Abstract
Purpose
Translocator protein (TSPO) is a promising biomarker for neuroinflammation. We developed two new PET ligands, 18F-PBR06 and 11C-PBR28, to image TSPOs. Although our prior studies suggest that either of the two ligands could be used to quantify TSPOs in human brain, the studies were done in different sets of subjects. In this study, we directly compared 18F-PBR06 and 11C-PBR28 in eight human subjects to determine (1) whether either ligand provides more precise measurements of TSPOs and (2) whether the higher in vitro affinity of PBR06 compared to PBR28 led to higher in vivo binding of 18F-PBR06 compared to 11C-PBR28.
Methods
In vivo binding was calculated as total distribution volume (VT), using an unconstrained two-tissue compartment model. VT was corrected for plasma free fraction (fP) to measure ligand binding based on free ligand concentration in brain.
Results
Both ligands measured VT with similar precision, as evidenced by similarly good identifiability. However, VT for both radioligands increased with increasing lengths of data acquisition, consistent with the accumulation of radiometabolites in brain. Despite its higher lipophilicity and higher in vitro affinity, VT/fP of 18F-PBR06 was similar to that of 11C-PBR28.
Conclusion
Both 18F-PBR06 and 11C-PBR28 are similar in terms of precision, sensitivity to accumulation of radiometabolites, and magnitude of in vivo binding. Thus, selection between the two radioligands will be primarily determined by the logistical impact of the different half-lives of the two radionuclides (110 vs 20 min).
Keywords: Compartment model, Kinetic analysis, Inflammation, Translocator protein (18 kDa), PET
Introduction
Translocator protein1 (18 kDa) (TSPO) is upregulated on activated microglia and is therefore a biomarker for inflammation [2]. For the past two decades, TSPO has been imaged using the positron emission tomographic (PET) ligand 11C-PK11195, but in the last several years, a number of improved PET ligands with higher ratios of specific to nondisplaceable binding have been developed from new structural classes [3]. We developed two new aryloxyanilide-based ligands, 18F-PBR06 [4] and 11C-PBR28 [5], to image TSPO (Fig. 1). In monkey brain, the specific binding of each ligand was greater than 90% of its total uptake [6, 7].
Fig. 1.
Chemical structures of 18F-PBR06 and 11C-PBR28
In human brain, both ligands precisely quantified TSPOs as distribution volumes using compartmental modeling [8, 9]. However, the distribution volumes of both ligands increased with longer duration of data, compatible with the accumulation of radiolabeled metabolites over time. Across brain regions, distribution volumes of 18F-PBR06 had somewhat better identifiability than those of 11C-PBR28. These prior findings raise the possibility that 18F-PBR06 may quantify TSPOs more precisely than 11C-PBR28, particularly in small brain regions; however, this conclusion is uncertain, since the two radioligands were studied in different subjects.
PBR06 and PBR28 differ in terms of lipophilicity and in vitro affinity for TSPO, and both properties can significantly affect in vivo binding of the cognate radioligands. The lipophilicity of PBR06 is about ten times greater than that of PBR28: log D=4.l vs 3.0, respectively, measured in octanol/phosphate buffer, pH 7.4 [4, 5]. Although increasing lipophilicity enhances blood-brain barrier penetration, it also tends to increase nondisplaceable uptake in brain, which has a high content of fat.
The in vitro assays performed in our lab showed that PBR06 had slightly but significantly greater affinity for TSPO than PBR28 in human brain tissue (Ki=1.0 vs 2.5 nM, respectively) [4, 5]. If the same relationship holds also in vivo, 18F-PBR06 would be expected to have higher specific binding to TSPO than 11C-PBR28. Since total distribution volume (VT) is the sum of specific (VS) and nondisplaceable (VND) distribution volumes, the higher lipophilicity and higher in vitro affinity of PBR06 compared to PBR28 suggests that the VT of 18F-PBR06 will be greater than that of 11C-PBR28. However, because the in vivo environment can differ markedly from in vitro conditions, PET measurements may not reflect in vitro properties.
In this study, we compared 18F-PBR06 and 11C-PBR28 in the same healthy subjects to determine (1) whether either ligand provides more precise measurements, as indirectly measured as identifiability of distribution volume, and (2) whether 18F-PBR06 and 11C-PBR28 show different amounts of in vivo binding. To answer these questions, we scanned eight healthy subjects with both ligands and analyzed the same length of the data using compartmental modeling with metabolite-corrected arterial input function.
Materials and methods
Eight healthy subjects (two women and six men; age 32±11 years) had PET scans with both 18F-PBR06 and 11C-PBR28. The mean interval between the two scans was 7.6 months (range 2–21 months). Data from all eight 18F-PBR06 scans and from three 11C-PBR28 scans were included in previous publications, where detailed descriptions of data acquisition and analyses are provided [8, 9].
18F-PBR06 and 11C-PBR28 were produced with high radiochemical purity (100%) and had specific radioactivities of 246±93 and 100±45 GBq/µmol, respectively, at the time of injection. After a bolus intravenous injection of 18F-PBR06 (170±26 MBq) or 11C-PBR28 (567±84 MBq), dynamic PET scans were acquired on a GE Advance scanner (GE Healthcare, Waukesha, WI, USA). Concurrent blood samples were drawn from the radial artery, and the plasma concentration of parent radioligand, separated from its radiometabolites by radio-HPLC, was measured to generate a metabolite-corrected input function for kinetic modeling. In addition, plasma free fraction (fP) was measured in each scan. From the dynamic PET scans, we measured radioactivity in ten brain regions (anterior cingulate, cerebellum, caudate, putamen, thalamus, and frontal, parietal, temporal, occipital, and medial temporal cortices) [9]. Radioactivity concentrations in both brain and plasma were decay-corrected and expressed as standardized uptake values (SUVs) to normalize for injected activity and body weight.
TSPO binding was measured as VT normalized to fP, which equals the ratio at equilibrium of radioactivity in brain to the concentration of free (i.e., non-protein-bound) parent radioligand in plasma [10]. VT was first calculated using an unconstrained two-tissue compartment model and metabolite-corrected input function [8, 9] and then normalized to fP. Identifiability (SE) of VT, an indicator of precision, was expressed as a percentage of VT itself, with a lower percentage corresponding to better identifiability. Data were acquired for 300 and 120 min for 18F-PBR06 and 11C-PBR28, respectively. In the current study, the same length of 120 min data were used for both ligands to allow direct comparison. Furthermore, in our prior study with 18F-PBR06 data acquired over 120 min provided the most accurate and precise measurement based on the time stability and the best identifiability [8]. Because only free ligand can enter the brain, we calculated VT/fP as our primary outcome measure of ligand binding. Analyses were performed using pixelwise modeling software (PMOD 2.85, PMOD Technologies Ltd., Zurich, Switzerland) and Statistical Parametric Mapping 5 (SPM 5, Wellcome Department of Cognitive Neurology, London, UK).
We compared VT/fP measurements by the two ligands to determine whether the measurements had similar precision and were consistent. First, we compared the precision of binding measurements in terms of identifiability of VT. We considered VT values with SE≤10% to be well identified. Second, we examined the time stability of VT by truncating PET data in 10-min increments until the scan length reached 30 min and then normalizing VT to the terminal value at 120 min. To further study the precision of the measurements by the two ligands, we examined the correlation between VT/fP values in ten brain regions. Because the two ligands bind to the same molecule, if errors in the measurement of each ligand are small, VT/fP values should correlate well between the two ligands.
In addition to examining precision and consistency of the measurements, we compared the in vivo affinities (1/KD) of the ligands using the regional differences in VT/fP values in analogy to the Lassen plot [11]. A ratio of the in vivo affinities of the two ligands was obtained from the slope of a regression line fitted to VT/fP values in ten brain regions by making two assumptions. The first assumption was that the VND (free plus nonspecific) of both ligands is uniform across brain regions, because our monkey scans under binding blockade showed uniform distribution of activity [6, 7]. Consequently, because VT/fP is a summation of VS and VND (VS/fP and VND/fP, respectively) the difference in VT/fP between any two brain regions corresponds to the difference in VS/fP. The second assumption was that both ligands have the same receptor density (Bmax), since they are expected to bind to the identical target site given their structural similarity. It then follows from the equation VS/fP=Bmax/KD that the ratio of the difference between any two brain regions in VT/fP of 18F-PBR06 to that of 11C-PBR28 (i.e., the slope of the regression line) corresponds to a ratio of the affinity of 11C-PBR28 to that of 18F-PBR06 because the regional differences of Bmax between the two ligands cancel out.
Data are expressed as mean ± SD. Brain data are reported as the mean of ten brain regions, each averaged across eight subjects. For statistical comparisons of 18F-PBR06 and 11C-PBR28, we used a paired samples t test to compare fP and repeated measures analysis of variance (ANOVA) to compare VT, VT/fP, identifiability, and time stability.
Results
Kinetics in plasma and brain
18F-PBR06 and 11C-PBR28 showed overall similar plasma total (= free plus protein-bound) parent levels, but the former showed lower brain activity. Plasma concentrations of total parent 18F-PBR06 and 11C-PBR28 were similar in the first 15 min after injection: concentrations peaked within 2 min and then rapidly declined (Fig. 2a). After the first 15 min, 18F-PBR06 was cleared from plasma more slowly than 11C-PBR28 (p<0.05; Fig. 2b). Brain uptake of radioactivity peaked within 5 min of injection for both ligands and then declined for the remainder of the scan (Fig. 3). 18F-PBR06 showed about one third less uptake than 11C-PBR28, with peak concentrations of 1.2 [mean of ten regions in eight subjects (range 1.0–1.6)] and 1.8 (range 1.5–2.1) SUV, respectively. As from plasma, 18F-PBR06 washed out from brain slightly more slowly than 11C-PBR28.
Fig. 2.
Concentration and percentage composition of unchanged total (= free+protein-bound) radioligand and percentage composition of radiometabolites in plasma after injection. a Concentrations of total 18F-PBR06 and 11C-PBR28 are decay-corrected SUV. Error bars representing SD begin at 20 min after injection for clarity. b Percentage of total plasma activity representing unchanged radioligand (○, ●) and radiometabolites (△, ▲) of 18F-PBR06 (open symbols) and 11C-PBR28 (closed symbols). Values are means of eight subjects
Fig. 3.
Concentration of radioactivity in frontal cortex after injection of 18F-PBR06 or 11C-PBR28. Values are mean (symbols) and SD (error bars) of decay-corrected SUV values from eight subjects
Measurement of TSPO binding in the brain
Consistent with the lower brain uptake of 18F-PBR06, VT of 18F-PBR06 was about half that of 11C-PBR28 (2.1±0.4 vs 4.2±1.0, p<0.001; Table 1). However, the fP of 18F-PBR06 was also only half that of 11C-PBR28 (2.2±0.6 vs 4.4±1.1%, p<0.001). Despite the twofold difference in VT, VT/fP, the primary measure of ligand binding, did not differ between 18F-PBR06 and 11C-PBR28 (100.7±24.0 vs 96.8±16.7, p=0.75; Table 1). In other words, after normalizing VT to fP to reflect only free ligand available to bind to receptors, 18F-PBR06 and 11C-PBR28 showed similar binding levels. Not only was the mean value of binding (VT/fP) in brain similar for both radioligands, the relative regional distribution was also quite similar. VT/fP values of 18F-PBR06 in ten brain regions correlated closely with those of 11C-PBR28 (r=0.92, p<0.0005), indicating that the measurements were consistent and did not contain large errors to make the correlation nonsignificant.
Table 1.
VT of 18F-PBR06 and 11C-PBR28 with and without correction by fP
| Region | VT (ml × cm−3) | VT/fP (ml × cm−3) | ||
|---|---|---|---|---|
| 18F-PBR06* | 11C-PBR28 | 18F-PBR06 | 11C-PBR28 | |
| Frontal cortex | 2.10±0.40 | 4.06±0.74 | 100.3±22.6 | 94.9±16.3 |
| Parietal cortex | 2.10±0.39 | 4.13±0.83 | 100.3±20.4 | 95.6±13.3 |
| Occipital cortex | 2.17±0.40 | 4.44±1.13 | 104.0±24.3 | 101.6±14.8 |
| Temporal cortex | 2.24±0.43 | 4.29±0.92 | 107.4±27.7 | 99.5±16.0 |
| Medial temporal cortex | 2.05±0.46 | 4.12±1.00 | 97.7±22.8 | 95.0±15.0 |
| Anterior cingulate | 2.05±0.42 | 4.24±0.87 | 97.7±21.5 | 98.5±15.4 |
| Thalamus | 2.37±0.44 | 4.67±1.33 | 113.6±25.7 | 106.6±20.1 |
| Caudate | 1.72±0.28 | 3.68±0.93 | 83.2±20.3 | 86.9±26.6 |
| Putamen | 1.98±0.36 | 3.92±1.02 | 94.8±21.2 | 90.0±15.6 |
| Cerebellum | 2.26±0.61 | 4.34±1.10 | 108.0±33.4 | 99.2±14.1 |
Data are means ± SD in eight subjects
p<0.001 vs 11 C-PBR28, by repeated measures ANOVA
We also compared 18F-PBR06 and 11C-PBR28 with regard to precision of measuring VT and the time stability of VT. Both 18F-PBR06 (SE 3%, mean of ten regions in eight subjects) and 11C-PBR28 (SE 5%) identified VT well in all regions (Table 2), and we did not find a clear difference between the two ligands in identifiability of VT (p=0.36 by repeated measures ANOVA). Similarly, the two ligands did not differ with respect to time stability of VT (p=0.12 by repeated measures ANOVA), though VT of both ligands increased as scan duration increased (Fig. 4).
Table 2.
Standard errors of VT for 18F-PBR06 and 11C-PBR28
| Region | Standard errors (%) | |
|---|---|---|
| 18F-PBR06 | 11C-PBR28 | |
| Frontal cortex | 2.58±1.53 | 4.27±3.00 |
| Parietal cortex | 2.39±1.48 | 4.74±3.97 |
| Occipital cortex | 2.18±0.48 | 4.16±2.75 |
| Temporal cortex | 2.17±0.94 | 3.97±3.31 |
| Medial temporal cortex | 3.84±3.09 | 4.54±4.26 |
| Anterior cingulate | 2.81±1.10 | 5.33±6.08 |
| Thalamus | 2.45±0.94 | 2.74±1.27 |
| Caudate | 6.18±5.84 | 10.36±14.24 |
| Putamen | 3.30±2.04 | 3.88±1.68 |
| Cerebellum | 2.03±0.62 | 3.44±3.02 |
Data are means ± SD in eight subjects
Standard errors are inversely proportional to identifiability of VT
Fig. 4.
VT values of 18F-PBR06 (○) and 11C-PBR28 (●) as a function of scan duration. Values are means from eight subjects across ten brain regions
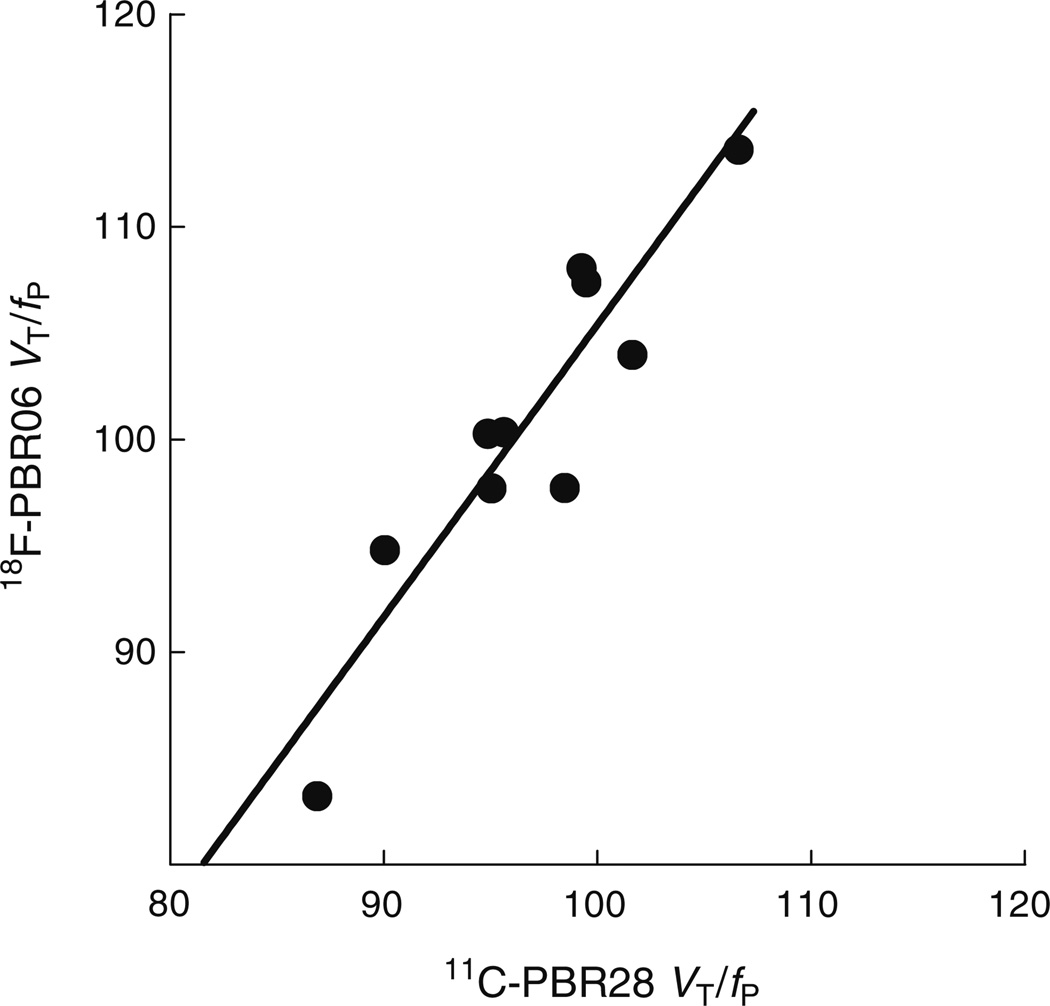
Based on the slope of the regression line (Fig. 5), we also compared the in vivo affinities (1/KD) of the two ligands. The slope, which equals the ratio of the affinity of 18F-PBR06 to that of 11C-PBR28, was 1.37 and did not significantly deviate from unity (95% confidence interval 0.89–1.84). The y-intercept was −32.
Fig. 5.
Correlation between VT/fP of 18F-PBR06 and 11C-PBR28. Each symbol (●) represents the mean of eight subjects in a single brain region. Pearson’s r=0.92 (p<0.0005). The best-fit linear regression line (solid line) had a slope of 1.37
Discussion
The present comparison of 18F-PBR06 and 11C-PBR28 in vivo in human brain shows that these two ligands provide similarly precise measurements of TSPOs (measured as the identifiability in compartmental analysis), similar amounts of total brain uptake after correction of fP, and similar regional distribution in brain. With regard to precision, although in our prior studies 18F-PBR06 had somewhat better identifiability than 11C-PBR28, when we studied these two ligands in the same subjects, we did not find a significant difference in the identifiability of VT across brain regions. Consistent with our prior studies, both ligands identified VT well (SE≤10%). Also consistent with prior findings, both ligands suffered from relatively poor time stability, as VT increased with scan duration, which is compatible with accumulation of radiolabel in the brain. However, the time dependence of VT was not significantly different between the two ligands.
We predicted that 18F-PBR06 would have higher total brain uptake than 11C-PBR28, because PBR06 has almost tenfold higher lipophilicity (which tends to increase nonspecific binding) and 2.5-fold higher in vitro affinity (which increases specific binding). In fact, total brain uptake corrected for plasma protein binding (VT/fP) was very similar for both radioligands. Please note that correction for plasma protein binding is important for a valid comparison. That is, higher lipophilicity and affinity imply higher nonspecific and specific binding, respectively, at equilibrium and in relation to the equilibrium concentration of free radioligand in plasma or brain. This failed prediction demonstrates that in vitro properties do not directly correlate with in vivo performance. Furthermore, we could estimate the in vivo affinity of the radioligands using a modification of the Lassen plot [11]. The slopes of linear regression of VT/fP values showed that two ligands had similar in vivo affinities, contrary to the higher in vitro affinity of 18F-PBR06 relative to that of 11C-PBR28.
Although not studied as part of this paper, both 18F-PBR06 and 11C-PBR28 are probably sensitive to variable affinity states of TSPO. We initially discovered the low-affinity state using 11C-PBR28 in healthy subjects whose images suggested they had essentially no specific binding in brain or in peripheral organs. We found that about 10% of the population (11 subjects of ~120 scanned to date) appeared to be non-binders. In vitro receptor binding to membranes of white blood cells showed that non-binders do have TSPOs, but their affinity for PBR28 was about 50-fold lower than that in the majority (90%) of the population. Owen and colleagues [12] found this low-affinity state directly in postmortem brain of ~20% of subjects. They also found that ~30% of subjects had mixed affinity, consistent with about half of all TSPOs in the high-affinity state and half in the low-affinity state. Since discovering the low-affinity state (“non-binders”) with PET using 11C-PBR28, we then excluded such subjects from scans using 18F-PBR06. We excluded subjects based on whole-body imaging using 11C-PBR28. Although we have not directly identified a subject with low-affinity TSPOs using 18F-PBR06 PET imaging, we think that subjects with low affinity for PBR28 will also show low affinity for PBR06. Preliminary results from Owen and colleagues (personal communication) using postmortem human brain shows that PBR06 is sensitive to high- and low-affinity states but to a lesser degree than PBR28. In patients with neuroinflammation, both 18F-PBR06 and 11C-PBR28 are expected to detect an increase in TSPO expression with similar magnitude and precision based on the current study in healthy humans. However, under the circumstances where the levels of specific binding are markedly higher than those in normal tissue, it is possible that these two ligands reveal small differences.
Conclusions
Because the present study of 18F-PBR06 and 11C-PBR28 did not reveal notable differences in their quantification of TSPOs, the major point of consideration for selecting one of the two ligands derives from its radionuclide. 11C-PBR28 offers the advantages of nearly threefold lower radiation exposure than 18F-PBR06, with effective doses of 6.6 and 18.5 µSv/MBq, respectively [13, 14]. On the other hand, because 18F-PBR06 has a longer half-life (110 min) than 11C-PBR28 (20 min), it can be produced at a more distant cyclotron and then transported to other facilities. Thus, 18F-PBR06 has greater potential than 11C-PBR28 for widespread clinical use in patients with neuropsychiatric disorders.
Acknowledgements
This research was supported by the Intramural Program of NIMH (project #Z01-MH-002795-07 and #Z01-MH-002793-07). We thank Kacey Anderson, M. Desiree Ferraris Araneta, Robert L. Gladding, Kimberly Jenko, William C. Kreisl, Barbara Scepura, Cheryl Wallisch, and the staff of the PET Department for successful completion of the studies, PMOD Technologies (Zurich, Switzerland) for providing its image analysis and modeling software, and Jussi Hirvonen for assisting statistical analyses.
Footnotes
Translocator protein was formerly named peripheral benzodiazepine receptor (PBR) because it was originally found as a binding site for diazepam in peripheral organs. To reflect its distribution in both central nervous system and peripheral organs and also to reflect its functions, PBR has been renamed as translocator protein [1].
Conflicts of interest None.
References
- 1.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–322. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauveau F, Boutin H, Van Camp N, Dollé F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C] PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 4.Briard E, Zoghbi SS, Siméon FG, Imaizumi M, Gourley JP, Shetty HU, et al. Single-step high-yield radiosynthesis and evaluation of a sensitive 18F-labeled ligand for imaging brain peripheral benzodiazepine receptors with PET. J Med Chem. 2009;52:688–699. doi: 10.1021/jm8011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, et al. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- 6.Imaizumi M, Briard E, Zoghbi SS, Gourley JP, Hong J, Musachio JL, et al. Kinetic evaluation in nonhuman primates of two new PET ligands for peripheral benzodiazepine receptors in brain. Synapse. 2007;61:595–605. doi: 10.1002/syn.20394. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi M, Briard E, Zoghbi SS, Gourley JP, Hong J, Fujimura Y, et al. Brain and whole-body imaging in nonhuman primates of [11C]PBR28, a promising PET radioligand for peripheral benzodiazepine receptors. Neuroimage. 2008;39:1289–1298. doi: 10.1016/j.neuroimage.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimura Y, Zoghbi SS, Simèon FG, Taku A, Pike VW, Innis RB, et al. Quantification of translocator protein (18 kDa) in the human brain with PET and a novel radioligand, (18)F-PBR06. J Nucl Med. 2009;50:1047–1053. doi: 10.2967/jnumed.108.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 11.Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, et al. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab. 1995;15:152–165. doi: 10.1038/jcbfm.1995.17. [DOI] [PubMed] [Google Scholar]
- 12.Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown AK, Fujita M, Fujimura Y, Liow J-S, Stabin M, Ryu YH, et al. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28, a PET radioligand to image inflammation. J Nucl Med. 2007;48:2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura Y, Kimura Y, Siméon FG, Dickstein LP, Pike VW, Innis RB, et al. Biodistribution and radiation dosimetry in humans of a new PET ligand, (18)F-PBR06, to image translocator protein (18 kDa) J Nucl Med. 2010;51:145–149. doi: 10.2967/jnumed.109.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]