Abstract
Objective
There is no proven regimen to reduce the severity of stroke in patients with acute cerebral infarction presenting beyond the thrombolytic time window. Ozagrel sodium, a selective thromboxane A2 synthetase inhibitor, has been known to suppress the development of infarction. The antiplatelet effect is improved when aspirin is used together with a thromboxane synthetase inhibitor.
Methods
Patients with non-cardiogenic acute ischemic stroke who were not eligible for thrombolysis were randomly assigned to two groups; one group received ozagrel sodium plus 100 mg of aspirin (group 1, n=43) and the other 100 mg of aspirin alone (group 2, n=43). Demographic data, cardiovascular risk factors, initial stroke severity [National Institute of Health Stroke Scale (NIHSS) and motor strength scale] and stroke subtypes were analyzed in each group. Clinical outcomes were analyzed by NIHSS and motor strength scale at 14 days after the onset of stroke.
Results
There were no significant differences in the mean age, gender proportion, the prevalence of cardiovascular risk factors, stroke subtypes, and baseline neurological severity between the two groups. However, the clinical outcome for group 1 was much better at 14 days after the onset of stroke compared to group 2 (NIHSS score, p=0.007, Motor strength scale score, p<0.001). There was one case of hemorrhagic transformation in group 1, but there was no statistically significant difference in bleeding tendency between two groups.
Conclusion
In this preliminary study, thromboxane A2 synthetase inhibitor plus a low dose of aspirin seems to be safe and has a favorable outcome compared to aspirin alone in patients with acute ischemic stroke who presented beyond the thrombolytic time window.
Keywords: Thromboxane A2 synthetase, Stroke, Aspirin, Tissue Plasminogen activator
INTRODUCTION
Ozagrel sodium is a thromboxane A2 (TXA2) synthetase inhibitor3,8,14,25). TXA2 acts as a vasoconstrictor and platelet aggregator in the ischemic brain18). Ozagrel sodium reduces hypoperfusion and cerebral edema by restricting the generation of TXA2 while facilitating the generation of prostacyclin (PGI2). Due to this mechanism, ozagrel sodium has neuroprotective properties and it has been shown to have efficacy for motor recovery in animal models of cerebral ischemia3,8,14,25).
Although this product has been approved for clinical use in Korea and is widely used, its clinical efficacy in patients with acute ischemic stroke has not yet been studied. Therefore, the aim of this was to evaluate the safety, feasibility, and clinical efficacy of ozagrel sodium in acute ischemic stroke.
MATERIALS AND METHODS
Eligibility criteria
The patients with non-cardiogenic acute ischemic stroke who were not eligible for thrombolysis were randomly assigned to two groups; one group to receive ozagrel sodium plus 100 mg of aspirin (group 1, n=43) and the other to receive 100 mg of aspirin alone (group 2, n=43). The exclusion criteria were; 1) bleeding tendency in laboratory findings including patients with anticoagulation therapy, 2) recent (<4 weeks) history of major operation, trauma, or intracranial hemorrhage, 3) the presence of a severe systemic illness at the time of the study, 4) ischemic strokes secondary to major medical events, invasive procedures, or surgeries, and 5) patients presenting symptoms longer than 2 days after the onset of symptoms or uncommunicative patients with an unknown time for the onset of symptoms.
Initial assessment and management
Evaluation and treatment were done for all subjects according to the protocol of the National Institute of Neurological Disorders and Stroke26). When the patients came to the emergency room, the first action was to evaluate the state of their airways, breathing, and circulation followed by immediate intervention.
History taking and a physical examination including a full neurological examination was done. National Institute of Health Stroke Scale (NIHSS) scores and motor strength scale score were thoroughly measured upon admission. Baseline laboratory tests included serum glucose, electrolytes, renal/hepatic function, complete blood count, coagulation test, electrocardiography, and chest radiography. All patients underwent non-enhanced brain CT immediately after the initial neurologic examination and diffusion-weighted MR within 48 hours after admission. The stroke subtype was classified according to the TOAST classification1) and vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, smoking, history of myocardiac, and cerebral ischemia) were evaluated for each group.
All subjects received a low dose of aspirin (100 mg) within 48 hours from symptom onset. For group 1, ozagrel sodium was additionally administered within 48 hours of admission with a dosage of 80 mg twice a day, and the average administration period was 11.1 days.
Outcome assessment
The NIHSS score and motor strength scale score (on the day of admission and 14 days later) were evaluated and compared between the two groups. Adverse drug reactions and hemorrhagic complications such as gastrointestinal bleeding, hematuria, or hemorrhagic transformation were also evaluated.
Statistical analysis
SPSS 15.0 (SPSS Inc., Chicago, IL, USA) was utilized for the statistical analysis. The mean standard deviations or median values were used as the basic risk factors while Fisher's exact tests and chi-square tests were used to secure the significance of a single variable. Mann-Whitney U tests were used for category variables while student t-tests were used for the continuous variables within a p-value of 0.05. Gender, age, hypertension, diabetes mellitus, dyslipidemia, smoking, and a history of myocardiac and cerebral ischemia are among the independent variables.
RESULTS
The average age of the subjects was 61.67 in group 1 and 60.44 in group 2. Group 1 consisted of 28 males and 15 females and group 2 had 29 males and 14 females. The prevalence of hypertension was 65.1% (28/43) in group 1 and 77.4% in group 2 (32/43). The prevalence of diabetes mellitus in group 1 and group 2 was 39.5% (17/43) and 20.9% (9/43), respectively. Dyslipidemia upon admission was present in 27.9% (12/43) of group 1 and 32.6% (14/43) of group 2. The percentage of smokers was 44.2% (19/43) and 62.8% (27/43) in group 1 and group 2, respectively. History of myocardiac ischemia and cerebral ischemia was 7% and, 14% (3/43, 6/43) in group 1, and 9.3% and, 11.6% (4/43, 5/43) in group 2, respectively. None of these vascular risk factors were statistically significantly different between the two groups (Table 1).
Table 1.
Characteristics of the patients
*Standard deviation
According to the TOAST Classification, 20 subjects had large artery atherosclerosis and 23 had small vessel occlusions in group 1, while 22 subjects had large artery atherosclerosis and 21 subjects had small vessel occlusions in group 2. As mentioned in the eligibility criteria, cardioembolism was excluded during initial recruitment (Table 2).
Table 2.
Stroke subtype classification
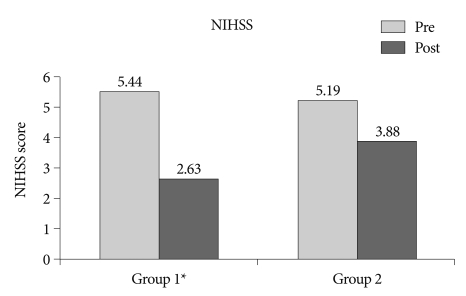
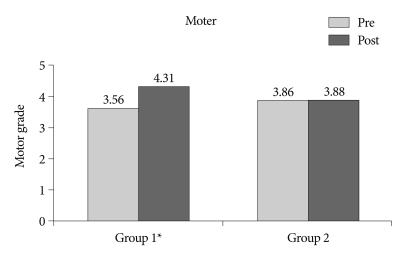
The mean NIHSS score upon admission was 5.44 (range : 0-20) in group 1 and 5.19 (range : 1-13) in group 2. The mean motor power score upon admission in group 1 and group 2 was 3.56 and 3.81, respectively. Although there was no significant difference in the initial NIHSS score and motor power score, the mean NIHSS score measured two weeks later was 2.63 in group 1 and 3.88 in group 2, with more improvement in group 1 (p=0.007) (Fig. 1). In addition, the mean motor score measured two weeks later was 4.31 in group 1 and 3.88 in group 2, with respective changes of 0.74 and 0.07, indicating more improvement in motor power for group 1 (p<0.001) (Fig. 2).
Fig. 1.
Comparison of mean NIHSS scores between treatment with thromboxane A2 synthetase inhibitor with a low dose of aspirin (group 1) and aspirin alone (group 2). pre : initial NIHSS score, post : NIHSS score at 14 days after onset of symptoms, p<0.007. NIHSS : National Institute of Health Stroke Scale.
Fig. 2.
Comparison of mean motor power score between treatment with thromboxane A2 synthetase inhibitor with a low dose of aspirin (group 1) and aspirin alone (group 2). pre : initial NIHSS score, post : NIHSS score at 14 days after onset of symptoms, p<0.001. NIHSS : National Institute of Health Stroke Scale.
One patient (1/43, 2.3%) in group 1 showed hemorrhagic transformation on the fourth day of admission, which resulted in a transient worsening of their mental status to a deep drowsy state. Four patients (4/43, 9.3%) in group 1 and two patients (2/43, 4.7%) in group 2 showed micro-hematuria, although no one showed gross hematuria in any of groups. All those with micro-hematuria had indwelling urinary catheters. There was no gastrointestinal bleeding in any of groups (Table 3).
Table 3.
Hemorrhagic complications
There was no adverse drug reaction for group 1 subjects except that two of subjects (2/43, 4.6%) showed slight increases in their glutamic oxaloacetate transaminase and glutamic pyruvate transaminase values. No one suffered from nausea, vomiting, diarrhea, or constipation. The complete blood count, blood urea nitrogen, and creatine values of all the patients were normal. No one complained of drug fever, hair loss, or any pain in the areas through which the drug was administered.
DISCUSSION
Ischemic stroke has been one of the leading causes of morbidity and mortality worldwide16). To reduce tissue damage from acute cerebral ischemia and to improve the neurological status of patients, cerebral reperfusion therapy including intravenous tissue plasminogen activator and intra-arterial thrombolysis within an eligible time window has been widely accepted2,4,5,7,9-11,17,27). However, the actual rate of giving intravenous or intra-arterial thrombolysis to patients who visit an emergency room is sadly low, since most patients fail to enter the hospital within the time window. Much research has been done to improve the long-term functional outcome of patients with ischemic stroke who have passed the time window20,28), however, the clinical implications have not been realized yet.
Brain damage by cerebral infarction occurs not only during an ischemic period but also after reperfusion takes place. Increased TXA2 in brain tissue after cerebral reperfusion reduces the cerebral blood flow by promoting vasoconstriction and platelet aggregation25). On the other hand, PGI2 expands blood vessels, prevents platelet aggregation, and plays an important role in controlling cerebral vasospasms12,22). Ozagrel sodium, a selective TXA2 synthetase inhibitor, reduces the TXA2 levels and increases the PGI2 levels14,25) whereas aspirin reduces both PGI2 and TXA2 as a cyclooxygenase inhibitor13). Therefore, Ozagrel sodium theoretically prevents a decrease in the cerebral blood flow during an ischemic period14,23) and improves the delayed hypoperfusion during the reperfusion period24,25).
Aspirin curbes the generation of cyclooxygenase, which decreases the formation of TXA2 and PGI213). Such sensitivity by platelet aggregation-related activity increases when a low dose of aspirin is used3). In contrast, the thromboxane synthetase inhibitor depresses the generation of TXA2 by preventing endoperoxide utilization. The antiplatelet effect depends on how effectively the two types of prostaglandin metabolism are reduced. The combined therapy of a thromboxane synthetase inhibitor and aspirin produces the best effect by restraining the platelet aggregation response and TXA2 synthesis and encouraging PGI2 formation15). Due to the differentiated antagonism of the two medications on prostaglandin, the combined therapy of a low dose of aspirin and a thromboxane synthetase inhibitor can be an effective antithrombotic treatment. Moreover, the synergistic effect by the two medications is more powerful in producing an antiplatelet effect compared to when each of them is used separately15).
A brain afflicted by cerebral infarction develops cytotoxic edema. The condition of brain edema itself causes brain damage and doubles the brain damage after cerebral infarction by reducing the cerebral blood flow during the reperfusion period19). Ozagrel sodium reduces brain swelling by acting during both the ischemic and reperfusion periods. It also prevents an expansion of cerebral infarction by curbing brain cell death in the penumbra6,14,18,21). For these reasons, ozagrel sodium can provide an additional effect to aspirin21).
Ozagral sodium, a selective TXA2 synthetase inhibitor, was approved by the Korean Food and Drug Administration for use in cerebral ischemia with motor deficit and it has been a familiar drug with the members of the Korean neurological and neurosurgical society. However, there have been few clinical studies regarding its efficacy in ischemic stroke. This study reaffirmed the effects of ozagrel sodium, which have been proven through a previously described animal test. Through vasodilatation, restraint of platelet aggregation, and a decrease in brain swelling, ozagrel sodium prevents hypoperfusion. In this study, patients with acute cerebral infarction had a better NIHSS score and motor power score when treated with ozagrel sodium and aspirin together compared to those who were treated only with aspirin. There was no major adverse reaction to the drugs or hemorrhagic tendency except for one case of hemorrhagic transformation. However, that case had a large area of middle cerebral arterial territorial infarction with a high risk of hemorrhagic transformation in its natural course. The authors could not find any clue of whether this event resulted from the use of the TXA2 synthetase inhibitor or not.
Our study has several limitations such as a small number of patients and a short term follow up period. Thus, a further larger study may be necessary to prove the efficacy of the combination therapy of ozagrel sodium and aspirin at low doses.
CONCLUSION
The combination therapy of ozagrel sodium and aspirin at low doses is safe, feasible, and effective in the treatment of acute cerebral infarction beyond the thrombolytic time window. It could especially help to improve motor functional outcome in the short term period. Further study on a larger scale might be needed to determine the long term outcomes.
References
- 1.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke : American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:630S–669S. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- 3.Bertelé V, Falanga A, Tomasiak M, Dejana E, Cerletti C, de Gaetano G. Platelet thromboxane synthetase inhibitors with low doses of aspirin : possible resolution of the "aspirin dilemma". Science. 1983;220:517–519. doi: 10.1126/science.6682245. [DOI] [PubMed] [Google Scholar]
- 4.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 5.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 6.Cole DJ, Patel PM, Schell RM, Drummond JC, Osborne TN. Brain eicosanoid levels during temporary focal cerebral ischemia in rats : a microdialysis study. J Neurosurg Anesthesiol. 1993;5:41–47. doi: 10.1097/00008506-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cross DT, 3rd, Moran CJ, Akins PT, Angtuaco EE, Diringer MN. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol. 1997;18:1221–1228. [PMC free article] [PubMed] [Google Scholar]
- 8.Defreyn G, Deckmyn H, Vermylen J. A thromboxane synthetase inhibitor reorients endoperoxide metabolism in whole blood towards prostacyclin and prostaglandin E2. Thromb Res. 1982;26:389–400. doi: 10.1016/0049-3848(82)90311-5. [DOI] [PubMed] [Google Scholar]
- 9.Del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M PROACT Investigators; Prolyse in Acute Cerebral Thromboembolism. a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Prolyse in Acute Cerebral Thromboembolism. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study : a randomized controlled trial. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hacke W, Zeumer H, Ferbert A, Brückmann H, del Zoppo GJ. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19:1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 12.Hallenbeck JM, Leitch DR, Dutka AJ, Greenbaum LT, Jr, McKee AE. Prostaglandin I2, indomethacin and heparin promote postischemic neuronal recovery in dogs. Ann Neurol. 1982;12:145–156. doi: 10.1002/ana.410120204. [DOI] [PubMed] [Google Scholar]
- 13.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa K, Tazawa S, Hamano S, Kojima M, Hiraku S. Effect of ozagrel on locomotor and motor coordination after transient cerebral ischemia in experimental animal models. Pharmacology. 1999;59:257–265. doi: 10.1159/000028328. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan S, Sauvage LR, Marcoe KF, Zammit M, Wu HD, Mathisen SR, et al. A new combination therapy for selective and prolonged antiplatelet effect : results in the dog. Stroke. 1986;17:450–454. doi: 10.1161/01.str.17.3.450. [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, Min BK, Hwang SN, Suk JS, Choi DY. A clinical analysis of cerebral infarction. J Korean Neurosurg Soc. 1989;18:885–892. [Google Scholar]
- 17.Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome : the NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. Role of platelet-activating factor and thromboxane A2 in radical production during ischemia and reperfusion of the rat brain. Brain Res. 1996;709:296–302. doi: 10.1016/0006-8993(95)01324-5. [DOI] [PubMed] [Google Scholar]
- 19.Mellergård P, Bengtsson F, Smith ML, Riesenfeld V, Siesjö BK. Time course of early brain edema following reversible forebrain ischemia in rats. Stroke. 1989;20:1565–1570. doi: 10.1161/01.str.20.11.1565. [DOI] [PubMed] [Google Scholar]
- 20.Mendioroz M, Fernández-Cadenas I, Rosell A, Delgado P, Domingues-Montanari S, Ribó M, et al. Osteopontin predicts long-term functional outcome among ischemic stroke patients. J Neurol. 2011;258:486–493. doi: 10.1007/s00415-010-5785-z. [DOI] [PubMed] [Google Scholar]
- 21.Nishigaya K, Yoshida Y, Sasuga M, Nukui H, Ooneda G. Effect of recirculation on exacerbation of ischemic vascular lesions in rat brain. Stroke. 1991;22:635–642. doi: 10.1161/01.str.22.5.635. [DOI] [PubMed] [Google Scholar]
- 22.Nosko M, Schulz R, Weir B, Cook DA, Grace M. Effects of vasospasm on levels of prostacyclin and thromboxane A2 in cerebral arteries of the monkey. Neurosurgery. 1988;22:45–50. doi: 10.1227/00006123-198801010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Ohyama H, Hosomi N, Takahashi T, Mizushige K, Kohno M. Thrombin inhibition attenuates neurodegeneration and cerebral edema formation following transient forebrain ischemia. Brain Res. 2001;902:264–271. doi: 10.1016/s0006-8993(01)02354-x. [DOI] [PubMed] [Google Scholar]
- 24.Roy MW, Dempsey RJ, Cowen DE, Donaldson DL, Young AB. Thromboxane synthetase inhibition with imidazole increases blood flow in ischemic penumbra. Neurosurgery. 1988;22:317–323. doi: 10.1227/00006123-198802000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Sadoshima S, Ooboshi H, Okada Y, Yao H, Ishitsuka T, Fujishima M. Effect of thromboxane synthetase inhibitor on cerebral circulation and metabolism during experimental cerebral ischemia in spontaneously hypertensive rats. Eur J Pharmacol. 1989;169:75–83. doi: 10.1016/0014-2999(89)90819-4. [DOI] [PubMed] [Google Scholar]
- 26.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 27.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ The rt-PA Acute Stroke Study Group. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita T, Deguchi K, Nagotani S, Abe K. Vascular protection and restorative therapy in ischemic stroke. Cell Transplant. 2011;20:95–97. doi: 10.3727/096368910X532800. [DOI] [PubMed] [Google Scholar]