Summary
Background
Efficient metabolic function in mammals depends on the circadian clock, which drives temporal regulation of metabolic processes. Nocturnin is a clock-regulated deadenylase that controls its target mRNA expression post-transcriptionally through poly(A) tail removal. Mice lacking Nocturnin (Noc−/− mice) are resistant to diet-induced obesity and hepatic steatosis, yet are not hyperactive or hypophagic.
Results
Here we show that Nocturnin is expressed rhythmically in the small intestine, is induced by olive oil gavage and that the Noc−/− mice have reduced chylomicron transit into the plasma following the ingestion of dietary lipids. Genes involved in triglyceride synthesis, storage and chylomicron formation have altered expression and large cytoplasmic lipid droplets accumulate in the apical domains of the Noc−/− enterocytes. The physiological significance of this deficit in absorption is clear since maintenance of Noc−/− mice on diets that challenge the chylomicron synthesis pathway result in significant reductions in body weight, while diets that bypass this pathway do not.
Conclusions
Therefore we propose that Nocturnin plays an important role in the trafficking of dietary lipid in the intestinal enterocyes by optimizing efficient absorption of lipids.
Introduction
Diverse classes of organisms employ biological clocks to temporally coordinate their physiology and behavior, and to enable accurate predictions of external environmental conditions including timing of meals and subsequent nutrient uptake. Recent studies have demonstrated extensive links between the circadian clock and metabolic regulatory networks, and loss of clock function has serious negative effects on metabolic health (reviewed in [1]). Among humans with disrupted clocks due to jet lag or shift work, gastrointestinal disturbances are a major complaint [2] and humans exposed to simulated shift work have disrupted postprandial responses to fatty meals [3]. However, the role of the clock in regulating intestinal function is still not well understood.
One link between the circadian clock and metabolic function in mammals is the protein Nocturnin (gene name, Ccrn4l), which is expressed with high amplitude rhythms in many tissues, peaking during the night [4]. Nocturnin is a deadenylase; a class of exonuclease that specifically degrades the poly(A) tail of target mRNAs, usually leading to mRNA turnover or translational silencing [5, 6]. Noc−/− mice are resistant to diet-induced obesity and this is not due to increased physical activity, decreased food intake, or a higher metabolic rate [7]. These and other pieces of evidence suggest that these animals have deficits in lipid metabolism or uptake, presumably due to loss of rhythmic post-transcriptional regulation of genes necessary for lipid uptake, metabolism, and/or storage.
Although little is known about the mechanism by which circadian clocks control digestion, several reports suggest that they play an important role in regulating timing of digestive function to ready the correct components for maximum uptake of nutrients. The core clock genes are expressed with rhythmic profiles throughout the digestive tract [8–11] and a number of digestive processes and products are rhythmic (reviewed in [12]). These include diurnal variations in circulating lipids and proteins related to lipid uptake, circadian production of serum lipids and apolipoproteins, colonic motility, gastric emptying and rhythms in cell proliferation and radiation sensitivity. Many important genes in the small intestine and colon are expressed rhythmically [8, 9, 11, 13–15], resulting in circadian rhythms of macronutrient absorption and lipoprotein synthesis and secretion that are lost in mice with genetic disruptions of the circadian clock mechanism [11, 15].
One nutrient of primary importance is lipid. Within vertebrates, lipids are valued as a high caloric energy source, and also as conveyors of lipid soluble vitamins, such as vitamin A and vitamin E. The path that triglycerides take from the gut to the circulation involves a series of processes, beginning with emulsification and hydrolysis in the lumen, followed by uptake of hydrolyzed products by enterocytes. Short or medium chain fatty acids diffuse across the enterocyte and enter the portal vein blood while longer chain fatty acids are re-synthesized into triglyceride (TG) in the ER. The majority of the TG is either secreted as part of chylomicrons into the lymphatic system or stored in the cytosol as lipid droplets [16, 17]. Consequently, circadian regulation of this process could potentially occur at a variety of levels.
Here we demonstrate that the circadian deadenylase Nocturnin is an important link between the circadian clock and the lipid-absorption pathway in the small intestine. The lack of Nocturnin in mice causes increased retention of dietary lipids in cytoplasmic stores within the intestinal enterocytes and reduced secretion of chylomicron lipoprotein particles into the circulation. The physiological significance of this change in storage/secretion dynamics is evident by the inability of these mice to maintain their body weight on lipid-rich diets that depend on rapid lipid flux through the chylomicron secretion pathway.
Results
Nocturnin expression in the proximal intestine shows diurnal variations
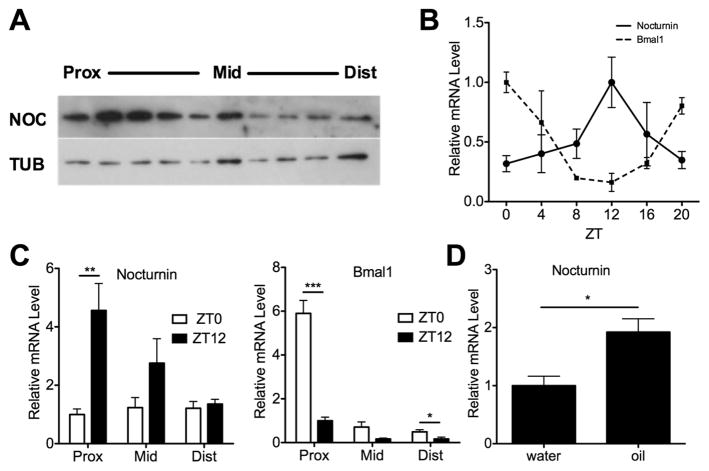
Noc−/− mice are resistant to diet-induced obesity and yet do not have reduced food intake, increased activity, or significant changes in energy expenditure [7]. Therefore, we sought to determine whether Nocturnin plays a role in the intestinal absorption of nutrients. We found that Nocturnin protein is expressed throughout the small intestine, with highest levels in the proximal portion (duodenum and proximal jejunum; Figure 1a), the region where the majority of lipid absorption takes place. Gene expression analysis of proximal jejunum samples collected every four hours throughout the day demonstrated that Nocturnin mRNA is expressed with a high amplitude rhythm, peaking in the early night at ZT12 (‘ZT’ refers to Zeitgeber Time in hours, where ZT0 is defined as light onset and ZT12 is defined as dark onset; Figure 1b). The central clock gene Bmal1 is shown for comparison and is also robustly rhythmic, but peaks with an opposite phase, at ZT0. Consistent with the protein data, Nocturnin mRNA levels are lower in the more distal sections of the small intestine (ileum; Figure 1c, left panel). And in contrast to the high amplitude rhythms in the proximal intestine, Nocturnin mRNA levels did not significantly differ between ZT0 and ZT12 in the distal small intestine. Bmal1 mRNA is expressed at higher levels at ZT0 than ZT12 in all three regions of the small intestine (Figure 1c, right panel). We have previously reported that Nocturnin is acutely inducible by various stimuli with “immediate early gene” induction properties [6, 18], and so we tested the effect of an olive oil gavage during the day when Nocturnin mRNA levels are normally low. We observed a significant acute induction of Nocturnin mRNA expression 2 hours after an olive oil gavage given at ZT 3 (Figure 1d). These data suggest that Nocturnin plays a role in intestinal responses to lipids.
Figure 1. Nocturnin is expressed rhythmically in the small intestine and is induced by lipid feeding.
(a) Mouse small intestine was harvested at ZT 12, dissected into 10 equally sized portions, from proximal to distal, and NOCTURNIN (NOC) protein expression was measured by western blot. TUBULIN (TUB) was used as a loading control. Prox, proximal; Mid, middle; and Dist, distal regions of the intestine. (b) Proximal sections of the small intestine (approximately the upper third) were collected from mice at different circadian times throughout the day as shown. Nocturnin and Bmal1 mRNA expression were assessed by quantitative RT-PCR (n=3 per time point, means ± SEM) (c) Small intestines were collected from mice at ZT0 and ZT12 and dissected into 3 equally sized portions called “proximal” (prox), “middle” (mid) and “distal” (dist). Nocturnin and Bmal1 mRNA expression were measured as described in (b). (n=4 per time point, means ± SEM) asterisks denote statistically significant differences in gene expression between sample times: * P < 0.05, ** P < 0.01, ***P < 0.001 by Student’s T-test. (d) A gavage of olive oil or water was administered at ZT 3 followed by isolation of the proximal small intestine 2 hours later at ZT 5. Nocturnin mRNA expression was measured as in (b) (n=5 per treatment, means ± SEM) and asterisks denote statistically significant differences (* P < 0.05) between treatments. The beta-2-microglobin (B2M) mRNA was used for normalization (b–d).
Noc−/− mice absorb less triglyceride and cholesterol than Noc+/+ counterparts
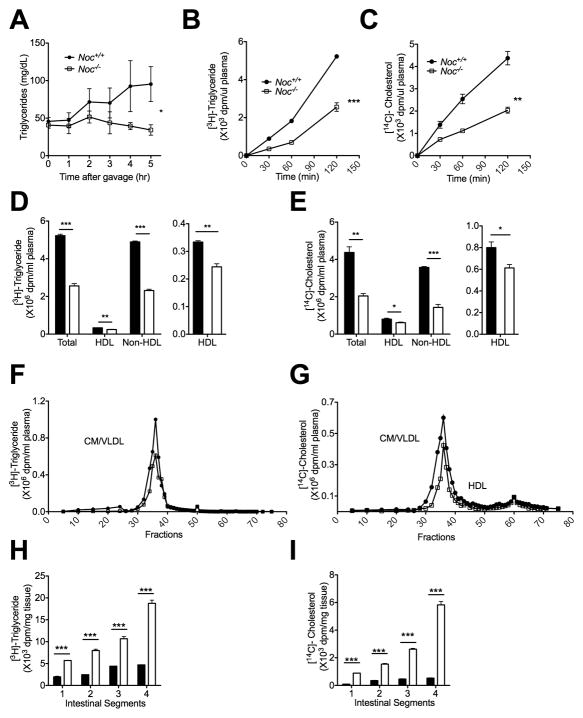
We examined Nocturnin’s role in the postprandial TG response in fasted Noc+/+ and Noc−/− mice by measuring TG levels in plasma following an olive oil gavage (Figure 2a). The plasma TG levels increase significantly following gavage in the Noc+/+ mice, but not in the Noc−/− mice. To understand mechanisms for the reduced TG levels in the Noc−/− mice, we studied lipid absorption by feeding the Noc+/+ and Noc−/− mice radiolabeled triglycerides and cholesterol. Plasma collected at time points over the next two hours demonstrated that the Noc−/− mice absorbed significantly less triglyceride and cholesterol (Figs. 2b and 2c) and had a significant reduction of both the HDL and non-HDL class of lipoproteins with the largest deficit in the non-HDL class (Figs. 2d and 2e) and specifically in the chylomicron/VLDL fraction (Figs. 2f and 2g). In contrast, the Noc−/− intestines retained significantly higher amounts of radiolabeled lipid than the Noc+/+ intestines two hours after feeding, with increasing amounts in the more distal sections of the intestine (Figs. 2h and 2i). This is likely the result of the reduced secretion of fat taken up by the enterocytes in the proximal intestinal regions, causing excess lipids to accumulate in the distal regions. Together, these data show that lipids are absorbed inefficiently in proximal jejunum of the Noc−/− mice.
Figure 2. Noc−/− mice have deficits in triglyceride and cholesterol transport into blood.
(a) Noc+/+ and Noc−/− mice were given an olive oil gavage and then plasma was collected at various timepoints following the gavage, as shown. Total TG content was measured from each plasma sample. (b and c) Mice were fed radiolabeled TG and cholesterol and plasma was collected at various times post-gavage and assayed for radioactivity. Shown are the plasma levels of [3H]-triglyceride (b) or [14C]-cholesterol (c) after an oral gavage. (d – g) Plasma samples from (b) and (c) were fractionated into total, HDL and non-HDL plasma fractions (d and e) or separated into different lipoprotein density fractions by FPLC (f and g). To the right of each graph, the HDL fractions are replotted with expanded y-axes for better visualization. (h and i) Graphs show the level of [3H]-triglyceride (h) and [14C]-cholesterol (i) that remained within the intestine after an oral gavage. Segment 1 is proximal and Segment 4 is distal part of intestine. All the graphs are means ± SEM, and asterisks denote statistically significant differences between genotypes: * P < 0.05, ** P < 0.01, ***P < 0.001 by linear mixed effects model (a), repeat measures ANOVA (b and c) or Student’s T-test (d, e, h, i). Samples are n=6 per genotype (a) and n=3 for both genotypes per time point (b and c), or pooled (d, e, h, i).
Primary enterocytes from the Noc−/− mice also have reduced lipoprotein secretion
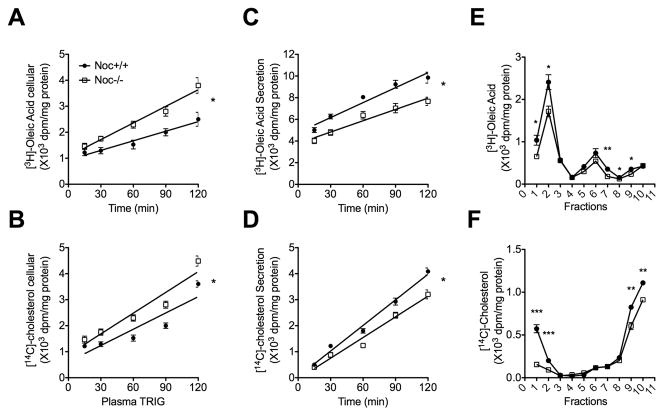
Based on the in vivo data, we examined the lipid secretion from isolated enterocytes following a pulse labeling with radiolabeled oleic acid and cholesterol. As seen in vivo, the primary enterocyte cultures from Noc−/− mice retained more radiolabeled lipids intracellularly (Figs. 3a and 3b) and secreted less (Figs. 3c and 3d) than Noc+/+ enterocytes. Density gradient fractionation analysis of the secreted oleic acid–derived lipids and cholesterol from the enterocyte culture medium indicated that the secreted lipoproteins were primarily deficient in low-density chylomicron fractions (fraction 1 and 2) in the Noc−/− cells (Figs. 3e and 3f). Therefore, consistent with the data from the in vivo analysis (Fig. 2), the Noc−/− enterocytes are deficient in efficiently secreting the absorbed lipid.
Figure 3. Primary enterocyte cultures from Noc−/− mice exhibit decreased lipoprotein secretion.
Primary enterocytes were isolated from Noc+/+ and Noc −/− mice and cultured with radiolabeled oleic acid or cholesterol, washed and chased for 2 hours in the presence of 1.5mM oleic acid-containing micells. (a and b) Shown is intracellular content of [3H]-Oleic acid (a) and [14C]-Cholesterol (b) in primary enterocytes. (c and d) and secretion of [3H]-Oleic acid (c) and [14C]-Cholesterol (d) from primary enterocytes. (e and f) The radioactivity of [3H]-Oleic acid (e) and [14C]-Cholesterol (f) in conditioned culture medium was separated into different lipoprotein density fractions by density gradient ultracentrifugaion. All the graphs are means ± SEM (n=3), and asterisks in (a, b, c, and d) denote statistically significant differences (* P < 0.05) between genotypes by repeat measures ANOVA, and asterisks in (e and f) denote statistically significant differences (* P < 0.05, ** P < 0.01, *** P < 0.001) by Student’s T-test.. Slopes of regression lines are as follows: (a) WT: 0.012 +/− 0.002; KO: 0.021+/− 0.002; (b) WT: 0.021 +/− 0.003; KO: 0.027+/− 0.003; (c) WT: 0.046 +/− 0.004; KO:. 0.034 +/− 0.004; (d) WT: 0.033 +/− 0.001; KO: 0.026 +/− 0.002.
Decreased lipoprotein secretion in Noc−/− mice is not due to microsomal triglyceride transfer protein deficiency
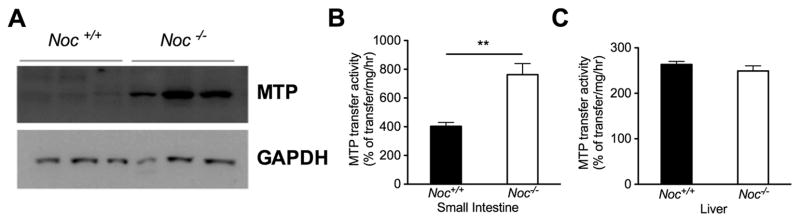
Microsomal triglyceride transfer protein (MTP) is required for the processing of dietary lipid to blood soluble chylomicrons through the direct facilitation of chylomicron assembly and is under circadian control [11, 13, 19]. Therefore, we measured the activity and protein levels of MTP in the Noc+/+ and Noc−/− mice to determine whether a deficiency in this activity could explain the reduced chylomicron secretion in the Noc−/− intestines. Contrary to this hypothesis, MTP protein levels and activity were both higher in proximal small intestines from Noc−/− mice (Figs. 4a and 4b). This increase in activity was specific to the intestine, as no changes in MTP levels or activity were detected in liver samples from the same animals (Fig. 4c). However, even with this enhanced MTP activity, chylomicron secretion in the Noc−/− mice is still significantly reduced relative to Noc+/+ mice (Figure 2), suggesting that some other component of this pathway is rate limiting.
Figure 4. Activity and abundance of MTP protein is significantly upregulated in the small intestine of Noc−/− mice.
(a) The proximal portions of small intestines from Noc+/+ and Noc −/− mice were collected at ZT12 after a 24-hr fast. MTP protein levels were measured by western blot. Shown are results from three individual mice for each genotype. (b and c) Extracts from intestine (b) or liver (c) were assayed for MTP activity. All the graphs are means ± SEM. (n=4 for both genotypes). Asterisks denote statistically significant differences (** P < 0.01) between genotypes by Student’s T-test.
Noc−/− enterocytes exhibit increased lipid storage
In order to further investigate the deficiencies in lipid absorption in the Noc−/− mice, we examined the expression levels of genes that play a role in lipid transport, storage, or chylomicron synthesis and secretion upon olive oil gavage (Supplementary Figure S1). Of particular interest was a significant decrease in the Noc−/− mice in the levels of Adipophilin (also known as Perilipin2, Adph, Adrp or Adfp), a protein known to be associated with the periphery of cytoplasmic lipid droplets (CLDs) and thought to be involved in regulation of CLD formation and size [20, 21]. We also observed a decrease in adipose triglyceride lipase (also known as ATGL or Pnpla2), a lipase important for the depletion/degradation of CLDs in adipose and non-adipose cells [22, 23] and in Diacylglycerol acetyltransferase 2 (Dgat2), but not in Dgat1. The Dgats are two enzymes that play non-redundant roles in the synthesis of triglycerides (TGs) for chylomicron formation and for CLD storage [24]. We also observed reductions in Apolipoprotein A-IV (ApoA IV), a protein important for the efficient transport of fat with chylomicrons, and small but significant reductions in Apolipoprotein B (ApoB), a structural protein required for chylomicron assembly. We did not detect any deficits in ApoB mRNA editing (data not shown). In all these cases, these changes were observed following oil gavage, while no significant change was seen between genotypes when the animals were gavaged with water. Notably, mRNAs encoding proteins involved in the secretory process such as the GTPase Sar1b, and a protein critical for vesicle trafficking; Vesicle transport through interaction with t-SNAREs homolog 1A (Vti1a) were not changed in the Noc−/− intestines. There were also no differences between genotypes in the mRNAs encoding the sterol transporters ABCG5 and ABCG8, which are involved in lipid excretion back into the gut lumen.
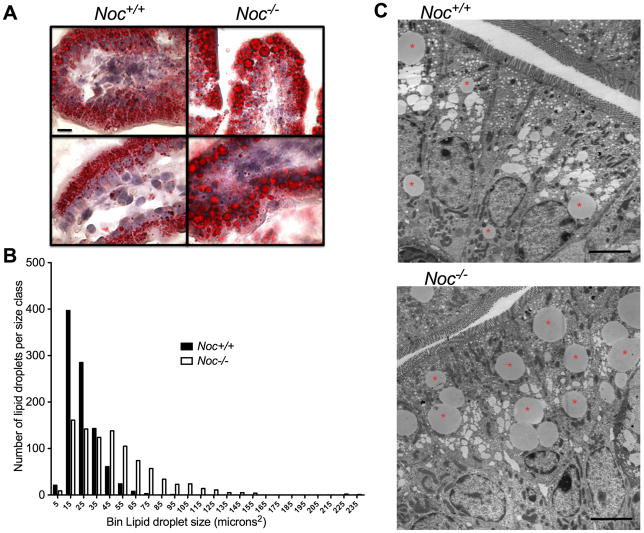
Since the genes that show expression changes in the Noc−/− enterocytes include those that encode proteins involved in the pathways leading to synthesis of TGs (DGAT2), regulation of CLD size (Adipophilin, DGAT2, ATGL) and flux into the chylomicron synthesis pathway in the endoplasmic reticulum (ApoA IV), we microscopically examined the enterocytes from mice that had been gavaged with olive oil. Oil Red O staining of sections of the duodenum revealed lipid droplets in both the Noc+/+ and Noc−/− cells, however, the droplets in the Noc−/− intestines appeared larger (Figure 5a). Quantitative analysis of multiple sections from multiple mice confirmed that this was the case, with fewer small droplets and more numerous large droplets in the Noc−/− as compared to the Noc+/+ sections (Figure 5b). Transmission electron microscopy clearly shows the morphology of these large cytoplasmic lipid droplets (Figure 5c). Together, these data implicate Nocturnin as an important regulator of intestinal lipoprotein processing, suggesting that the absence of Nocturnin increases storage of lipids and decreases formation and secretion of lipoproteins.
Figure 5. Lipid accumulates in larger droplets in the Noc−/− enterocytes.
(a) Representative pictures of intestines stained with Oil-Red O (Noc+/+ left, and Noc−/− right) two hours after olive oil gavage (ZT5). Examples from 2 different mice are shown for each genotype. All images were taken at the same magnification and the bar in the upper left panel represents 10 μm. (b) Oil-Red O stained sections were analyzed by counting the number of oil droplets in different size “bins”. Shown is a histogram detailing number and size of lipid droplets stained with Oil-Red O in proximal small intestine (n=7 mice per genotype). The values on the x-axis refer to the largest size oil droplet assigned to that bin (for example, “5” refers to the number of oil droplets between 0–5 microns). (c) Representative TEM images of Noc+/+ (top) and Noc−/− (bottom) in proximal small intestine (n=4 mice per genotype). Samples were taken at ZT14, two hours after an olive oil gavage. Asterisks denote large CLDs. Scale bar represents 5μm.
The changes in chylomicron secretion have significant ramifications on body weight
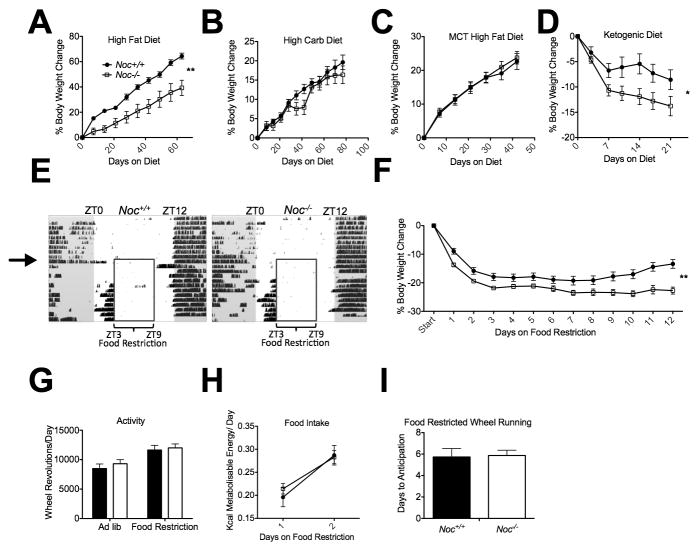
The idea that Noc−/− mice absorb TGs and cholesterol less efficiently than Noc+/+ mice is consistent with our findings that Noc−/− mice remain lean on a high fat diet (40% kcal fat), although they show no increase in respiration or activity and no decrease in food intake ([7] and Figure 6a). In contrast, feeding the Noc−/− mice either a standard diet (17% kcal fat; [7]), or a high carbohydrate diet (70% kcal carb; Figure 6b) or a high fat diet containing medium chain (60% C8, 40% C10) fatty acids (45% kcal fat), which can be absorbed without being packaged into chylomicrons [25] (Figure 6c), results in weight gains indistinguishable from Noc+/+ mice. To further test the physiological significance of the reduced lipid uptake in the Noc−/− mice, we maintained the mice on feeding regimens that challenge the efficiency of the normal lipoprotein packaging pathways. In the first of these, we fed Noc+/+ and Noc−/− mice a ketogenic diet (95% kcal fat), in which nearly all of the calories come from fat [26]. Noc+/+ mice lose weight rapidly on this diet, but eventually stabilize, while the Noc−/− mice lose more weight and do not stabilize (Figure 6d). This suggests that the decreased efficiency of lipid uptake in the Noc−/− mice results in the inability to maintain body weight when forced to rely on a diet in which lipids are the primary energy source.
Figure 6. Noc −/− mice cannot maintain body weight on diets that challenge the lipoprotein secretion pathway.
Body weights were recorded from Noc+/+ and Noc −/− mice maintained on (a) a high fat diet (Noc+/+ n=8, Noc−/− n=6), (b) a high carbohydrate diet (Noc+/+ n=14, Noc−/− n=10), (c) a medium-chain triglyceride high fat diet (Noc+/+ n=13, Noc−/− n=19) and (d) a ketogenic diet (Noc+/+ n=14, Noc−/− n=17). Shown are percent body weight change (means +/− SEMs). (e) Representative actograms of continuous running wheel recordings of adult male Noc+/+ (left) or Noc−/− (right) mice. Each horizontal line represents 24 hours and each day is plotted blow the previous day. Mice are individually housed in 12:12 Light-Dark cycle (white and gray background, respectively) with ad lib access to food for 7 days, followed by restriction of food to 6 hours in the middle of the day (insert box). Black hash marks indicate wheel-running behavior. (f) Percent body weight change (means ± SEM) over 12 days of food restriction (Noc+/+ n=29–35, Noc−/− n=31–37 for each time point, Linear Mixed Effect model of repeat measures ANOVA F=8.1, p<0.01 for genotype). (g) Overall activity levels (wheel revolutions/day) before and during food restriction. (h) Energy consumed per day while on food restriction (Noc+/+ n=35, Noc−/− n=37 for each time point). (i) Days to anticipation of food presentation as measured by sustained wheel running under food restriction diet (Noc+/+ n=15, Noc−/− n=15).
Finally, we examined the mice under restricted feeding (RF) conditions, in which standard chow was available for only 6 hours during the middle of the light period (ZT3–9). Under this regimen, both the Noc+/+ and Noc−/− mice entrain to the food availability after a few days, anticipating the food with increased bouts of locomotor activity just prior to the feeding period, and have indistinguishable overall levels of activity (Figure 6e,g). The Noc+/+ mice lose weight and then as they entrain to the new food schedule, they begin to stabilize and even regain some weight. However, as with the ketogenic diet, the Noc−/− mice cannot maintain their body weight on this regimen. The Noc−/− mice lose significantly more weight than Noc+/+ mice during the first 2 days of RF (Figure 6f), although their food intake is the same as Noc+/+ over this period (Figure 6h). Unlike the Noc+/+ mice, the Noc−/− mice do not recover following entrainment and continue to lose weight. This is not due to an inability to entrain to the food, since anticipatory activity onset does not differ between genotypes (Figure 6i). These results suggest that even on standard chow, the reduced efficiency of dietary lipid absorption in the Noc−/− mice results in significant deficits in body weight maintenance when food availability is limited to a few hours during the day.
Discussion
These studies demonstrate that the circadian clock-controlled gene, Nocturnin, plays an important role in the normal utilization of dietary lipids, providing a new link between the circadian timing system and this important metabolic process. Our data support the idea that the deficiency in nutrient uptake is due to decreased flux of dietary lipids going through the enterocyte lipoprotein synthesis and secretion pathway and increased storage in CLDs. This is reflected in the significant weight loss by the Noc−/− mice relative to their wild-type (Noc+/+) counterparts when maintained on a ketogenic diet, but not when maintained on a high carbohydrate diet or on high fat diets containing medium chain fatty acids which can be transported into the plasma without assembly into chlomicrons [25]. Our studies using radiolabeled lipids proved that the reduced lipid mobilization capacity is happening at the level of the enterocyte in the Noc−/− mice, most likely at the level of regulation of resynthesis and storage of TGs, upstream of the lipoprotein assembly process in the endoplasmic reticulum. The reduced lipid secretion is not due to a decrease in the critical chylomicron assembly mediator, MTP, since MTP activity levels are significantly increased in the intestines of these mice. There is evidence that MTP may be up-regulated by fatty acids [27] and therefore we interpret this to be a compensatory response to the increased lipid content present in these cells, consistent with our observation that this up-regulation does not occur in the non-steatotic livers of the Noc−/− mice (Figure 4c). Previous studies reported that the amount of lipid found in the enterocyte can be manipulated by changes in bile presence [28]. Although there is a small change in bile acid pH in the Noc−/− mouse (Supplementary Fig. S2), emulsification of lipids and transport across the luminal surface are probably not major components of the Noc−/− lipid malabsorption phenotype since primary enterocyte experiments demonstrated that Noc−/− cells could sequester lipid within these cells and Oil Red O and TEM studies demonstrate that there is significant accumulation of lipid mass in the intestine, residing in large lipid droplets inside the cells near the apical border. Therefore, although lipid uptake by these cells is not limiting, secretion of lipid from the Noc−/− enterocytes is attenuated compared to Noc+/+ enterocytes.
Nocturnin’s robust rhythmicity suggests that it is an important mediator of circadian control of intestinal lipid absorption, presumably through circadian post-transcriptional control of mRNA decay and/or translation. A recent study [11] demonstrated a clear role for the clock in these pathways and revealed that significantly more TG and cholesterol could be absorbed into the circulation at night as compared to day, and the lower daytime levels of radiolabeled lipids in the plasma could be accounted for by increased retention of counts in the intestine, similar to our observations in the Noc−/− mice. These differences were not simply due to time of food intake since these rhythms were lost in Clock mutant mice [11]. The clock has previously been shown to regulate nuclear receptors such as LXR, RXR, PPARa and SREBP in other metabolic tissues [29, 30] and these receptors have known roles in cholesterol and fatty acid absorption and metabolism [31]. Furthermore, intestinal expression of many mRNAs encoding key enzymes involved in TG synthesis and storage and chylomicron assembly are regulated by both the circadian clock and food, while a few were only regulated by food [11]. Interestingly, the genes that are changed in the Noc−/− enterocytes, as well as Nocturnin itself, fall into the class that are both clock and food regulated, providing another link between these important processes.
It is clear from our data that Nocturnin is required for optimal dietary lipid absorption into the circulation. The reduced secretion from the Noc−/− enterocytes results in excess fat accumulation in CLDs within the enterocytes and increased transport of fat to the distal regions of the intestine under conditions of high fat feeding. Increased CLD size has also been observed in other mice with defects in the TG re-synthesis or chylomicron assembly pathways [32–34], presumably reflecting the need to store the excess lipids that accumulate due to the reduced secretion rate. However, a more active and regulated role for CLDs in this process cannot be ruled out. Although CLDs were originally thought to be inert intracellular sites of TG storage, they have recently been shown to be dynamic organelles with organized structures and changing composition involved in highly regulated lipid storage/mobilization [21, 35, 36] and lipid droplet proteins are regulated in a dynamic fashion in enterocytes in response to acute and chronic high fat diets, suggesting that droplet size and function are under careful modulation [20, 37]. Whether active regulation of CLD size and function contributes in a significant way to the flux of lipids going through the lipoprotein pathways in enterocytes is not known, but chylomicrons secreted from enterocytes can contain TGs from stored cytoplasmic pools rather than from the most recently ingested lipids [37–42] and it has recently been suggested that the postprandial TG response may be modulated by regulatory steps that control CLD size and therefore dictate the balance between storage and secretion in these cells, rather than simply reflecting the rate of direct transit of dietary lipids [24].
The lipid absorption defects seen in the Noc−/− mice are mild relative to chylomicron assembly deficiencies such as seen in mice with reduced expression of MTP or intestinal ApoB [43–45] or in some human disorders such as Abetalipoproteinemia (Bassen-Kornzweig syndrome) or chylomicron retention disease (Anderson’s disease) [46, 47]. In each of these cases the constitutive alteration of chylomicron assembly had severe health consequences. In contrast, the Noc−/− mice are able to form and secrete chylomicrons but do so less efficiently. As such, when this system is unchallenged, such as on ad lib standard diet, or high carbohydrate diet, a weight phenotype is not observed. On a high fat “western-style” diet, they have a lean phenotype, but this develops slowly over several weeks. But, when forced to rely almost solely on fat as in the ketogenic diet, or when forced to obtain their daily energy in a short period of time (such as on RF) this deficit in lipid absorption into the circulation has more profound effects on body weight. It is likely that the reduced uptake of dietary lipids is a major causative factor in at least some aspects of the Noc−/− lean phenotype and is consistent with the Noc−/− mouse’s resistance to weight gain on high fat diets, their lower constitutive body temperature, decreased lipid deposits in the liver and smaller white adipocytes [7]. However, Nocturnin is widely expressed in many metabolically relevant tissues where it has been implicated in other processes, such as in the stimulation of PPARγ transcriptional activity and adipogenesis [48]. Therefore, the relative contribution of the changes in the small intestine to the whole body lean phenotype in the Noc−/− mice will have to wait for the testing of mice with intestine-specific ablation of Nocturnin function.
These findings suggest that the circadian clock in the enterocytes regulates the postprandial TG response, at least in part, by controlling the relative flux of dietary lipids that are either diverted into transient storage within the enterocytes or into the chylomicron assembly process for secretion into the plasma. Our data support the idea that high levels of Nocturnin during the night normally contribute to the maximization of lipid absorption through the chylomicron pathway. In its absence, this pathway is less optimal and lipids accumulate in cytosolic droplets resulting in inefficient utilization of dietary lipids by the animal. It is worth noting that mice lacking Nocturnin do not accumulate measurable excess lipids in their feces (data not shown). Therefore gastrointestinal tract-specific inhibition of Nocturnin enzyme activity may be an attractive therapeutic strategy to prevent obesity in humans through reduction of dietary fat absorption without the negative side effects of currently available compounds such as olestra.
Experimental Procedures
Animals and Diets
Animal experiments were conducted according to relevant national and international guidelines and following the protocols approved by the Institutional Animal Care and Use Committees. Eight-week old male Noc−/− and Noc+/+ mice on a congenic C57BL/6J background (N9 or N10) were maintained on a 12:12 LD cycle and fed ad lib unless otherwise stated. Olive oil gavage and restricted feeding procedures and compositions of the diets are in Supplementary Experimental Procedures.
Small Intestine Histology
Following olive oil gavage, 3 cm segments of intestine starting 1 cm distal to the pyloric valve were taken for further histological analysis by light and electron microscopy as described in Supplemental Experimental Procedures.
Plasma TG measurements and Lipid Absorption Studies
For postprandial TG measurements, blood was collected from tail bleeds at various time points following the gavage as indicated and TG levels were measured from plasma using a Serum Triglyceride Determination Kit (Sigma, St. Louis, MO). Short-term in vivo lipid absorption studies were done as described in Supplemental Experimental Procedures based on methods from [49–51]. Lipoproteins carrying TG and cholesterol were identified from primary enterocytes from Noc−/− and Noc+/+ mice as described in Supplementary Experimental Procedures. MTP activity measurements were done as previously described [52, 53] (Supplementary Experimental Procedures).
Supplementary Material
Highlights.
Nocturnin mRNA is rhythmic in the small intestine and is induced by dietary lipids
Mice lacking Nocturnin have increased accumulation of lipid in enterocytes
Rates of chylomicron secretion are reduced in Nocturnin knockout mice
Nocturnin knockout mice are unable to maintain body weight under dietary challenges
Acknowledgments
We thank the other members of the Green laboratory for many useful discussions of our data, with special thanks to Jeremy Terrien for help with statistical analysis and to Hyun Jeung Sung for excellent management of our mouse colony. This work was supported by National Institutes of Health Grant GM076626 (to C.B.G.), DK81879 (to M.M.H.), by the Cell and Molecular Biology Predoctoral Training Grant 2T32 GM008136-21 from the National Institutes of Health (to N.D.), and Scientist Development Grant from the American Heart Association (to X.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vener KJ, Szabo S, Moore JG. The effect of shift work on gastrointestinal (GI) function: a review. Chronobiologia. 1989;16:421–439. [PubMed] [Google Scholar]
- 3.Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. The Journal of endocrinology. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Osterbur DL, Megaw PL, Tosini G, Fukuhara C, Green CB, Besharse JC. Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev Biol. 2001;1:9. doi: 10.1186/1471-213X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggs JE, Green CB. Nocturnin, a Deadenylase in Xenopus laevis Retina. A Mechanism for Posttranscriptional Control of Circadian-Related mRNA. Curr Biol. 2003;13:189–198. doi: 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 6.Garbarino-Pico E, Niu S, Rollag MD, Strayer CA, Besharse JC, Green CB. Immediate early response of the circadian polyA ribonuclease nocturnin to two extracellular stimuli. RNA. 2007;13:745–755. doi: 10.1261/rna.286507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci U S A. 2007;104:9888–9893. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133:1250–1260. doi: 10.1053/j.gastro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology. 2008;135:2019–2029. doi: 10.1053/j.gastro.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sladek M, Rybova M, Jindrakova Z, Zemanova Z, Polidarova L, Mrnka L, O’Neill J, Pacha J, Sumova A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology. 2007;133:1240–1249. doi: 10.1053/j.gastro.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res. 2009;50:1800–1813. doi: 10.1194/jlr.M900085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bron R, Furness JB. Rhythm of digestion: keeping time in the gastrointestinal tract. Clin Exp Pharmacol Physiol. 2009;36:1041–1048. doi: 10.1111/j.1440-1681.2009.05254.x. [DOI] [PubMed] [Google Scholar]
- 13.Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem. 2007;282:24707–24719. doi: 10.1074/jbc.M701305200. [DOI] [PubMed] [Google Scholar]
- 14.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord. 2009;10:293–300. doi: 10.1007/s11154-009-9119-3. [DOI] [PubMed] [Google Scholar]
- 15.Hoogerwerf WA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol. 2010;298:G143–150. doi: 10.1152/ajpgi.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansbach CM, II, Dowell RF, Pritchett D. Portal transport of absorbed lipids in rats. Am J Physiol. 1991;261:G530–538. doi: 10.1152/ajpgi.1991.261.3.G530. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert MR, Douris N, Tongjai S, Green CB. Nocturnin expression is induced by fasting in the white adipose tissue of restricted fed mice. PLoS One. 2011;6:e17051. doi: 10.1371/journal.pone.0017051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol. 2008;19:277–284. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Zhu J, Wolins NE, Cheng JX, Buhman KK. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim Biophys Acta. 2009;1791:1173–1180. doi: 10.1016/j.bbalip.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1−/− mice. J Lipid Res. 2010;51:1770–1780. doi: 10.1194/jlr.M002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132:329–332. doi: 10.1093/jn/132.3.329. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 27.Hussain MM, Nijstad N, Franceschini L. Regulation of microsomal triglyceride transfer protein. Clinical Lipidology. 2011;6:293–303. doi: 10.2217/clp.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevin P, Koelsch D, Mansbach CM., 2nd Intestinal triacylglycerol storage pool size changes under differing physiological conditions. J Lipid Res. 1995;36:2405–2412. [PubMed] [Google Scholar]
- 29.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 30.Lemberger T, Saladin R, Vazquez M, Assimacopoulos F, Staels B, Desvergne B, Wahli W, Auwerx J. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 31.Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA. Intestinal cholesterol transport proteins: an update and beyond. Curr Opin Lipidol. 2007;18:310–318. doi: 10.1097/MOL.0b013e32813fa2e2. [DOI] [PubMed] [Google Scholar]
- 32.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng D, Iqbal J, Devenny J, Chu CH, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM, et al. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J Biol Chem. 2008;283:29802–29811. doi: 10.1074/jbc.M800494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem. 2006;281:4075–4086. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 35.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 36.Dugail I, Hajduch E. A new look at adipocyte lipid droplets: towards a role in the sensing of triacylglycerol stores? Cell Mol Life Sci. 2007;64:2452–2458. doi: 10.1007/s00018-007-7277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Lee B, Buhman KK, Cheng JX. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez Vallejo SJ, Alqub M, Luquet S, Cruciani-Guglielmacci C, Delerive P, Lobaccaro JM, Kalopissis AD, Chambaz J, Rousset M, Lacorte JM. Short-term adaptation of postprandial lipoprotein secretion and intestinal gene expression to a high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2009;296:G782–792. doi: 10.1152/ajpgi.90324.2008. [DOI] [PubMed] [Google Scholar]
- 39.Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr. 1996;63:36–41. doi: 10.1093/ajcn/63.1.36. [DOI] [PubMed] [Google Scholar]
- 40.Jackson KG, Robertson MD, Fielding BA, Frayn KN, Williams CM. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am J Clin Nutr. 2002;76:942–949. doi: 10.1093/ajcn/76.5.942. [DOI] [PubMed] [Google Scholar]
- 41.Mansbach CM, Dowell R. Effect of increasing lipid loads on the ability of the endoplasmic reticulum to transport lipid to the Golgi. J Lipid Res. 2000;41:605–612. [PubMed] [Google Scholar]
- 42.Mansbach CM, 2nd, Nevin P. Intracellular movement of triacylglycerols in the intestine. J Lipid Res. 1998;39:963–968. [PubMed] [Google Scholar]
- 43.Young SG, Cham CM, Pitas RE, Burri BJ, Connolly A, Flynn L, Pappu AS, Wong JS, Hamilton RL, Farese RV., Jr A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96:2932–2946. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung HR, Turner SM, Neese RA, Young SG, Hellerstein MK. Metabolic adaptations to dietary fat malabsorption in chylomicron-deficient mice. Biochem J. 1999;343(Pt 2):473–478. [PMC free article] [PubMed] [Google Scholar]
- 45.Leung GK, Veniant MM, Kim SK, Zlot CH, Raabe M, Bjorkegren J, Neese RA, Hellerstein MK, Young SG. A deficiency of microsomal triglyceride transfer protein reduces apolipoprotein B secretion. J Biol Chem. 2000;275:7515–7520. doi: 10.1074/jbc.275.11.7515. [DOI] [PubMed] [Google Scholar]
- 46.Shoulders CC, Stephens DJ, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opin Lipidol. 2004;15:191–197. doi: 10.1097/00041433-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 48.Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc Natl Acad Sci U S A. 2010;107:10508–10513. doi: 10.1073/pnas.1000788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res. 2005;46:1491–1501. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Anwar K, Iqbal J, Hussain MM. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J Lipid Res. 2007;48:2028–2038. doi: 10.1194/jlr.M700207-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764–772. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779–1785. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.