there were substantial increases of death of stage 1 to 3 CA-IKKβ iNKT cells as compared to WT controls (Fig 5E). Together, the observations demonstrate that CA-IKKβ causes severe decreases of iNKT cells correlated with decreased ICOS expression and increased iNKT cell death.
Figure 5. iNKT cell developmental defects in thymocytes expressing constitutively active IKKβ.
Thymocytes, splenocytes, and liver mononuclear cells from age- and sex-matched CA-IKKβ mice and WT controls were subjected to flow cytometric analysis. Data shown are representative of five mice per group. (A) Flow cytometry of cells stained with CD1d-Tet and anti-TCRβ. (B) Percentage (left) and number (right) of live CD1d-Tet+ cells (mean, s.e.m.). (B) Percentage (left) and number (right) of live CD1d-Tet+ TCRβ+ cells (mean, s.e.m.). (C) Expression of CD44 vs. NK1.1 on live CD1d-Tet+CD24− gated thymocytes. (D) Percentage (left) and number (right) of CD1d-Tet+CD24- live thymocytes in different iNKT developmental compartments (mean, s.e.m.). (E) Percentage of cell death in different iNKT developmental compartments (mean, s.e.m.). (F) ICOS, CD127, and Nur77 expression in iNKT cells, DP thymocytes, and TCRβ+ thymocytes. *, p<0.05; **, p<0.01; ***, p<0.001.
To determine the cell-intrinsic role of IKKβ signaling in the development and homeostasis of iNKT cells, we reconstituted sub-lethally irradiated TCRβ−/−δ −/− mice with a 1:1 mixture of WT and CA-IKKβ BM cells (Fig S3A–D). About 98% of total thymocytes in the recipient mice were derived from CD45.1+ WT BM, indicating that CA-IKKβ progenitors have a severe competitive disadvantage. Nevertheless, CD1dTet+ iNKT cells were notably absent from the CA-IKKβ compartment, suggesting that the profound block in early iNKT development in the CA-IKKβ mice was also cell-intrinsic. A similar trend was observed in spleen and liver of the recipient mice. Akin to DGKαζDKO mice, normal level of V α 14 to Jα 18 recombination was also observed in CA-IKKβ DP thymocytes (Fig S3E). CD1d, SLAM and SLAM6 expression on CA-IKKβ DP thymocytes was similar to WT controls. SLAM and SLAM6 expression in CA-IKKβ iNKT cells was slightly increased as compared to WT iNKT cells (Fig S3F). Moreover, we did not observe a significant reduction of various factors known to affect early iNKT development, such as SAP, Fyn, RORγt, RUNX1, cMyc, and HEB, between CA-IKKβ and WT DP thymocytes (Fig S3G).
While it is known that some activity of the PKCθ-Carma1/Bcl10-IKK-NFκB pathway is necessary for normal iNKT cell development, our data shows that elevated IKK signaling also proves detrimental to this process, thereby suggesting the need to maintain an optimal amount of signaling.
DISCUSSION
It has been well established that the iVα14TCR signal plays a crucial role in iNKT cell development. Among TCR signaling pathways downstream of DAG and IP3, the PKCθ-Carma1/Bcl10/Malt-IKK-NFκB pathway (13–18) and the Ca++-calcineurin-NFAT pathway (43) have been demonstrated to be essential for iNKT cell development. However, the mechanisms regulating these TCR signaling cascades and the importance of such regulation during iNKT development have been poorly understood. In this report, we demonstrate that dysregulation of DAG-mediated signaling pathways hinders iNKT cell maturation and that DGKα and ζ redundantly promote iNKT cell development by fine-tuning these DAG-mediated signaling pathways.
Although the PKCθ-Carma1/Bcl10/Malt-IKK-NFκB pathway is not essential for cαβT cell development, it is critical for the development of iNKT cells as well as regulatory T cells (Treg) (15, 44–46). It has been proposed that activation of NFκB induces transcription of molecules promoting cell survival. In this regard, it is surprising that enhanced IKK-NFκB signaling in the CA-IKKβ mice leads to severe decrease of iNKT cells. iNKT cells appear to be more sensitive to an increase of IKKβ activity than conventional αβ T cells. It has been reported that deficiency of CYLD, a tumor suppressor with deubiquitinase function, also causes decrease of iNKT cell numbers due to increased NFκB activation and cell death (24, 42). Our data are consistent with these observations and further support the importance of tight regulation of the IKK-NFκB pathway through multiple mechanisms as dysregulation of this pathway is detrimental to iNKT cells by inducing increased cell death.
Different from CA-IKKβ, enhanced Ras activity in the CA-KRas mice does not appear to inhibit early iNKT cell development, suggesting that the RasGRP1-Ras pathway may not be involved in negative selection of iNKT cells. Yet the iNKT terminal maturation from stage 2 to stage 3, often referred to as the checkpoint 2, was dramatically blocked in the CA-KRas mice, resulting in a severe loss of mature CD44+NK1.1+ cells and a simultaneous accumulation of the CD44+NK1.1− cells in the thymus, accompanied by selectively reduced T-bet and IL15Rβ expression at the CD44+NK1.1− stage. Previous studies have shown that CD1d expression is required for iNKT terminal maturation (47), suggesting that continued signaling from iVα 14TCR remains critical at this checkpoint. In addition, IL-15 signaling and T-bet have been demonstrated to play essential and selective roles in promoting the iNKT maturation from stage 2 to stage 3. Our data suggested that strict regulation of the Ras-Erk pathway downstream of iVα 14TCR at checkpoint 2 is essential for iNKT terminal maturation, possibly by ensuring efficient IL-15 signaling and/or proper T-bet-mediated transcription programming. Moreover, the fact that enhanced Ras-Erk activity only affects late stage iNKT maturation but not early development suggests that qualitatively and/or quantitatively different TCR signals are involved in these two developmental checkpoints.
We demonstrate that DGKα and ζ perform redundant roles during early development and terminal maturation of iNKT cells. DGK activity regulates both DAG and PA concentrations. Our data suggest that the major impact of DGKα and ζ deficiency on iNKT development may be caused by uncontrolled DAG signaling since hyperactivation of either IKKβ or KRas can also cause iNKT cell developmental defects. CA-IKKβ and CA-KRas cause iNKT cell developmental defects that can mimic DGKαζDKO to certain degrees. For example, both CA-IKKβ and DGKαζDKO mice display severe decreases of iNKT cells from stage 1 to 3, correlated with increased cell death. Similar to CA-IKKβ mice, ICOS expression in DGKαζDKO iNKT cells and cαβT cells is decreased (supplemental Fig S4A). In addition, stage 2 iNKT cells were relatively enriched in the remaining iNKT of DGKαζDKO mice and a defect in stage 2 to stage 3 maturation can be seen in CA-KRas mice. However, there are obvious differences between DGKαζDKO mice and CA-IKKβ or CA-KRas mice. Different from CA-KRas iNKT cells, there is no decrease of T-bet or CD-122 expression on DGKαζDKO stage 2 iNKT cells (supplemental Fig S4A). In contrast, CD122 expression is upregulated in CA-IKKβ iNKT cells (supplemental Fig S4B). Nur77 experssion is slightly increased in DGKαζ iNKT cells but not in CA-IKKβ iNKT cells. Furthermore, the relative percentages of CD4+, CD4−, and CD8+ populations within iNKT cells also appear different among these mice. While these populations in CA-KRas mice are not obviously different from WT mice, the CD4+ to the CD4− iNKT cell ratio is slightly increased in DGKαζDKO mice. In contrast, the relative percentage of CD4+ iNKT cells is decreased while the percentage of CD8+ iNKT cells is increased in CA-IKKβ mice (supplemental Fig S4C). Such differences may reflect the fact that DGKα and ζ double deficiency affects both the Ras-Erk1/2 and the PKCθ-IKK-NFκB pathways. Furthermore, PA has also been reported to regulate the activities of multiple signaling molecules such as mTOR, Sos, and PI5K (20, 48–50). A reduction of DGK-derived PA could also contribute to the developmental abnormality in DGKαζDKO mice as well as the differences of DGKαζDKO mice to the CA-IKKβ and CA-KRas mice.
The semi-fixed nature of the iV α 14TCR and the existence of likely limited endogenous ligands presented by CD1d to this TCR suggest that iV α 14TCR signaling must be tightly regulated to ensure proper development of iNKT cells. Based on our data and other published studies, we propose that DAG signaling is not only essential but also needs to be tightly controlled for normal iNKT cell development. Under physiological conditions, DGKα and ζ may play a redundant role to ensure normal development and homeostasis of iNKT cells by tuning down DAG-mediated activation of the PKCθ-IKK-NFκB and the RasGRP1-Ras-Erk1/2 pathways. Absence of DGKα and ζ activities leads to dysregulated DAG-mediated signaling and defective iNKT cell development and homeostasis.
Supplementary Material
Acknowledgments
We thank Nancy Martin in Duke Cancer Center Flow Cytometry Core Facility for providing sorting services, the NIH Tetramer Core Facility for providing the CD1d tetramer, and Tommy O’Brien for critically reviewing the manuscript.
Abbreviations
- α-Galcer
α-galactosyl ceramide
- DAG
Diacylglycerol
- DGK
DAG kinase
- IKK
IκB kinase
- iNKT
invariant natural killer T cell
- iVα14TCR
invariant Vα14-Jα18 T cell receptor
Footnotes
This study is supported by funding from the National Institute of Health (R01AI076357, R01AI079088, and R21AI079873), the American Cancer Society, and the American Heart Association to X-P.Z.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Brutkiewicz RR, Sriram V. Natural killer T (NKT) cells and their role in antitumor immunity. Crit Rev Oncol Hematol. 2002;41:287–298. doi: 10.1016/s1040-8428(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 7.Berzins SP, McNab FW, Jones CM, Smyth MJ, Godfrey DI. Long-term retention of mature NK1.1+ NKT cells in the thymus. J Immunol. 2006;176:4059–4065. doi: 10.4049/jimmunol.176.7.4059. [DOI] [PubMed] [Google Scholar]
- 8.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn-deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 10.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanic AK, Bezbradica JS, Park JJ, Matsuki N, Mora AL, Van Kaer L, Boothby MR, Joyce S. NF-κB controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004;172:2265–2273. doi: 10.4049/jimmunol.172.4.2265. [DOI] [PubMed] [Google Scholar]
- 14.Stanic AK, Bezbradica JS, Park JJ, Van Kaer L, Boothby MR, Joyce S. Cutting edge: the ontogeny and function of Vα14Jα18 natural T lymphocytes require signal processing by protein kinase C theta and NF-κB. J Immunol. 2004;172:4667–4671. doi: 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M. NIK-dependent RelB activation defines a unique signaling pathway for the development of Vα14iNKT cells. J Exp Med. 2003;197:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallabhapurapu S, Powolny-Budnicka I, Riemann M, Schmid RM, Paxian S, Pfeffer K, Korner H, Weih F. Rel/NF-kappaB family member RelA regulates NK1.1− to NK1.1+ transition as well as IL-15-induced expansion of NKT cells. Eur J Immunol. 2008;38:3508–3519. doi: 10.1002/eji.200737830. [DOI] [PubMed] [Google Scholar]
- 18.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor κB family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 20.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol. 2008;180:5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 23.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA, Merida I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 24.Lee AJ, Zhou X, Chang M, Hunzeker J, Bonneau RH, Zhou D, Sun SC. Regulation of natural killer T-cell development by deubiquitinase CYLD. EMBO J. 2010;29:1600–1612. doi: 10.1038/emboj.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 26.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 27.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 31.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 32.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veillette A, Dong Z, Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007;27:698–710. doi: 10.1016/j.immuni.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 34.D’Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci U S A. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Vα14iNKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, MacDonald HR. Selective requirement for c-Myc at an early stage of Vα14iNKT cell development. J Immunol. 2009;182:4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 38.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Vα14iNKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 39.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- 41.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 42.Joyce S, Gordy LE. Natural killer T cell-a cat o’ nine lives! EMBO J. 2010;29:1475–1476. doi: 10.1038/emboj.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, Luster AD, Xavier RJ. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 47.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 49.Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- 50.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.