Abstract
Objective
The aim of the study is to determine the efficacy of indocyanine green (ICG) videoangiography for confirmation of vascular anastomosis patency in both extracranial-intracranial and intracranial-intracranial bypasses.
Methods
Intraoperative ICG videoangiography was used as a surgical adjunct for 56 bypasses in 47 patients to assay the patency of intracranial vascular anastomosis. These patients underwent a bypass for cerebral ischemia in 31 instances and as an adjunct to intracranial aneurysm surgery in 25. After completion of the bypass, ICG was administered to assess the patency of the graft. The findings on ICG videoangiography were then compared to intraoperative and/or postoperative imaging.
Results
ICG provided an excellent visualization of all cerebral arteries and grafts at the time of surgery. Four grafts were determined to be suboptimal and were revised at the time of surgery. Findings on ICG videoangiography correlated with intraoperative and/or postoperative imaging.
Conclusion
ICG videoangiography is rapid, effective, and reliable in determining the intraoperative patency of bypass grafts. It provides intraoperative information allowing revision to reduce the incidence of technical errors that may lead to early graft thrombosis.
Keywords: Intracranial bypass, ICG videoangiography, Intraoperative imaging
INTRODUCTION
Since the original description of the superficial temporal artery (STA) to middle cerebral artery (MCA) bypass by Donagy and Yasargil in 1967 a number of extracranial to intracranial (EC-IC) and intracranial to intracranial (IC-IC) bypasses have been used to augment cerebral blood flow in a variety of conditions25). Adherence to meticulous microsurgical techniques is essential for high short-term and long-term patency rates28). Methods to verify intraoperative patency may identify technical deficiencies and allow the surgeon to correct those problems and improve patency rates. The established methods for intraoperative assessment include observation under an operating microscope, microvascular Doppler and intraoperative digital subtraction angiography (DSA)1,27). More recently, indocyanine green (ICG) video angiography has been applied to neurovascular procedures as a simple and safe technique to evaluate the intracranial circulation before and after neurosurgical management14,16). Combined with the use of near-infrared video technology in operating microscopes, surgeons can evaluate intraoperative blood flow with a simple intravenous injection.
We present our experience with the use of ICG fluorescence video angiography in the management of patients undergoing bypass procedures. ICG video angiography was used to verify patency of the bypasses after performance of the procedure and to assist in correcting technical errors. The results of ICG videoangiography were compared to intraoperative DSA, postoperative DSA, or postoperative computed tomography angiography (CTA).
MATERIALS AND METHODS
We began using ICG video angiography in Emory University Hospital in late 2006. Between January 30, 2007 and December 30, 2009 59 consecutive bypass procedures were performed by the senior author at Emory University Hospital. Fifty-six of these 59 bypass procedures were performed in 47 patients and were evaluated with intraoperative ICG video angiography as part of their procedure. Thirty-one of these procedures were performed for ischemia and 25 as an adjunct to aneurysm surgery. All patients received the standard 25 mg intravenous dose dissolved in 10 mL of aqueous solvent and given as a bolus. The dye was visualized using an OPMI Pentero Microscope (Carl Zeiss, Inc., Oberkochen, Germany). The 3 patients excluded had surgery during a time of unavailability of ICG due to manufacturing constraints. In four cases ICG demonstrated either poor filling or occlusion of the bypass. Two of the four were able to be successfully revised, the other two were occluded. All patients underwent intra-or postoperative DSA or CTA to verify the results.
The bypass procedures included in this series that underwent intraoperative evaluation with ICG included 43 STA-MCA bypasses, 1 occipital artery to posterior inferior cerebellar artery (PICA) bypass, 9 radial artery bypass grafts, 1 saphenous vein graft and 5 other IC-IC bypasses. The five IC-IC procedures included 2 PICA-PICA side to side bypasses, an anterior temporal artery to MCA bypass and 2 re-implementations of the PICA into the vertebral artery.
The findings on ICG videoangiography were compared to either intraoperative and/or postoperative DSA or CTA. Table 1, 2 outline the clinical data, bypass type, and imaging findings for patients undergoing bypasses for ischemia and as an adjunct for aneurysm surgery, respectively.
Table 1.
Patient demographics and results for bypasses completed as an adjunct to aneurysm surgery
POA : postoperative angiogram, ICG : indocyanine green, STA : superficial temporal artery, MCA : middle cerebral artery, PICA : posterior inferior cerebellar artery, CTA : computed tomography angiography
Table 2.
Patient demographics and results for bypass procedures done for ischemia
STA : superficial temporal artery, MCA : middle cerebral artery, DSA : digital subtraction angiography, CTA : computed tomography angiography, ICG : indocyanine green, ICA : inferior cerebellar artery
RESULTS
Videoangiography was useful in demonstrating patency (Fig. 1) or potential technical deficiencies for which correction was attempted (Fig. 2). In our series, 4 of 56 bypasses were demonstrated to be occluded or have inadequate flow in the graft at the time of surgery and were revised at that time. Despite revision, two STA-MCA bypass remained occluded. In each case, a double bypass had been performed and one bypass was patent. In the other two cases, the bypass was revised successfully. ICG videoangiography added less than 10 minutes to operative time. There were no complications related to the use of ICG. At last followup, all grafts that were demonstrated to be patent intraoperatively remained patent.
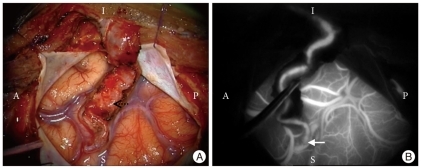
Fig. 1.
Patent STA-MCA bypass. A : Intraoperative photo of STA-MCA bypass. Solid arrow denotes anastomosis and dotted arrow denotes STA. A : anterior, P : posterior, I : inferior, S : superior. B : ICG videoangiography demonstrates patency. Arrow denotes anastomosis. STA : superficial temporal artery, MCA : middle cerebral artery, ICG : indocyanine green.
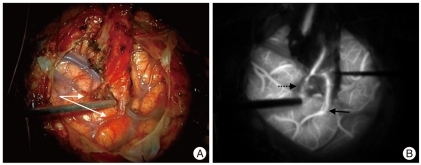
Fig. 2.
Occluded STA-MCA bypass. A : Intraoperative photo of "double barrel" STA-MCA bypass. B : ICG videoangiography documents patency of the bypass on the right side (solid arrow) of image and occlusion of the bypass on the left (dotted arrow). STA : superficial temporal artery, MCA : middle cerebral artery, ICG : indocyanine green.
Illustrative case reports
Case one
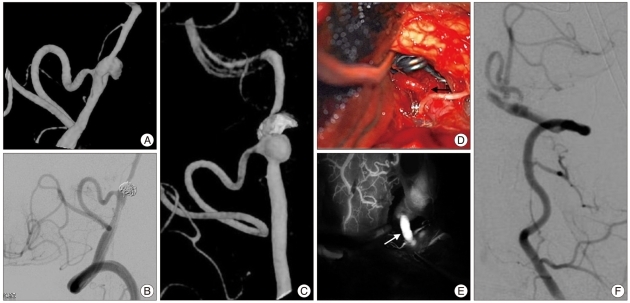
A 59-year-old male presented with a subarachnoid hemorrhage and was discovered to have a dissecting pseudo-aneurysm of the left vertebral artery (Fig. 3A). This was treated by partial endovascular coiling with sparing of the dominant PICA (Fig. 3B). Follow-up angiography three months following his endovascular procedure demonstrated recurrence of the pseudo-aneurysm (Fig. 3C). The patient's aneurysm was exposed through a left transcondylar approach with trapping of the left vertebral artery dissecting aneurysm and division of the proximal PICA with reimplantation into the vertebral artery proximal to the aneurysm (Fig. 3D). ICG videoangiography demonstrated patency of the re-implanted PICA (Fig. 3E) and verified by both intraoperative and postoperative DSA (Fig. 3F). The patient developed some transient worsening of his preoperative left-sided weakness. An MRI demonstrated no evidence of ischemia. At two month follow-up he had made a complete recovery.
Fig. 3.
Case One. A : Oblique 3-D DSA illustrates a left vertebral dissecting aneurysm at the origin of PICA. B : Oblique left vertebral angiogram after endovascular coiling of the aneurysm. C : Follow-up 3-D DSA demonstrates significant recurrence 3 months after endovascular treatment. D : Intraoperative photograph of PICA re-implanted into the left vertebral artery with clip distal to the bypass trapping the vertebral artery and aneurysm. Solid arrow denotes PICA-left vertebral anastomosis. E : ICG videoangiography demonstrates patency of re-implanted PICA (solid arrow). F : Intraoperative left vertebral DSA confirms patency of re-implanted PICA. DSA : digital subtraction angiography, PICA : posterior inferior cerebellar artery, ICG : indocyanine green.
Case two
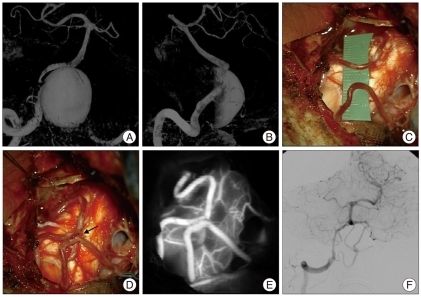
A 64-year-old woman was known to have a giant intracavernous aneurysm on the right internal carotid artery with a smaller contralateral intracavernous aneurysm (Fig. 4A). She presented with progressively worsening retro-orbital pain and diplopia. The patient underwent a right frontotemporal craniotomy and exposure of the cervical carotid with harvesting of a radial artery graft for a common carotid to MCA bypass in preparation for endovascular occlusion of the internal carotid artery (Fig. 4B, C, D). Intraoperative ICG videoangiography and DSA demonstrated patency of the radial artery bypass graft. Two days later she underwent carotid artery and aneurysm occlusion by endovascular coiling. With progressive thrombosis of the aneurysm, she developed transient worsening of her cranial neuropathy including the 3rd and 6th cranial nerves. At determitwo month follow-up the ophthalmoparesis was improving and her headaches had resolved. CTA demonstrated patency of the bypass and complete thrombosis of the aneurysm (Fig. 4E).
Fig. 4.
Case Two. A : Lateral right common carotid angiogram shows giant intracavernous aneurysm. B : Intraoperative photograph of radial artery bypass from common carotid to middle cerebral artery. Radial artery denoted by solid arrow and anastomotic site shown by dotted arrow. C : ICG videoangiography of bypass demonstrates patency. D : Intraoperative DSA confirms patency of bypass. Solid arrow points to radial graft and dotted arrow points to filling MCA branches. E : Postoperative CTA AP verifies patency of the bypass and thrombosis of the aneurysm. ICG : indocyanine green, DSA : digital subtraction angiography, MCA : middle cerebral artery, CTA : computed tomography angiography.
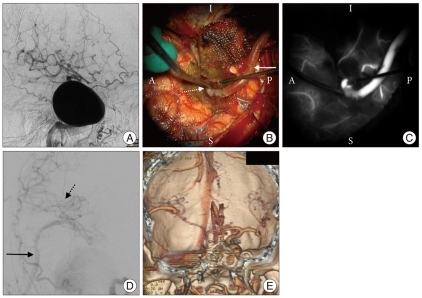
Case three
A 77-year-old man presented with progressive dizziness and ataxia for one year. He had experienced a number of falls more recently. CT and MRI demonstrated a giant right vertebral artery aneurysm causing significant brainstem compression. DSA illustrated the angioarchitecture of the aneurysm which arose just beyond the origin of PICA (Fig. 5A). Endovascular occlusion of the right vertebral artery was considered but would likely have occluded the PICA.
Fig. 5.
Case Three. A : AP and B : Lateral vertebral 3-D angiogram demonstrates a giant left vertebral aneurysm. C : Intraoperative photograph of bilateral PICAs prepared for side-to side. D : Intraoperative photograph of PICA side-to side anastomosis (solid arrow) after completion. E : ICG videoangiography of bypass demonstrates patency. F : AP DSA showing patency of the bypass and trapping of the aneurysm. PICA : posterior inferior cerebellar artery, ICG : indocyanine green, DSA : digital subtraction angiography.
The patient underwent a right transcondylar approach to the aneurysm. A PICA-PICA side-to-side bypass was performed and documented by intraoperative ICG videoangiography and DSA to be patent before trapping the giant aneurysm (Fig. 5B-E). Postoperative angiography confirmed patency of the bypass (Fig. 5F).
DISCUSSION
The role of intracranial bypasses grew after the introduction of microsurgical techniques in the 1960s and 1970s25). In 1967, Yasargil performed the first STA-MCA bypass in a patient with complete occlusion of the MCA28,29). Subsequently, the indications for EC-IC bypasses expanded to include inaccessible extracranial carotid stenosis or occlusion, occlusive intracranial disease, complex aneurysms not amenable to simple clip ligation, skull base tumors, cerebral vasospasm, carotid dissection, carotid-cavernous fistulas, and moya-moya disease7,20,25). The EC-IC bypass trial in 1985 failed to show surgical benefit over medical therapy leading to a decrease in the performance of the procedure23). Though used in a limited fashion today, this procedure remains vital in many cases.
At the present time, intracranial bypass grafts are performed primarily by cerebrovascular neurosurgeons. Through evaluation of the blood flow demand, the surgeon may utilize a low flow bypass such as the superficial temporal artery or occipital artery with flow rates between 15 and 30 mL/min8). In cases requiring higher demands, the surgeon may employ a radial artery graft with flow rates of 40-70 mL/min or a saphenous vein graft with rates between 70 and 140 mL/min8,21). Finally, a side-to-side or end-to-side anastomoses within the vessels of the circle of Willis can be completed without the need of graft harvest13,21). The majority of bypasses are performed for complex aneurysms or tumors, moyamoya disease, and recently, occlusive disease in a specific subpopulation of atherosclerotic patients12,13,17,19). The benefit of the bypass is dependent on low rates of surgical complications and high patency rates of the graft5).
In efforts to minimize early graft occlusion, multiple adjuncts can be employed to test patency. In the past, the majority of studies were performed postoperatively. With improvements in technology, intraoperative assessment can be completed in a reliable manner allowing higher early patency rates than the current range of 90 and 96%9,18,23).
Currently, the primary means of graft assessment after completion of the procedure include 32 and 64 slice multidetector CTA, quantitative magnetic resonance (MR) angiography, and conventional cerebral angiography6,24,27). Both CTA and MR angiography provide noninvasive means of assessing bypass patency with results comparable to conventional angiography. Unfortunately, both methods are compromised in patients where there is clip artifact in complex aneurysm cases21). In all postoperative imaging, the information is acquired at a point where it is difficult to correct the technical error.
Numerous techniques are available to the surgeon for intraoperative confirmation of graft patency at the time of surgery. The most simple means of which is direct visualization. Though the surgeon can easily determine the pulsatility of the donor vessel and recipient under the microscope, this method is known to be unreliable21). With varying results, surgeons have also employed duplex sonography, thermal imaging, and flow determination by ultrasonic transit times1,3,10).
Intraoperative DSA was first described in the 1960s for cranial procedures and has been used with excellent results for neurovascular disorders since that time2,22,27). Used primarily as an adjunct in aneurysm surgery, DSA can reliably evaluate the patency of bypass grafts. The use of intraoperative DSA requires a dedicated neuroangiography team and operative time for both sheath placement and the angiogram itself. Complication rates remain small (0.4%), but not inconsequential, as this is an invasive test using ionizing radiation and contrast agents22).
Originally approved for cardiac flow studies, ICG is a fluorescent tracer with applications in ophthalmology, cardiology, and gastroenterology11). The dye is highly protein bound with intense fluorescence and a short half life of 150 to 180 seconds4). Cherrick et al.4) found the dye produced no adverse effects when introduced subcutaneously or intravenously. The dye does contain iodine and care should be taken in patients with iodine allergies. To view the fluorescence, a specialized near-infrared light source and camera must be used. Its first use in neurosurgical procedures was by Raabe et al.14) in 2003.
At that time, a separate camera was needed to view the dye. Presently, intraoperative microscopes can be modified to view ICG15). Surgeons have reported good results using ICG for intraoperative assessment in intracranial aneurysms, arteriovenous malformations, and EC-IC bypasses16,26). The low cost, high spatial resolution, and ease of use makes ICG an attractive tool in the treatment of neurovascular disorders.
We report here the results from our use of ICG as an adjunct for assessment of the patency of IC-IC and EC-IC bypasses. We found the procedure to be reliable and comparable to intraoperative DSA. The ICG can be dosed in two to five minutes as compared to about 30 minutes for standard intraoperative DSA. The dye can also be easily redosed after the fluorescence has washed out of the vessels. The dosing of ICG resulted in no adverse effects compared to the 0.4% risk of stroke associated with intraoperative angiography. In 4 cases, ICG videoangiography determined that the graft was compromised or occluded or there was a technical error that could be corrected. In 2 of the cases, intraoperative revision of the graft resulted in graft patency. These results were comparable to Woitzik et al.26) in their series of 45 patients who received ICG for EC-IC bypass grafts.
Additionally, ICG provides benefits over duplex sonography. The primary disadvantages of intraoperative doppler is the lack of a live dynamic picture of blood flow and is limited in small caliber vessels1). ICG provides excellent visualization of small caliber vessels, though it also remains inferior to DSA in dynamic blood flow.
It is important to understand that ICG videoangiography provides an image limited to the size of the surgical field. If the view to the vessel is obstructed by bone or hematoma, the fluorescence cannot be visualized. Further studies are needed to determine to what extent ICG videoangiography can replace or supersede other imaging methods.
CONCLUSION
ICG videoangiography provides a surgeon with an effective means to determine graft patency at the time of surgery allowing the surgeon to correct any technical shortcomings. Use of this intraoperative technique provides a simple and reproducible means to assess intraoperative patency for both EC-IC and IC-IC bypasses.
References
- 1.Badie B, Lee FT, Jr, Pozniak MA, Strother CM. Intraoperative sonographic assessment of graft patency during extracranial-intracranial bypass. AJNR Am J Neuroradiol. 2000;21:1457–1459. [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow DL, Boyer KL, Joseph GJ. Intraoperative angiography in the management of neurovascular disorders. Neurosurgery. 1992;30:153–159. doi: 10.1227/00006123-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Charbel FT, Misra M, Clarke ME, Ausman JI. Computer simulation of cerebral blood flow in moyamoya and the results of surgical therapies. Clin Neurol Neurosurg. 1997;99(Suppl 2):S68–S73. doi: 10.1016/s0303-8467(97)00073-5. [DOI] [PubMed] [Google Scholar]
- 4.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green : observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Sciacca RR, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease : a systematic review. Clin Neurol Neurosurg. 2009;111:319–326. doi: 10.1016/j.clineuro.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Horn P, Vajkoczy P, Schmiedek P, Neff W. Evaluation of extracranial-intracranial arterial bypass function with magnetic resonance angiography. Neuroradiology. 2004;46:723–729. doi: 10.1007/s00234-004-1249-4. [DOI] [PubMed] [Google Scholar]
- 7.Little JR, Yamamoto YL, Feindel W, Meyer E, Hodge CP. Sperficial temporal artery to middle cerebral artery anastomosis. Intraoperative evaluation by fluorescein angiography and xenon-133 clearance. J Neurosurg. 1979;50:560–569. doi: 10.3171/jns.1979.50.5.0560. [DOI] [PubMed] [Google Scholar]
- 8.Liu JK, Kan P, Karwande SV, Couldwell WT. Conduits for cerebrovascular bypass and lessons learned from the cardiovascular experience. Neurosurg Focus. 2003;14:e3. [PubMed] [Google Scholar]
- 9.Mendelowitsch A, Taussky P, Rem JA, Gratzl O. Clinical outcome of standard extracranial-intracranial bypass surgery in patients with symptomatic atherosclerotic occlusion of the internal carotid artery. Acta Neurochir (Wien) 2004;146:95–101. doi: 10.1007/s00701-003-0154-7. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa A, Hirano T, Uenohara H, Sato M, Kusaka Y, Shirane R, et al. Intraoperative thermal artery imaging of an EC-IC bypass in beagles with infrared camera with detectable wave-length band of 7-14 microm: possibilities as novel blood flow monitoring system. Minim Invasive Neurosurg. 2003;46:231–234. doi: 10.1055/s-2003-42357. [DOI] [PubMed] [Google Scholar]
- 11.Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996;80:263–266. doi: 10.1136/bjo.80.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinones-Hinojosa A, Du R, Lawton MT. Revascularization with saphenous vein bypasses for complex intracranial aneurysms. Skull Base. 2005;15:119–132. doi: 10.1055/s-2005-870598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinones-Hinojosa A, Lawton MT. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2005;57:140–145. doi: 10.1227/01.neu.0000163599.78896.f4. discussion 140-145. [DOI] [PubMed] [Google Scholar]
- 14.Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography : a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132–139. doi: 10.1097/00006123-200301000-00017. discussion 139. [DOI] [PubMed] [Google Scholar]
- 15.Raabe A, Beck J, Seifert V. Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near-infrared video technology. Zentralbl Neurochir. 2005;66:1–6. doi: 10.1055/s-2004-836223. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 16.Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005;103:982–989. doi: 10.3171/jns.2005.103.6.0982. [DOI] [PubMed] [Google Scholar]
- 17.Regli L, Piepgras DG, Hansen KK. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83:806–811. doi: 10.3171/jns.1995.83.5.0806. [DOI] [PubMed] [Google Scholar]
- 18.Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einhupl K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg. 1994;81:236–244. doi: 10.3171/jns.1994.81.2.0236. [DOI] [PubMed] [Google Scholar]
- 19.Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms : techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646–658. doi: 10.1097/00006123-200109000-00023. discussion 658-659. [DOI] [PubMed] [Google Scholar]
- 20.Sundt TM, Jr, Siekert RG, Piepgras DG, Sharbrough FW, Houser OW. Bypass surgery for vascular disease of the carotid system. Mayo Clin Proc. 1976;51:677–692. [PubMed] [Google Scholar]
- 21.Surdell DL, Hage ZA, Eddleman CS, Gupta DK, Bendok BR, Batjer HH. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24:E21. doi: 10.3171/FOC.2008.25.2.E21. [DOI] [PubMed] [Google Scholar]
- 22.Tang G, Cawley CM, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery : a prospective evaluation of efficacy. J Neurosurg. 2002;96:993–999. doi: 10.3171/jns.2002.96.6.0993. [DOI] [PubMed] [Google Scholar]
- 23.The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: Results of an international randomized trial. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 24.Thines L, Agid R, Dehdashti AR, da Costa L, Wallace MC, Terbrugge KG, et al. Assessment of extracranial-intracranial bypass patency with 64-slice multidetector computerized tomography angiography. Neuroradiology. 2009;51:505–515. doi: 10.1007/s00234-009-0522-y. [DOI] [PubMed] [Google Scholar]
- 25.Vilela MD, Newell DW. Superficial temporal artery to middle cerebral artery bypass : past, present, and future. Neurosurg Focus. 2008;24:E2. doi: 10.3171/FOC/2008/24/2/E2. [DOI] [PubMed] [Google Scholar]
- 26.Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102:692–698. doi: 10.3171/jns.2005.102.4.0692. [DOI] [PubMed] [Google Scholar]
- 27.Yanaka K, Fujita K, Noguchi S, Matsumaru Y, Asakawa H, Anno I, et al. Intraoperative angiographic assessment of graft patency during extracranial-intracranial bypass procedures. Neurol Med Chir (Tokyo) 2003;43:509–512. doi: 10.2176/nmc.43.509. discussion 513. [DOI] [PubMed] [Google Scholar]
- 28.Yasargil MG. A legacy of microneurosurgery : memoirs, lessons, and axioms. Neurosurgery. 1999;45:1025–1092. doi: 10.1097/00006123-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Yasargil MG, Krayenbuhl HA, Jacobson JH., 2nd Microneurosurgical arterial reconstruction. Surgery. 1970;67:221–233. [PubMed] [Google Scholar]