Abstract
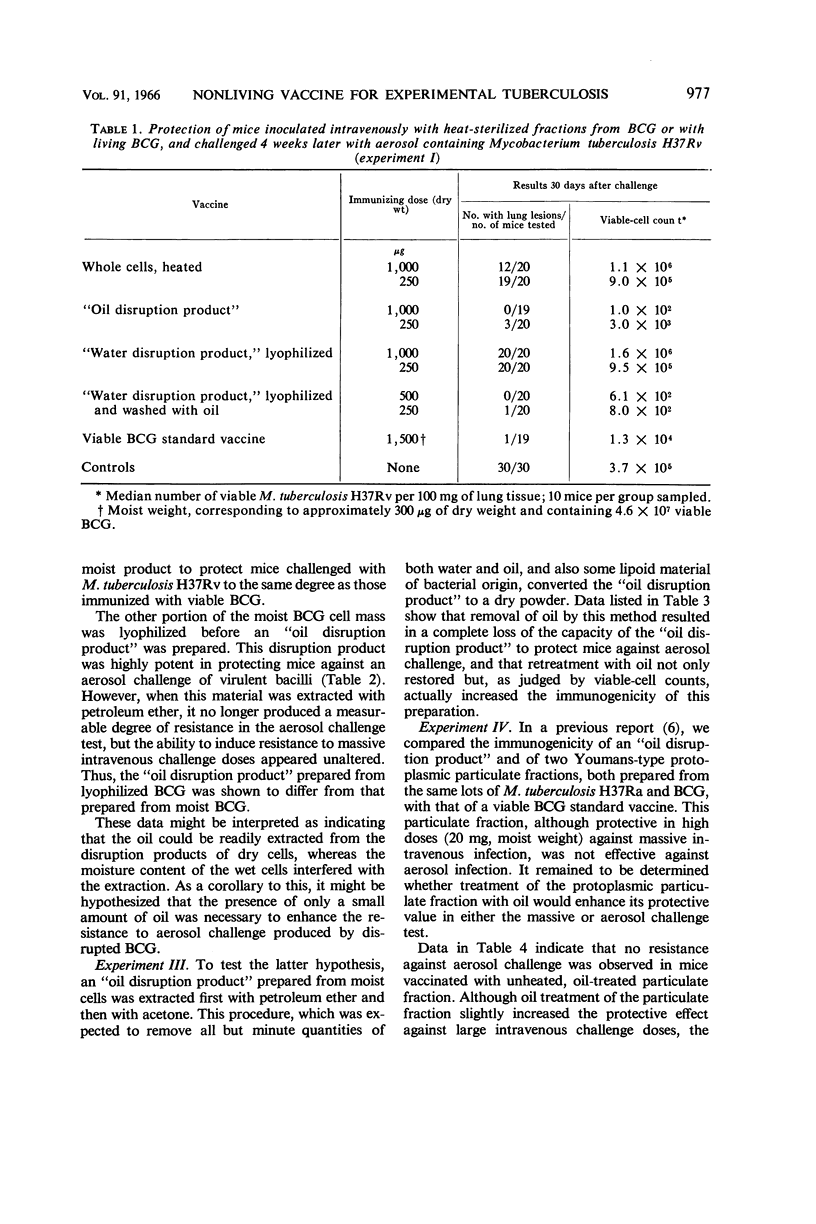
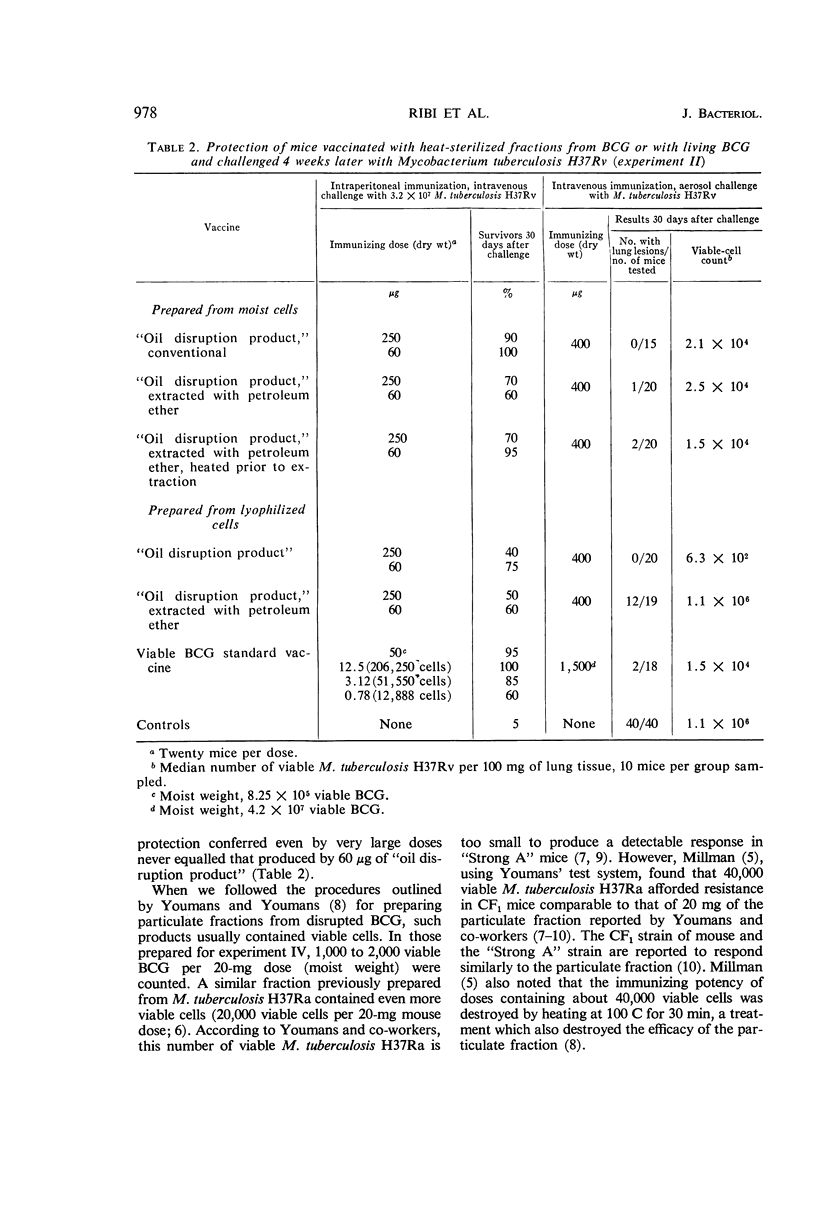
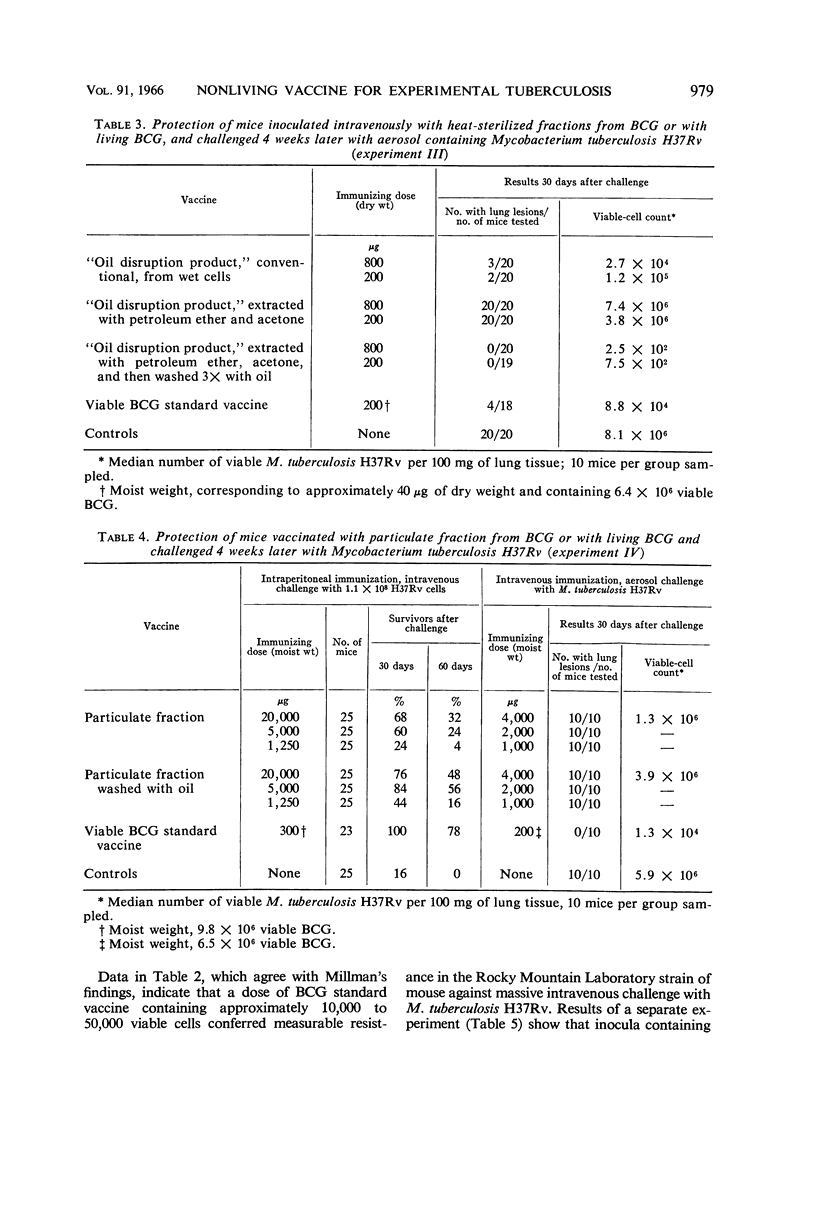
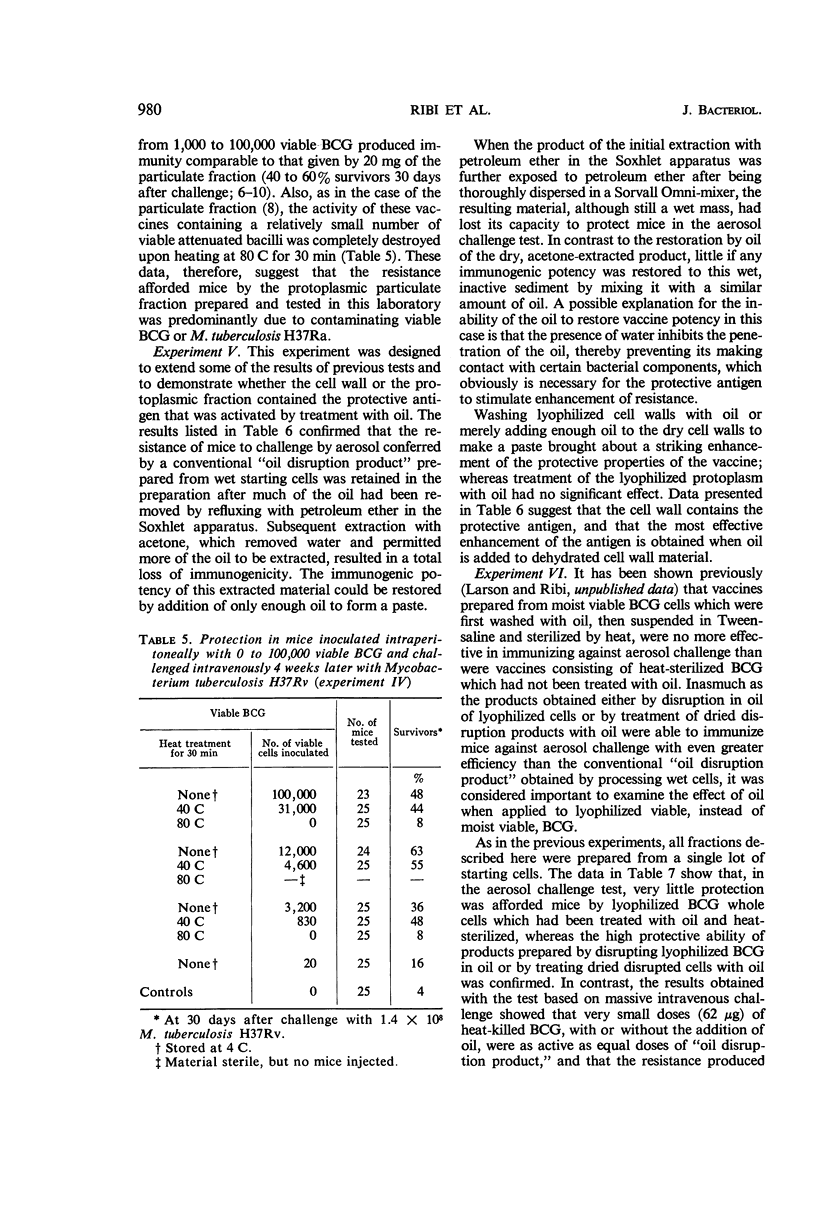
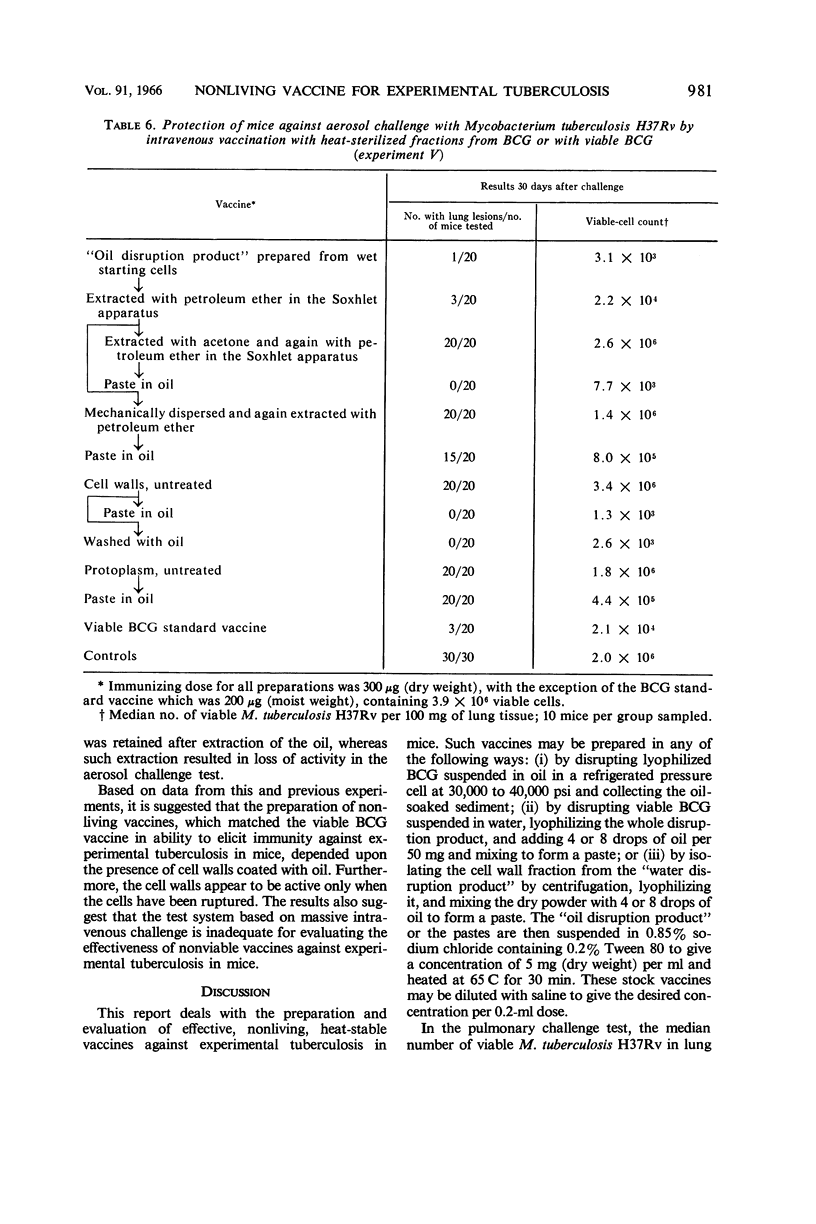
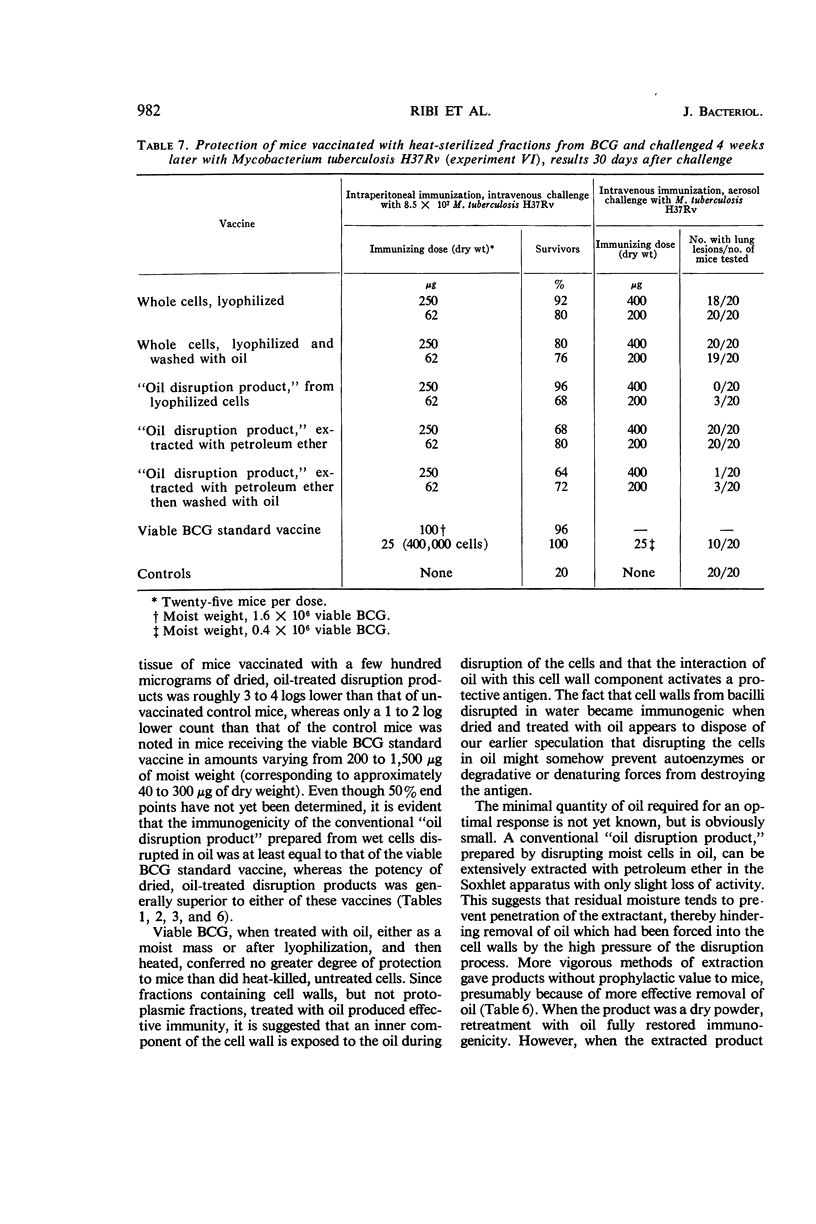
Ribi, Edgar (Rocky Mountain Laboratory, Hamilton, Mont.), Carl Larson, William Wicht, Robert List, and Granville Goode. Effective nonliving vaccine against experimental tuberculosis in mice. J. Bacteriol. 91:975–983. 1966.—Antituberculosis vaccines were prepared in one of three manners: lyophilized BCG suspended in light mineral oil was disrupted in a Sorvall pressure cell and the “oil disruption product” was collected by centrifugation; BCG was disrupted in water, lyophilized, and worked into a paste with a small amount of oil (about 0.16 ml per 50 mg); BCG was disrupted in water, and the cell wall fraction was isolated, lyophilized, and prepared in an oil paste. These vaccines were suspended in Tween-saline to a concentration of 5 mg/ml and heated at 65 C for 30 min. In protection tests based on pulmonary infection with Mycobacterium tuberculosis H37Rv, the median number of virulent organisms in lung tissue of mice immunized with a few hundred micrograms of these three vaccines was 3 to 4 logs lower than in unvaccinated control mice. A similar dose of viable BCG standard vaccine reduced the lung count 1 to 2 logs below the controls. Protection afforded by nonviable, whole BCG, with or without oil, was of only borderline significance. Since oil-treated fractions containing cell walls produced effective immunity, while the oil-treated protoplasm or whole cells were not active, the protective antigen appeared to be an inner component of the cell wall, exposed when the cell was disrupted, and activated by oil. Extraction of oil from immunogenic disruption products resulted in loss of ability of the products to confer protection against the aerosol challenge, whereas high protection against the conventional challenge by intravenous infection with up to 1.4 × 108 cells of M. tuberculosis H37Rv was retained. Retreatment with oil of these nonimmunogenic products restored the immunogenicity if the oil was applied to dried products. The consistent finding that moisture interferes with the enhancement of the vaccine potency by oil suggested that such enhancement may not be the same as that ordinarily produced by water-in-oil emulsions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOS R. J., SCHAEDLER R. W., BOHME D. Effects of bacterial endotoxins on susceptibility to infection with gram-positive and acid-fast bacteria. Fed Proc. 1957 Sep;16(3):856–857. [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W. C. Studies of resistance to experimental tuberculosis in mice vaccinated with living attenuated tubercle bacilli and challenged with virulent organisms. Am Rev Respir Dis. 1962 Jun;85:833–846. doi: 10.1164/arrd.1962.85.6.833. [DOI] [PubMed] [Google Scholar]
- MILLMAN I. Nonspecific resistance to tuberculosis. Am Rev Respir Dis. 1961 May;83:668–675. doi: 10.1164/arrd.1961.83.5.668. [DOI] [PubMed] [Google Scholar]
- RIBI E., LARSON C. L., WICHT W., LIST R., GOODE G. RESISTANCE TO EXPERIMENTAL TUBERCULOSIS STIMULATED BY FRACTIONS FROM ATTENUATED TUBERCLE BACILLI. Proc Soc Exp Biol Med. 1965 Apr;118:926–933. doi: 10.3181/00379727-118-30009. [DOI] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. FURTHER STUDIES ON A LABILE IMMUNOGENIC PARTICULATE SUBSTANCE ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Feb;87:278–285. doi: 10.1128/jb.87.2.278-285.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P., MILLMAN I. Immunogenicity of particles isolated from Mycobacterium tuberculosis. Proc Soc Exp Biol Med. 1957 Dec;96(3):762–768. doi: 10.3181/00379727-96-23602. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., MILLMAN I., YOUMANS A. S. The immunizing activity against tuberculous infection in mice of enzymatically active particles isolated from extracts of Mycobacterium tuberculosis. J Bacteriol. 1955 Nov;70(5):557–562. doi: 10.1128/jb.70.5.557-562.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S., KANAI K. The difference in response of four strains of mice to immunization against tuberculous infection. Am Rev Respir Dis. 1959 Nov;80:753–756. doi: 10.1164/arrd.1959.80.5.753. [DOI] [PubMed] [Google Scholar]



