Abstract
Dispiro 1,2,4-trioxanes and 1,2,4,5-tetraoxanes had superior efficacy against Fasciola hepatica than the corresponding ozonides (1,2,4-trioxolanes). For highest efficacy, spiroadamantane and carboxymethyl substructures were required. Three compounds completely cured F. hepatica-infected mice at single oral doses of 50 mg/kg and two were partially curative at single doses of 25 mg/kg.
Keywords: artemisinin, peroxide, Fasciola hepatica
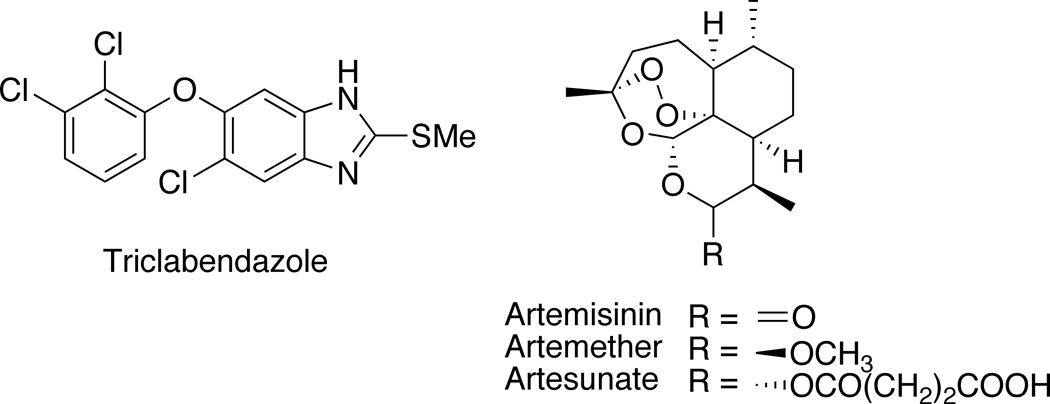
The liver flukes Fasciola hepatica and F. gigantica are pathogenic trematodes infecting an estimated 2.4–17 million people in the Andean countries, Cuba, Western Europe, Egypt and Iran.1 Moreover, the morbidity and mortality of fascioliasis in cattle and sheep results in considerable economic loss.2 The benzimidazole triclabendazole (Fig. 1) is the drug of choice used to treat veterinary fascioliasis, but it is registered in only four countries for the treatment of human fascioliasis.3,4 Evidence of drug resistance to triclabendazole in veterinary medicine5,6 provides an impetus for the discovery and development of new drugs against fascioliasis.
Figure 1.
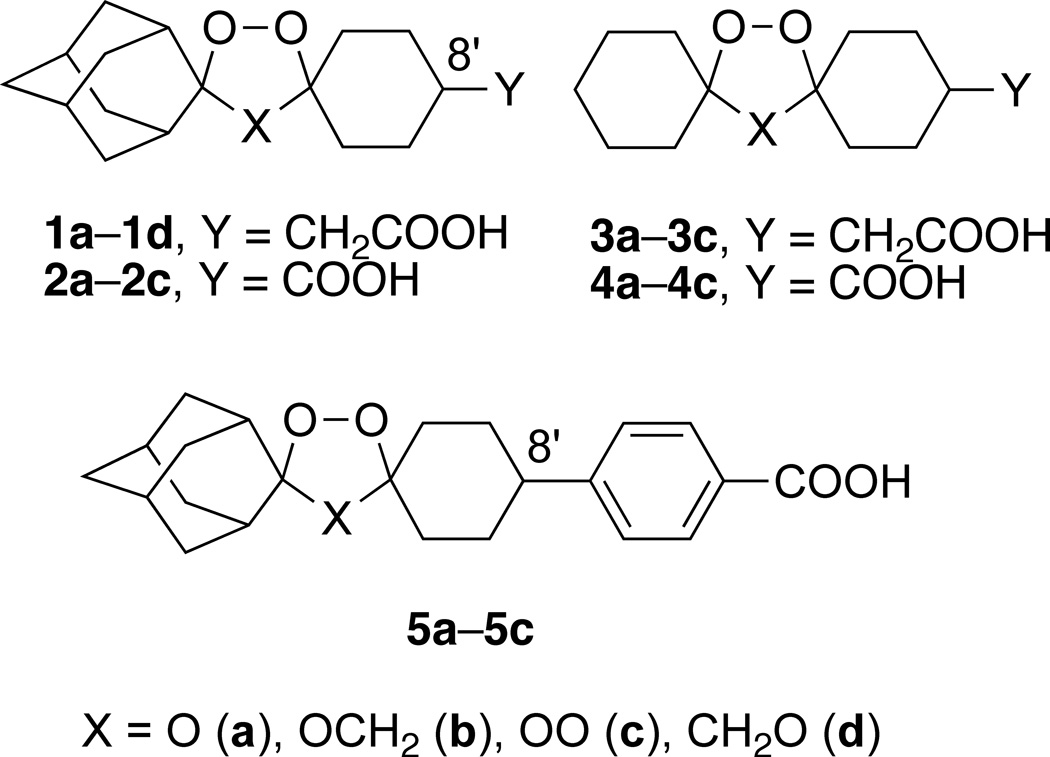
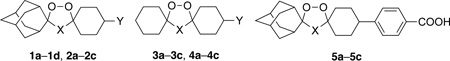
We have shown that semisynthetic artemisinins (Fig. 1) and synthetic ozonides (Fig. 2) have good efficacy against F. hepatica.7–10 It is postulated10,11 that such peroxidic compounds possess antiplasmodial12,13 and flukicidal activities because both plasmodia and Fasciola spp. degrade hemoglobin to generate free heme, a likely target14 of bioactive peroxides. In an effort to identify more effective synthetic peroxides, a structurally diverse ozonide library of OZ78 (cis-1a) analogues was recently studied.10 It was found that a spiroadamantane substructure, an acidic functional group (or ester prodrug), and the peroxide bond and non-peroxide oxygen atom of the ozonide heterocycle, were all required for high efficacy against F. hepatica.10 We now report an investigation of the 1,2,4-trioxane and 1,2,4,5-tetraoxane analogs of ozonides (1,2,4-trioxolanes) 1a–5a (Fig. 2).15 Target peroxide heterocycles 5a–5c were designed on the basis of the superior pharmacokinetic profiles of 8’-aryl ozonides compared to those of 8’-alkyl ozonides.16
Figure 2.
Target dispiro peroxides
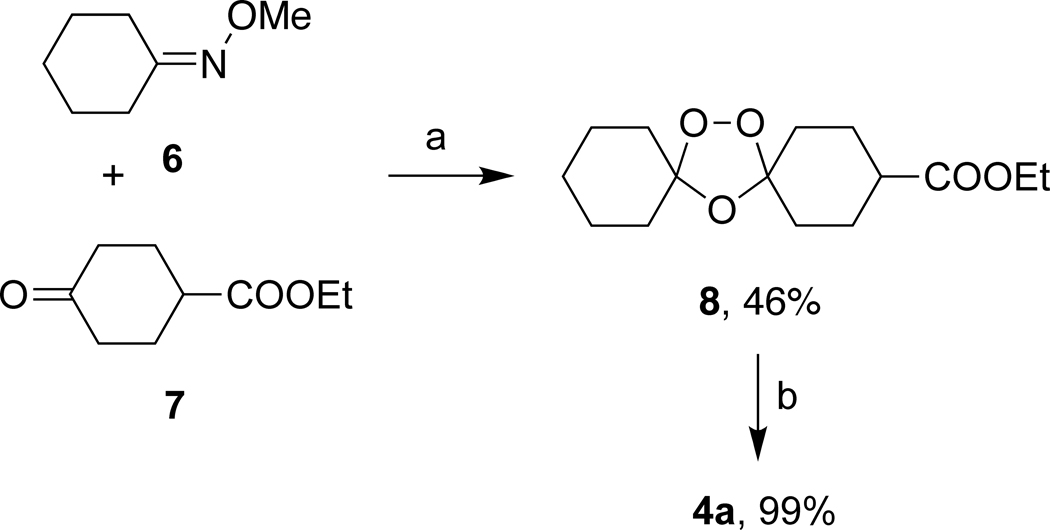
Griesbaum coozonolysis17 of oxime ether 618 and ketoester 7 afforded ozonide ester 8 as a 2.5:1 mixture of cis and trans isomers. Chromatographic separation into the individual isomers followed by hydrolysis of the cis isomer afforded 4a in high yield. Ozonides 1a–3a and 5a were obtained as previously described.19–21
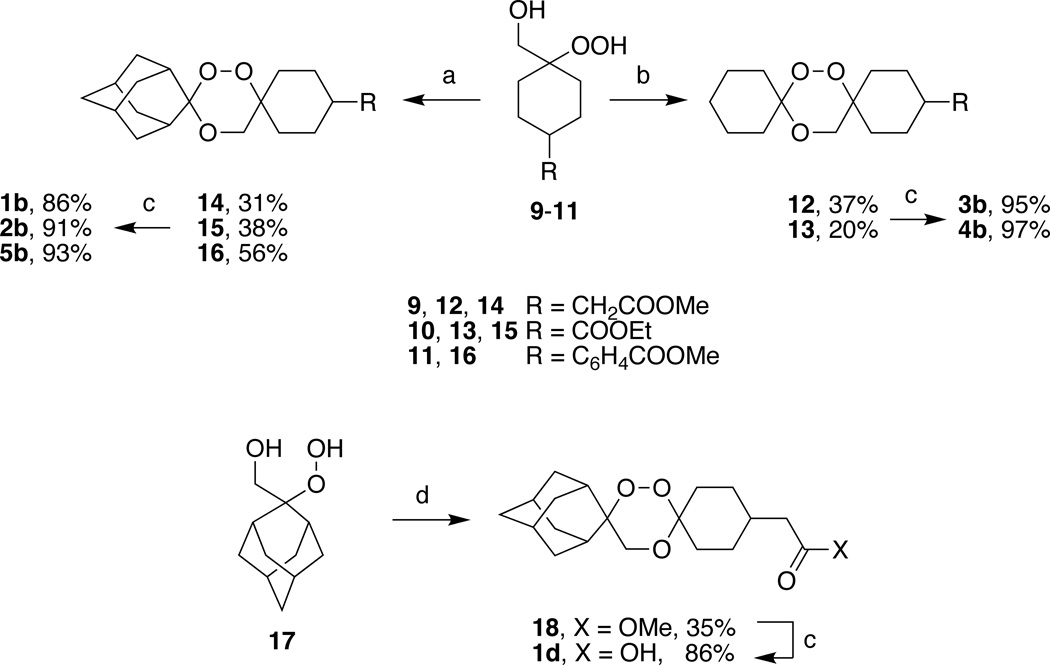
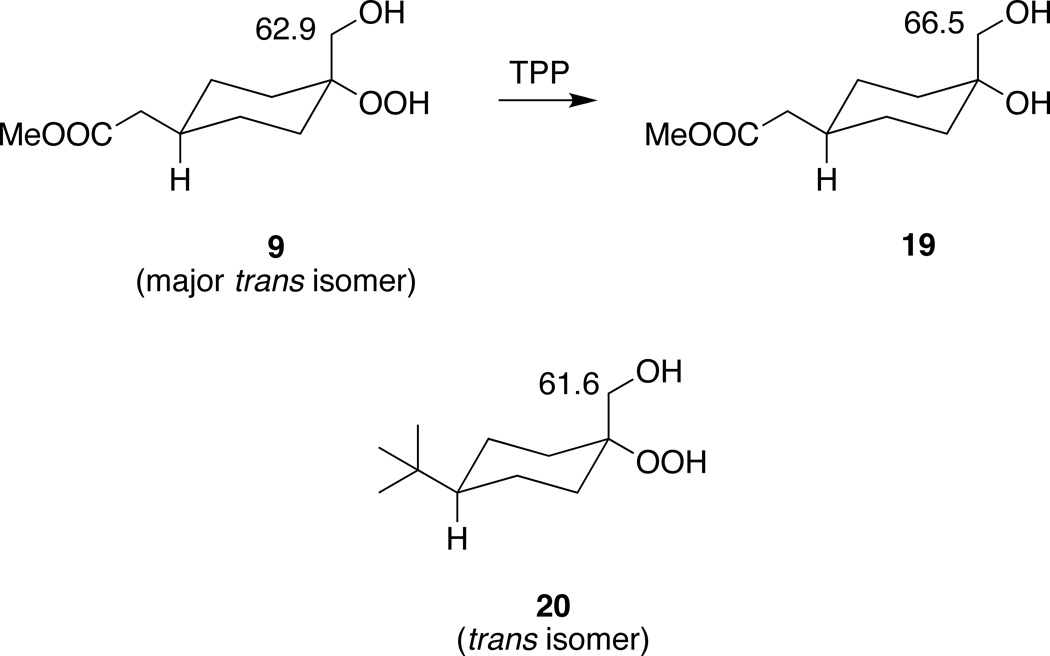
Acid-catalyzed condensation of β–hydroperoxy alcohols 9–11 with 2-adamantanone or cyclohexanone afforded trioxane esters 12–16 (20–56% yields) (Scheme 2) which were isolated as single trans isomers after crystallization (vide infra). Hydrolysis of 12–16 afforded trioxane acids 1b–5b. β–Hydroperoxy alcohols 9–11 were formed in 52–98% yields by regioselective perhydrolysis22,23 of the corresponding epoxides, which in turn, were formed predominantly as their cis isomers24 by treatment of their keto ester precursors20,21 with the sulfur ylid formed from trimethylsulfoxonium iodide and potassium tert-butoxide.25 Even though β–hydroperoxy alcohols 9–11 were formed as mixtures of cis and trans isomers, the trans isomers predominated as demonstrated by the triphenylphosphine reduction of 9 to its corresponding 1,2-diol 19 and NMR analysis of the latter (Scheme 3). Observation of a signal at 66.5 ppm in the 13C NMR spectrum of 1926 is consistent with a shielded axial hydroxymethyl group indicating that the hydroperoxide in 9 is equatorial. Similarly, we suggest that the signal at 62.9 ppm in the 13C NMR spectrum of 9 is that of a shielded axial hydroxymethyl group. By way of comparison, Li et al.23 report hydroxymethyl group 13C NMR signals at 61.6 and 67.2 ppm for the isomers of β–hydroperoxy alcohol 20. Finally, trioxane acid 1d was obtained by hydrolysis of trioxane ester 18; the latter was formed in low yield by acid-catalyzed condensation of β–hydroperoxy alcohol 1722 with methyl 2-(4-oxocyclohexyl)acetate.
Scheme 2.
Reagents and conditions: (a) 2-adamantanone, CSA, CH2Cl2, rt, 12 h; (b) cyclohexanone, CSA, CH2Cl2, rt, 12 h; (c) 15% aq. KOH, EtOH/THF, 50 °C, 4 h, then AcOH; (d) methyl 2-(4-oxocyclohexyl)acetate, CSA, CH2Cl2, rt, 12 h.
Scheme 3.
β–Hydroperoxy alcohol stereochemistry. Assigned hydroxymethyl group 13C NMR signals are indicated in ppm.
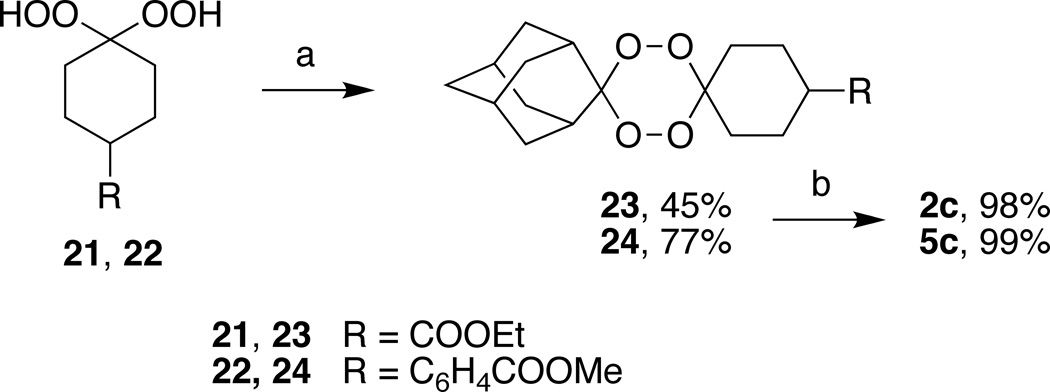
Tetraoxane acids 2c and 5c were obtained by hydrolysis of their respective tetraoxane esters 23 and 24; the latter were formed in 45 and 75% yields by Re2O7 catalyzed condensation27 of 1,1-dihydroperoxide esters 21 and 22 with 2-adamantanone. 1,1-Dihydroperoxide esters 21 and 22 were obtained in quantitative yields from the corresponding keto esters by treatment with 50% aq. H2O2 and I2 catalyst.28 Tetraoxane acids 1c, 3c, and 4c were synthesized as previously described.29,30
Target compound efficacy data against F. hepatica are shown in Tables 1 and 2. At eight to thirteen weeks post-infection, rats were treated with single 25–100 mg/kg oral doses of target compounds prepared as suspensions in 7% (v/v) Tween 80 and 3% (v/v) EtOH. At day 6 post-treatment, rats were sacrificed and adult flukes were recovered from the bile ducts and livers. Target compound efficacies were evaluated by comparing the mean total worm burdens of treated and untreated control rats. Statistical significance was calculated using the Kruskal-Wallis test.
Table 1.
Worm burden reductions in adult F. hepatica harbored in rats following the administration of dispiro peroxides at single oral doses of 100 mg/kg.
 | ||||
|---|---|---|---|---|
| Compd | X | Y | Worm Burden Reduction (%) |
Curesa |
| Control | ------ | ------ | ------ | 0/12 |
| ASb | ------ | ------ | 30 | 2/5 |
| 1ac | O | CH2COOH | 100e | 10/10 |
| 1b | OCH2 | CH2COOH | 100e | 3/3 |
| 1c | OO | CH2COOH | 100e | 4/4 |
| 1d | CH2O | CH2COOH | 0 | 0/3 |
| 2a | O | COOH | 52 | 0/4 |
| 2b | OCH2 | COOH | 61e | 1/3 |
| 2c | OO | COOH | 100e | 4/4 |
| 3ad | O | CH2COOH | 0 | 0/3 |
| 3b | OCH2 | CH2COOH | 95e | 2/3 |
| 3c | OO | CH2COOH | 29 | 0/3 |
| 4a | O | COOH | 26 | 0/3 |
| 4b | OCH2 | COOH | 100e | 3/3 |
| 4c | OO | COOH | 100e | 3/3 |
| 5a | O | ------ | 100e | 3/3 |
| 5b | OCH2 | ------ | 100e | 3/3 |
| 5c | OO | ------ | 92e | 2/4 |
Table 2.
Worm burden reductions in adult F. hepatica harbored in rats following the administration of selected dispiro peroxides at single oral doses of 50 and 25 mg/kg.
Like 1a, seven compounds were completely curative at 100 mg/kg doses (Table 1). These compounds were then tested at lower doses of 50 and 25 mg/kg (Table 2). Several trends can be seen from the combined efficacy data. First, we suggest that the complete loss of efficacy for 1,2,4-trioxane 1d compared to its active regioisomer 1b results from a different iron (II) reaction profile. Previous investigations22 with the unsubstituted (Y = H) analogs of 1d and 1b reveal that although both 1,2,4-trioxanes undergo a preferred attack of iron (II) on the less hindered peroxide oxygen atom, 1d forms a higher proportion of inactive carbonyl-containing reaction products, and, unlike 1b, does not form a secondary carbon-centered radical by β-scission of the spiroadamantane substructure. Second, for 1,2,4-trioxolane/1,2,4-trioxane/1,2,4,5-tetraoxane compound sets 1a–1c, 2a–2c, 3a–3c, 4a–4c, and 5a–5c, the trioxanes and tetraoxanes were superior to the trioxolanes. Third, the efficacies of 1a–1c compared to 2a–2c show the superiority of an 8’-carboxymethyl vs. 8’-carboxy substituent. Fourth, the efficacies of 1a–1c compared to 3a–3c and 2a–2c compared to 4a–4c show the superiority of a spiroadamantane vs. spirocyclohexane. Fifth, unlike the superior antimalarial efficacies of 8’-aryl vs. 8’-alkyl ozonides,16 the 8’-aryl 5a–5c were inferior to the corresponding 8’alkyl peroxide heterocycles 1a–1c. Finally, compounds 1b and 1c had the highest overall efficacies, but of the two, as previously determined by Kirchhofer et al.,31 1c had the best efficacy against juvenile F. hepatica and is easier to synthesize than 1b. Ongoing investigations will assess if the superior efficacies of the 1,2,4-trioxanes and 1,2,4,5-tetraoxanes vs. the corresponding 1,2,4-trioxolanes are due to pharmacokinetic differences.32
Supplementary Material
Scheme 1.
Reagents and conditions: (a) O3, CH2Cl2/cyclohexane, 0 °C then sg chromatography; (b) aq. NaOH/EtOH, 60 °C, 4 h, then 1 M HCl, 0 °C.
Scheme 4.
Reagents and conditions: (a) 2-adamantanone, Re2O7, CH2Cl2, rt, 12 h; (b) 15% aq. KOH, EtOH/THF, 50 °C, 4–20 h, then AcOH.
Acknowledgment
This investigation received financial support from NIH (R21AI076783) and the Swiss National Science Foundation (project no. PPOOA-114941).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Keiser J, Utzinger J. Clin. Microbiol. Rev. 2009;22:466. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schweizer G, Braun U, Deplazes P, Torgerson PR. Vet. Rec. 2005;157:188. doi: 10.1136/vr.157.7.188. [DOI] [PubMed] [Google Scholar]
- 3.Fairweather I. J. Helminthol. 2009;83:139. doi: 10.1017/S0022149X09321173. [DOI] [PubMed] [Google Scholar]
- 4.Keiser J, Utzinger J. Emerg. Infect. Dis. 2005;11:1507. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moll L, Gaasenbeek CP, Vellema P, Borgsteede FH. Vet. Parasitol. 2000;91:153. doi: 10.1016/s0304-4017(00)00267-3. [DOI] [PubMed] [Google Scholar]
- 6.Keiser J, Utzinger J. Expert Opin. Drug Discov. 2007;2:S9. doi: 10.1517/17460441.2.S1.S9. [DOI] [PubMed] [Google Scholar]
- 7.Keiser J, Utzinger J. Trends Parasitol. 2007;23:555. doi: 10.1016/j.pt.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Keiser J, Utzinger J, Tanner M, Dong Y, Vennerstrom JL. J. Antimicrob. Chemother. 2006;58:1193. doi: 10.1093/jac/dkl408. [DOI] [PubMed] [Google Scholar]
- 9.Keiser J, Utzinger J, Vennerstrom JL, Dong Y, Brennan G, Fairweather I. Trans. R. Soc. Trop. Med. Hyg. 2007;101:1219. doi: 10.1016/j.trstmh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Q, Vargas M, Dong Y, Zhou L, Wang X, Sriraghavan K, Keiser J, Vennerstrom JL. J. Med. Chem. 2010;53:4223. doi: 10.1021/jm100226t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser M, Wittlin S, Nehrbass-Stuedli A, Dong Y, Wang X, Hemphill A, Matile H, Brun R, Vennerstrom JL. Antimicrob. Agents Chemother. 2007;51:2991. doi: 10.1128/AAC.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White NJ. Science. 2008;320:330. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 13.Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FCK, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H, McIntosh K, Padmanilayam M, Santo Tomas J, Scheurer C, Scorneaux B, Tang Y, Urwyler H, Wittlin S, Charman WN. Nature. 2004;430:900. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 14.Robert A, Benoit-Vical F, Claparols C, Meunier B. Proc. Natl. Acad. Sci. USA. 2005;102:13676. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melting points are uncorrected. 1H and 13C NMR spectra were recorded in CDCl3 on a 500 MHz spectrometer. All chemical shifts are reported in parts per million (ppm) and are relative to internal (CH3)4Si (0 ppm) for 1H and 77.0 ppm for 13C NMR.trans-Adamantane-2-spiro-3'-9'-carboxymethyl-1',2',4'-trioxaspiro[5.5]undecane (1b). mp 178–179 °C. 1H NMR δ 0.96–2.20 (m, 21H), 2.29 (d, J = 5.0 Hz, 2H), 2.54 (brs, 1H), 2.95 (brs, 1H), 3.74 (s, 2H); 13C NMR δ 27.1, 27.6 (br), 28.6 (br), 29.3 (br), 31.5 (br), 33.4, 36.1 (br), 37.1, 39.7, 62.7, 77.6, 104.4, 177.5. Anal (C19H28O5) C, 67.83; H, 8.39. Found: C, 68.02; H, 8.36.Adamantane-2-spiro-3'-9'-carboxymethyl-1',2',5'-trioxaspiro[5.5]undecane (1d). mp 210–211 °C. 1H NMR δ 1.20–2.00(m, 19H), 2.04–2.26 (m, 2H), 2.27 (d, J = 6.0 Hz, 2H), 2.73 (brs, 1H), 2.92 (brs, 1H), 3.75 (brs, 1H), 4.07 (brs, 1H), 10.98 (brs, 1H); 13C NMR δ 27.4, 27.5, 28.5 (br), 29.3 (br), 31.9, 33.6, 34.3 (br), 37.7, 40.5, 63.9, 81.3, 101.4, 178.3. Anal (C19H28O5) C, 67.83; H, 8.39. Found: C, 68.01; H, 8.18.trans-Adamantane-2-spiro-3'-9'-carboxy-1',2',4'-trioxaspiro[5.5]undecane (2b). mp 180–181 °C. 1H NMR δ 1.20–2.20 (m, 21H), 2.52–2.63 (m, 1H), 2.94 (brs, 1H), 3.62 (brs, 1H), 3.72 (brs, 1H); 13C NMR δ 22.7 (br), 23.8 (br), 27.1, 27.9 (br), 28.5 (br), 30.0 (br), 33.0 (br), 33.3, 36.0 (br), 37.1, 40.5, 64.0, 76.9, 104.4, 181.2. Anal (C18H26O5) C, 67.06; H, 8.13. Found: C, 67.30; H, 8.28.Adamantane-2-spiro-3'-9'-carboxy-1',2',4',5'-tetraoxaspiro[5.5]undecane (2c). mp 175–176 °C. 1H NMR δ 1.44–2.12 (m, 20H), 2.42–2.52 (m, 1H), 2.93 (brs, 1H), 3.16 (brs, 1H); 13C NMR δ 23.6 (br), 24.5 (br), 27.0, 28.1 (br), 30.2 (br), 33.1, 34.3 (br), 36.9, 41.2, 107.0, 110.6, 179.8. Anal (C17H24O6) C, 62.95; H, 7.46. Found: C, 62.51; H, 7.50.trans-3-Carboxymethyl-7,8,15-trioxadispiro[5.2.5.2]hexadecane (3b). mp 196–197 °C. 1H NMR δ 0.96–1.22 (m, 2H), 1.32–2.00 (m, 15H), 2.29 (d, J = 6.5 Hz, 2H), 2.18 (brs, 1H), 2.50 (brs, 1H), 3.75 (s, 2H); 13C NMR δ 22.3, 25.5, 28.4 (br), 33.3, 39.8, 63.3, 77.8, 102.3, 177.7. Anal (C15H24O5) C, 63.36; H, 8.51. Found: C, 63.59; H, 8.48.cis-3-Carboxy-7,14,15-trioxadispiro[5.1.5.2]pentadecane (4a). mp 161–163 °C; 1H NMR δ 1.34–1.99 (m, 18H), 2.35–2.39 (m, 1H); 13C NMR δ 27.7, 24.8, 25.9, 33.1, 34.5, 41.0, 107.7, 109.1, 181.3. Anal (C13H20O5) C, 60.92; H, 7.87. Found: C, 60.74; H, 7.79.trans-3-Carboxy-7,8,15-trioxadispiro[5.2.5.2]hexadecane (4b). mp 157–158 °C. 1H NMR δ 1.20–2.40 (m, 22H), 2.53–2.64 (m, 1H), 3.61 (brs, 1H), 3.75 (brs, 1H); 13C NMR δ 22.3, 22.7 (br), 25.5, 28.2 (br), 29.9 (br), 34.6 (br), 40.4, 64.6, 102.3, 181.1. Anal (C14H22O5) C, 62.20; H, 8.20. Found: C, 62.26; H, 8.30.trans-Adamantane-2-spiro-3'-9'-(4'-carboxyphenyl)-1',2',4'-trioxaspiro[5.5]undecane (5b). mp 212–213 °C. 1H NMR δ 1.38–2.20 (m, 20H), 2.64–2.74 (m, 1H), 2.86 (brs, 1H), 2.98 (brs, 1H), 3.76–4.02 (m, 2H), 7.30 (d, J = 8.0 Hz, 2H), 8.04 (d, J = 8.0 Hz, 2H), 11.51 (brs, 1H); 13C NMR δ 27.1, 28.8 (br), 33.0 (br), 33.4, 36.4 (br), 37.1, 43.7, 62.3, 77.6, 104.5, 126.9, 127.2, 130.5, 152.2, 171.0. Anal (C24H30O5) C, 72.34; H, 7.59. Found: C, 72.22; H, 7.63.Adamantane-2-spiro-3'-9'-(4'-carboxyphenyl)-1',2',4',5'-tetraoxaspiro[5.5]undecane (5c). mp 194–195 °C; 1H NMR δ 1.56–2.14 (m, 20H), 2.66–2.76 (m, 1H), 3.20 (brs, 1H), 3.32 (brs, 1H), 7.34 (d, J = 8.5 Hz, 2H), 8.04 (d, J = 8.5 Hz, 2H); 13C NMR δ 27.0, 29.5 (br), 30.1 (br), 31.8 (br), 33.1, 34.2 (br), 36.9, 43.8, 107.3, 110.6, 127.1, 127.3, 130.5, 152.2, 171.4. Anal (C23H28O6) C, 68.98; H, 7.05. Found: C, 68.90; H, 6.96.
- 16.Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FCK, Chollet J, Craft CJ, Creek DJ, Dong Y, Matile H, Maurer M, Morizzi J, Nguyen T, Papastogiannidis P, Scheurer C, Shackleford DM, Sriraghavan K, Stingelin L, Tang Y, Urwyler H, Wang X, White KL, Wittlin S, Zhou L, Vennerstrom JL. Proc. Natl. Acad. Sci. 2011;108:4400. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griesbaum K, Liu X, Kassiaris A, Scherer M. Libigs Ann./Recueil. 1997:1381. [Google Scholar]
- 18.Marques CA, Selva M, Tundo P, Montanari F. J. Org. Chem. 1993;58:5765. [Google Scholar]
- 19.Tang Y, Dong Y, Karle JM, DiTusa CA, Vennerstrom JL. J. Org. Chem. 2004;69:6470. doi: 10.1021/jo040171c. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Tang T, Chollet J, Matile H, Wittlin S, Charman SA, Charman WN, Santo Tomas J, Scheurer C, Snyder C, Scorneaux B, Bajpai S, Alexander SA, Wang X, Padmanilayam M, Rao CS, Brun R, Vennerstrom JL. Bioorg. Med. Chem. 2006;14:6368. doi: 10.1016/j.bmc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Wittlin S, Sriraghavan K, Chollet J, Charman SA, Charman WN, Scheurer C, Urwyler H, Santo Tomas J, Snyder C, Creek DJ, Morizzi J, Koltun M, Matile H, Wang X, Padmanilayam M, Tang Y, Dorn A, Brun R, Vennerstrom JL. J. Med. Chem. 2010;53:481. doi: 10.1021/jm901473s. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Dong Y, Wang X, Sriraghavan K, Wood JK, Vennerstrom JL. J. Org. Chem. 2005;70:5103. doi: 10.1021/jo050385+. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Hao H-D, Wu Y. Org. Lett. 2009;11:2691. doi: 10.1021/ol900811m. [DOI] [PubMed] [Google Scholar]
- 24.Leslie CP, Bentley J, Biagetti M, Contini S, Di Fabio R, Donati D, Genski T, Guery S, Mazzali A, Merlo G, Pizzi DA, Sacco F, Seri C, Tessari M, Zonzini L, Caberlotto L. Bioorg. Med. Chem. Lett. 2010;20:6103. doi: 10.1016/j.bmcl.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Cleij M, Archelas A, Furstoss R. J. Org. Chem. 1999;64:5029. doi: 10.1021/jo982101+. [DOI] [PubMed] [Google Scholar]
- 26.Bellucci G, Chiappe C, Lo Moro G. J. Org. Chem. 1995;60:6214. doi: 10.1021/jo9620526. [DOI] [PubMed] [Google Scholar]
- 27.Ghorai P, Dussault PH. Org. Lett. 2009;11:213. doi: 10.1021/ol8023874. [DOI] [PubMed] [Google Scholar]
- 28.Zmitek K, Zupan M, Stavber S, Iskra J. Org. Lett. 2006;8:2491. doi: 10.1021/ol060590r. [DOI] [PubMed] [Google Scholar]
- 29.Opsenica I, Opsenica D, Smith KS, Milhous WK, Šolaja BA. J. Med. Chem. 2008;51:2261. doi: 10.1021/jm701417a. [DOI] [PubMed] [Google Scholar]
- 30.Amewu R, Stachulski AV, Ward SA, Berry NG, Bray PG, Davies J, Labat G, Vivas L, O’Neill PM. Org. Biomol. Chem. 2006;4:4431. doi: 10.1039/b613565j. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhofer C, Vargas M, Braissant O, Dong Y, Wang X, Vennerstrom JL, Keiser J. Acta Trop. 2011;118:56–62. doi: 10.1016/j.actatropica.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill PM, Amewu RK, Nixon GL, Bousejra ElGarah F, Mungthin M, Chadwick J, Shone AE, Vivas L, Lander H, Barton V, Muangnoicharoen S, Bray PG, Davies J, Park BK, Wittlin S, Brun R, Preschel M, Zhang K, Ward SA. Angew. Chem. Int. Ed. 2010;49:5693. doi: 10.1002/anie.201001026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.