Abstract
The major challenges in the delivery and therapeutic efficacy of nano-delivery systems in chronic obstructive airway conditions is airway defense, severe inflammation and mucous hypersecretion. Chronic airway inflammation and mucous hypersecretion are hallmarks of chronic obstructive airway diseases, including asthma, COPD (chronic obstructive pulmonary disease) and CF (cystic fibrosis). Distinct etiologies drive inflammation and mucous hyper secretion in these diseases, that is further induced by infection or components in cigarette smoke (CS). Controlling chronic inflammation is at the root of treatments such as corticosteroids, antibiotics or other available drugs, which pose the challenge of sustained delivery of drugs to target cells or tissues. In spite of the wide application of nano-based drug delivery systems, very few are tested to date. Targeted nanoparticle(NP)-mediated sustained drug delivery is required to control inflammatory cell chemotaxis, fibrosis, protease mediated chronic emphysema and/or chronic lung obstruction in COPD. Moreover, targeted epithelial delivery is indispensable for correcting the underlying defects in CF, and for controlling other chronic inflammatory lung diseases. We propose that the design and development of novel nano-based biodegradable therapeutic vehicles, capable of bypassing the airway defenses, will be invaluable in the search for novel treatments for chronic obstructive lung diseases. This paper discusses a novel nano-theranostic strategy that we are currently evaluating to treat the underlying cause of CF and COPD lung disease.
Keywords: Nano, Theranostic, Therapeutics, CF & COPD
Introduction
The major challenge in delivery and therapeutic efficacy of nano-delivery systems in chronic obstructive airway conditions is severe inflammation, airway defense and mucous hyper secretion[1–4]. Chronic airway inflammation and mucous hypersecretion is a hallmark of chronic obstructive airway diseases, including asthma, COPD (chronic obstructive pulmonary disease) and CF (cystic fibrosis). Distinct etiologies and inflammatory responses drive mucous hypersecretion in these diseases. In asthma, inflammation appears to be mediated by allergen-specific Th2 cells, leading to eosinophilia, while in COPD and CF, the inflammatory response is macrophage (COPD) or neutrophilic (CF & COPD) driven[3–7] that can be induced by infection and/or components in cigarette smoke (CS). The chronic stage of these diseases is also associated with widespread damage to the bronchial epithelium, due to excessive inflammation and apoptosis, and defective epithelial repair[4,5,7,8]. Controlling chronic inflammation is at the root of treatment using corticosteroids, bronchodilators or antibiotics in these chronic obstructive inflammatory conditions yet despite therapy, challenge is the sustained delivery of drugs to target cells or tissues. Moreover, COPD subjects have shown to develop a HDAC2 (histone deacetylase-2) mediated corticosteroid resistance[9] although anti-inflammatory bronchodilator and HDAC2 inducer, theophylline has been shown to overcome this[10–13] but have a significant side effects if given orally. Similarly in CF, correction of misfolded CFTR requires targeted drug/gene delivery as recently shown using a novel nano-system capable of overriding airway defense and providing sustained drug release[14]. These nano-based systems can overcome airway defense (like macrophage, neutrophils & mucus etc) in chronic obstructive lung diseases and variety of other pulmonary conditions but very few are tested till date [2,15,16].
1. Biodegradable nanoparticles for obstructive lung diseases
Recent studies have shown the efficacy of poly-lactide-co-glycolide (PLGA) based nano-systems for selective drug delivery[14]. A major drawback of PLGA nanoparticles (NPs) is that when formulated with the commonly used emulsifier polyvinyl alcohol (PVA), they are hydrophobic and have a high negative charge on their surface. As a result, such a system, when administered in experimental animals, is rapidly opsonized by the defense system of the body (Reticuloendothelial System, RES or Mononuclear Phagocyte System, MPS; systemic circulation or airway)[17]. The best way to overcome this challenge is coating of the drug delivery system with the outer layer of polyethyleneglycol (PEG) that endow these NPs with ‘stealth’, or RES/MPS evading properties[17]. PEGylation also increases the circulation time of the NPs, thereby enhancing their propensity of accumulation in target organs or cells by passive diffusion, taking aid of the enhanced permeability and retention (EPR) effect[18]. PEG chains, covalently attached with PLGA NPs using ring-opening polymerization method, results in increased residence in blood (intravenous) or airway (intranasal) that enhances the prospects of stable controlled release in target tissues or cells[19]. NP mediated drug delivery presents the added advantage of targeting the drug to specific organs or cells in the body, for example by conjugating it with a monoclonal antibody or IgA receptor (Fig 1, pending patent) that targets the system specifically to the airway inflammatory or epithelial cells which over express the complementary antigen (ongoing studies). However, until date, the use of drug loaded PLGA NPs synthesized using the popular emulsifier PVA has resulted in poor in vivo drug delivery efficiency. It has also been found that such a formulation can never be completely purified of the emulsifier PVA, which is suspected of non-specific toxicity[20]. In order to develop an improved, clinically viable formulation of PLGA NPs over existing PVA based ones, we adopted a strategy used in the synthesis of PEGylated liposomes and PEGylated immunoliposomes, employing commercially available PEGylated phospholipids (like Distereolylphos-phatidylethanolamine-mPEG2000, or DSPE-mPEG2000) as emulsifiers[14]. Such molecules have surfactant-like properties, and spontaneously self-aggregate in aqueous solutions forming micelles. As discussed above major challenges in delivery and therapeutic efficacy of nano-delivery systems in chronic obstructive airway conditions is airway defense (such as macrophage, neutrophils etc), severe inflammation and mucous hypersecretion[1,2]. We anticipate that development of biodegradable NP-based targeted drug delivery system that can overcome airway defense associated challenges will have enormous applications in the treatment of chronic pathophysiology of obstructive lung diseases.
Figure 1. Schematic of proposed nanotheranostic for targeting chronic inflammatory cascade in COPD.
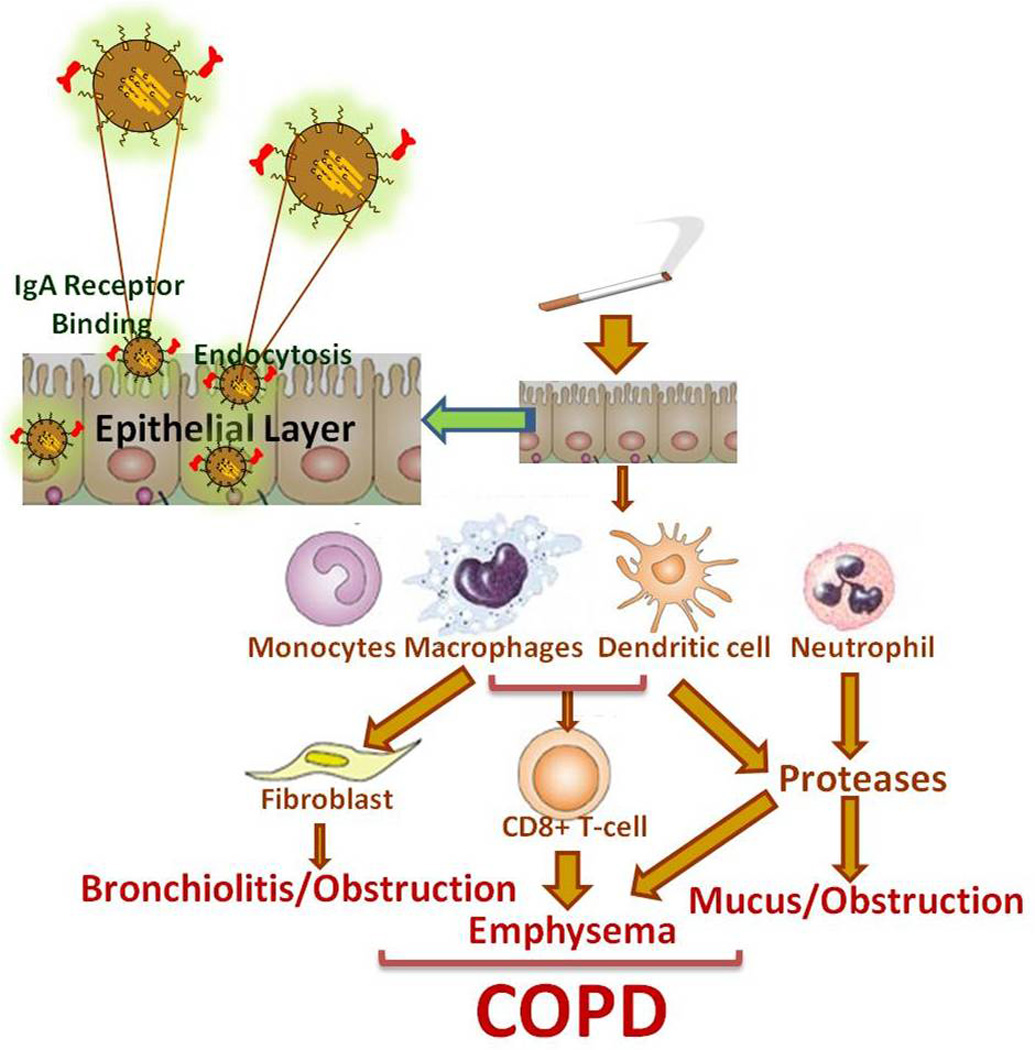
As an example, epithelial targeted nanoparticle (NP) providing sustained drug and molecular probe delivery (nano-theranostic) can control neutrophil chemotaxis, fibrosis and protease mediated chronic emphysema while providing real time assessment of COPD lung disease. Drug, molecular probe, gene and/or short interfering RNA sequences are encapsulated within a nanoparticle vesicle. Targeting antibodies are attached onto the surface for transport to specific epithelial or inflammatory cells. The proposed nano-theranostic incorporates many functionalities, such as targeting molecules, a polymer shield, therapeutic agents and molecular probes.
2. Theranostics
Theranostics, the pairing of a diagnostic test with a therapy is considered the pathway to personalized medicine, which will usher in an entirely new era of healthcare delivery. Nano based theranostics can offer many benefits for CF & COPD patients including real time diagnosis of lung inflammatory state and treatment, optimal therapy for individual patients and reduction in adverse drug effects[21]. To make theranostics possible for CF & COPD subjects, it is essential to develop multifunctional airway targeting nanocarriers for combined delivery of diagnostic and therapeutic agents. Innovative design of such nanoparticles (NPs) allows for the integration of multiple functions, such as cell targeting, imaging ultra sensitivity and therapy into one system. In particular, the integration of diagnostic imaging capability with therapeutic interventions is critical in addressing the challenges of chronic obstructive lung disease and increasing survival in CF and COPD subjects. A number of such multifunctional metal or metal oxide NPs have been reported for cancer. For instance, gold NPs with proper morphology (size and shape) and surface functionalization were used to simultaneously enhance the optical imaging ability and provide photo thermal cancer therapy[22]. In addition, multifunctional quantum dot-conjugated immunoliposomes were used for targeted anticancer drug delivery and fluorescence imaging[23]. Similarly multifunctional NPs loaded with both magnetic iron oxide and anticancer drugs encapsulated in a silica shell, offers the potential to deliver the payload (i.e. both the magnetic resonance imaging (MRI) contrast agent and hydrophobic anticancer drug) to the targeted tumor tissues thereby making simultaneous cancer therapy and diagnosis feasible[24]. Despite the recent progress in developing multifunctional nanosystems for combined cancer therapy and diagnosis, there is still an urgent need to further develop novel multifunctional nanosystems to face existing challenges in the current field of theranostics as well its application in chronic obstructive lung diseases. Moreover, metal oxide NPs developed as cancer theranostics have limited application in chronic airway diseases due to toxicity and chronic inflammation. As an alternate, amphiphilic block copolymers can form several types of nano-assembly in an aqueous solution including micelles and vesicles depending on their relative hydrophilicity/hydrophobicity and chemical structures. Similar to liposomes, polymeric vesicles can simultaneously deliver hydrophilic and hydrophobic agents encapsulated in their aqueous core and hydrophobic membrane. However, compared with liposomes, polymer vesicles offer numerous possibilities of controlling the physical, chemical and biological properties by tailoring the block lengths block chemistry and functionalization.
3. Theranostic nanoparticles for obstructive lung diseases
We are currently developing a multifunctional polymeric vesicle formed by a mixture of poly(ethylene glycol)– poly(lactic-co-glycolic acid)- (PLGAPEG) for combined delivery of COPD/CF drugs (ex- corticosteroid, prednisolone and/or anti-inflammatory bronchodilator, theophylline, ΔF508-CF correctors etc) and molecular probes that can be used for theranostic application in obstructive lung diseases. The application of such nanocarriers in clinics in near future would allow the use of non-invasive imaging modality to monitor the bio-distribution and anti-inflammatory effects of the PROBE/DRUG- loaded nanocarriers in real time thereby making theranostics possible for CF and COPD. PEG is the most commonly used hydrophilic polymer block in amphiphilic block co-polymers used for drug delivery due to its excellent water solubility, high flexibility, very low toxicity, low immunogenicity and lack of accumulation in the RES cells. While PLGA is a co-polymer that is used in a host of FDA approved therapeutic devices, owing to its biodegradability and biocompatibility. Current tools for monitoring inflammatory state and drug activity are not only costly and time consuming but also require significant resources for processing [17]. Moreover, in human subject’s development of such state of art real time imaging technologies can greatly improve the evaluation of Phase I-III drug development and reduce the costs of clinical procedures (biopsy, bronchoscopy etc). Most importantly for the study of CF/COPD lung disease, it is impossible to assess real time drug activity in lung epithelia or inflammatory cells. Moreover, the drug-specific responses in the same animal or subject at different time points are currently not feasible. Thus, non-invasive imaging technique to monitor correction of lung disease in the same group of animals or subjects is crucial for both drug development and evaluation of chronic lung disease state in the most cost-effective manner. Single photon emission computed tomography (SPECT/CT) and PET/CT (positron emission tomography/CT) are extensively used for non-invasive and real-time assessment of human diseases including lung cancer [25–32]. It relies on the detection of positrons emitted from radiolabeled tracers that accumulate at the site of the desired tissue. One such tracer is the radioactive marker [18F] FDG (fluorine-18- fluorodeoxyglucose). Under inflammatory conditions, the affinity of glucose transporters for deoxyglucose is increased by various cytokines and growth factors. FDG is known to accumulate in inflammatory cells such as activated macrophages at the site of inflammation [25–30,33,34], making it a desirable molecular probe for inflammatory lung diseases (such as CF&COPD). Development of other novel molecular probes that can detect inflammation, bacteria and apoptotic cells[35,36] hence chronicity of lung disease can be even more useful for chronic obstructive lung diseases.
Expert Opinion
Although the role of inflammatory signaling and oxidative stress in COPD and CF is apparent, the lack of efficient drug delivery and real time diagnosis of inflammatory-oxidative state results in improper treatment that leads to chronic and fatal lung pathophysiology. There is an immediate need to develop novel targeted nanosystem(s) that can effectively provide sustained delivery of potent CF and COPD therapeutics and molecular probes through obstructive airway for correction and real time assessment of lung disease (theranostics). The carefully designed safety, efficacy & preclinical studies will help translate these nano-based theranostics for further clinical evaluation in human subjects.
Acknowledgments
This paper has been supported by the FAMRI YCSA and NIH (CTSA UL RR 025005 and RHL096931) grants to NV.
Footnotes
Declaration of interest
The author declares no conflict of interest.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1. Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–312. doi: 10.1172/JCI6222. * Describes the pathogenesis of obstructive CF lung disease.
- 2. Roy I, Vij N. Nanodelivery in airway diseases: challenges and therapeutic applications. Nanomedicine. 2010;6:237–244. doi: 10.1016/j.nano.2009.07.001. ** This paper discusses the application of nanosystems in airway diseases.
- 3. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. ** Describes the mechanisms and cell types involved in pathogenesis of COPD.
- 4. Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. * A good review of mechanisms and cell types involved in pathogenesis of COPD.
- 5.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich M, Worlitzsch D, Viglio S, et al. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 8.Larsson K. Aspects on pathophysiological mechanisms in COPD. J Intern Med. 2007;262:311–340. doi: 10.1111/j.1365-2796.2007.01837.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Theophylline for COPD. Thorax. 2006;61:742–744. doi: 10.1136/thx.2006.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano T, Yamagata T, Gohda M, et al. Inhibition of reactive nitrogen species production in COPD airways: comparison of inhaled corticosteroid and oral theophylline. Thorax. 2006;61:761–766. doi: 10.1136/thx.200X.058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsdell J. Use of theophylline in the treatment of COPD. Chest. 1995;107:206S–209S. doi: 10.1378/chest.107.5_supplement.206s. [DOI] [PubMed] [Google Scholar]
- 13.Culpitt SV, de Matos C, Russell RE, et al. Effect of theophylline on induced sputum inflammatory indices and neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1371–1376. doi: 10.1164/rccm.2105106. [DOI] [PubMed] [Google Scholar]
- 14.Vij N, Min T, Marasigan R, et al. Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J Nanobiotechnology. 2010;8:22. doi: 10.1186/1477-3155-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Peters JI, Williams RO., 3rd Inhaled nanoparticles--a current review. Int J Pharm. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 16. Zhang W, Yang H, Kong X, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. ** The study describes intranasal efficacy of siRNA nanoparticles in targeting viral NS1 gene.
- 17.Davis SS. Biomedical applications of nanotechnology--implications for drug targeting and gene therapy. Trends Biotechnol. 1997;15:217–224. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Sawa T, Maeda H. Factors and mechanism of "EPR" effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol. 2003;519:29–49. doi: 10.1007/0-306-47932-X_2. [DOI] [PubMed] [Google Scholar]
- 19. Gref R, Minamitake Y, Peracchia MT, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. ** Discusses applications of biodegradable polymeric nanoparticles.
- 20.Sahoo SK, Panyam J, Prabha S, et al. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 21. Kurmi BD, Kayat J, Gajbhiye V, et al. Micro- and nanocarrier-mediated lung targeting. Expert Opin Drug Deliv. 7:781–794. doi: 10.1517/17425247.2010.492212. * Expert opinion on lung targeting using micro- and nano- particles
- 22.Verbavatz JM, Ma T, Gobin R, et al. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci. 1997;110(Pt 22):2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- 23.Weng KC, Noble CO, Papahadjopoulos-Sternberg B, et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008;8:2851–2857. doi: 10.1021/nl801488u. [DOI] [PubMed] [Google Scholar]
- 24.Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alavi A, Reivich M. Guest editorial: the conception of FDG-PET imaging. Semin Nucl Med. 2002;32:2–5. doi: 10.1053/snuc.2002.29269. [DOI] [PubMed] [Google Scholar]
- 26.Vos FJ, Bleeker-Rovers CP, Corstens FH, et al. FDG-PET for imaging of non-osseous infection and inflammation. Q J Nucl Med Mol Imaging. 2006;50:121–130. [PubMed] [Google Scholar]
- 27.Chen DL, Rosenbluth DB, Mintun MA, et al. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol. 2006;100:1602–1609. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 28.Love C, Tomas MB, Tronco GG, et al. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 29.Kuo PH, Carlson KR, Christensen I, et al. FDG-PET/CT for the evaluation of response to therapy of cutaneous T-cell lymphoma to vorinostat (suberoylanilide hydroxamic acid, SAHA) in a phase II trial. Mol Imaging Biol. 2008;10:306–314. doi: 10.1007/s11307-008-0161-4. [DOI] [PubMed] [Google Scholar]
- 30.Kuo PH, McClennan BL, Carlson K, et al. FDG-PET/CT in the evaluation of cutaneous T-cell lymphoma. Mol Imaging Biol. 2008;10:74–81. doi: 10.1007/s11307-007-0127-y. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Kotzer CJ, Makrogiannis S, et al. A Noninvasive [(99m)Tc]DTPA SPECT/CT Imaging Methodology as a Measure of Lung Permeability in a Guinea Pig Model of COPD. Mol Imaging Biol. doi: 10.1007/s11307-010-0423-9. [DOI] [PubMed] [Google Scholar]
- 32. Katyal S, Kramer EL, Noz ME, et al. Fusion of immunoscintigraphy single photon emission computed tomography (SPECT) with CT of the chest in patients with non-small cell lung cancer. Cancer Res. 1995;55:5759s–5763s. * Describes diagnostic application of SPECT-CT in NSCLC patients.
- 33. Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. * Discusses application of 18-FDG in detecting macrophage and granulation tissues.
- 34.Kubota R, Yamada S, Kubota K, et al. [Autoradiographic demonstration of 18F-FDG distribution within mouse FM3A tumor tissue in vivo] Kaku Igaku. 1992;29:1215–1221. [PubMed] [Google Scholar]
- 35.Leevy WM, Lambert TN, Johnson JR, et al. Quantum dot probes for bacteria distinguish Escherichia coli mutants and permit in vivo imaging. Chem Commun (Camb) 2008:2331–2333. doi: 10.1039/b803590c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leevy WM, Gammon ST, Jiang H, et al. Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe. J Am Chem Soc. 2006;128:16476–16477. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]


