Abstract
Objective
To evaluate the efficacy of scalene injection in patients with thoracic outlet syndrome.
Methods
We selected 142 patients diagnosed with thoracic outlet syndrome between January 2005 and October 2009. We performed a series of scalene injection with conservative treatment in all cases. Patients rated their pain degrees using a visual analogue scale. We also evaluated the time to return to everyday life and work, and patients' functional capacity.
Results
There were no complications or instances of inadvertent somatic or sympathetic ganglionic blockade after scalene injection. Overall, 111 patients (76.5%) experienced improved symptoms after the first set of scalene injection and 128 patients (88.2%) improved after scalene injection followed by conservative treatment. Of the 68 patients who returned to work during the study period, 54 returned within 1 week, and 62 within 2 weeks. Of those who returned to work, 61 reported nearly full functional capacity. We found that scalene injection was more effective in cases of thoracic outlet syndrome related to trauma than in those related to work-related repetitive stress.
Conclusion
In patients with thoracic outlet syndrome, scalene injection effectively reduces pain. We recommend scalene injection as an adjunct to conservative treatment.
Keywords: Thoracic outlet syndrome, Injury mechanism, Scalene injection
INTRODUCTION
Thoracic outlet syndrome (TOS) results from the compression of major neurovascular structures at their thoracic outlets, such as brachial plexus, subclavian arteries, and subclavian veins. The causes of compression include trauma (e.g., motor vehicle accidents and work-related repetitive injury) and several cases of congenital anomalies3,11). Despite being one of the earliest described and most discussed of the compression neuropathies, TOS remains controversial in terms of its etiology, diagnosis, and treatment3).
Although there is no "gold standard" for diagnosing TOS, scalene injection can help determine which patients may respond favorably to surgery, improving diagnostic accuracy in what remains a test that relies on subjective responses10,13). Temporary pain relief after the scalene injection support the diagnosis of TOS, but the relief is short-lived3,10). In this study, we investigated scalene injection as an initial treatment method for TOS.
MATERIALS AND METHODS
Among the patients diagnosed with TOS who underwent scalene injection between January 2005 and October 2009, we selected 145 patients who were followed over 1 year. We excluded patients with disorders such as diabetes or thyroid disease, which can induce peripheral neuropathy; cervical lesions such as cervical disc herniation or spinal stenosis, as diagnosed by magnetic resonance imaging; and rotator cuff disorders or shoulder impingement syndrome. We included 142 patients of the selected patients for the analysis. Three cases of TOS were idiopathic. By means of a chart review and telephone interview, we complied a thorough medical history, inquiring specifically about motor vehicle accidents, work-related repetitive injury, upper limb trauma. We investigated the exact mechanism of each injury to clarify the pathogenesis of TOS.
In this study, 3 criteria were required to diagnose TOS. The first criterion was either sensory dysfunction (pain or paresthesia) or muscle weakness in the face, upper limbs, shoulders, upper chest, or upper abdomen. The second was either tenderness of the anterior and middle scalene muscles or Tinel's sign of the brachial plexus. The third was a positive finding in the following 3 provocation tests; neck tilt test, hyperabduction test, and costoclavicular compression test13). All patients that received scalene injection experienced neck and arm pain and paresthesias that worsened with the provocation tests, with a point of maximal tenderness over the anterior and middle scalene muscles. Similar to trigger point muscle injection in chronic pain, we performed scalene injection for symptom relief as the patients proceeded with other medical therapies or surgery10). We defined a positive outcome as symptom relief for any period time.
Patients rated their pain using a visual analogue scale (VAS; 0=no pain, 10=worst pain imaginable) at the time of diagnosis, after the scalene injection, and during the follow-up period. We recorded VAS scores and clinical results immediately after the procedure and after 1 month, 6 months, and 1 year by telephone interview10). We also evaluated the time to return to everyday life and work and patients' functional capacity.
For statistical analysis, we used the STATA 11.1 program. Statistical significance was set at p<0.05. We used a t-test to compare difference in VAS score between repetitive injuries and 3 types of traumatic injuries. These VAS scores were calculated from the results of pre-injection VAS scores minus post-injection VAS score. We also used a paired t-test to evaluate the differences in VAS scores, calculated from pre-injection VAS scores minus post-injection VAS scores.
Technique of scalene injection
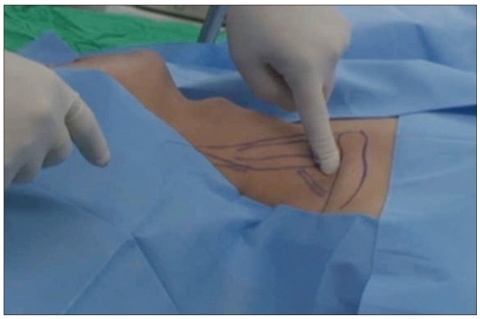
Scalene injections were performed with the following technique. As the patient laid supine, a pillow was inserted under the upper back to maintain hyperextension of the neck and upper chest. Vital signs were continuously monitored, including blood pressure, heart rate, and oxygen saturation. In preparation for the injection, the anterolateral neck and shoulder area were prepared and draped in a sterile manner. The anterior scalene muscle (ASM) descends inferolaterally from the anterior tubercle of the transverse processes of C3-C6 vertebrae to the scalene tubercle of the first rib, anterior to subclavian artery groove. The ASM was identified deep to the sternocleidomastoid muscle; it is activated when the patient lifts the neck. The point of maximal tenderness was identified by pressing gently along the ASM. Most patients felt 3 or 4 points of tenderness. Next, the middle scalene muscle (MSM) was identified. The MSM descends inferolaterally from the transverse process of C2 and the posterior tubercles of the transverse processes of C3-C7 vertebrae to the superior surface of the first rib, posterior to subclavian artery groove, which is posterolateral to the ASM. Again, the point of maximal tenderness was identified by gentle pressure along the muscle (Fig. 1, 2). Finally, the brachial plexus was located between ASM and MSM.
Fig. 1.
The anterior scalene muscle, middle scalene muscle, brachial plexus are identified and tenderness point is checked by pressing gently along anterior scalene muscle and middle scalene muscle. In most cases, tenderness at 3-4 points is found, especially around proximal, distal end and middle area of the muscle.
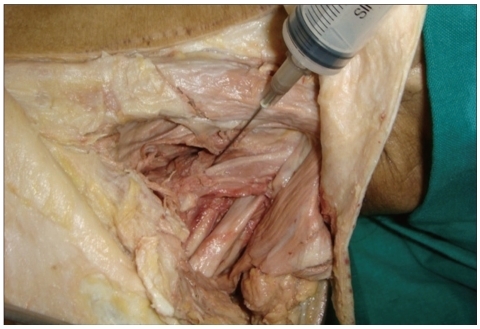
Fig. 2.
In cadevaric study, needle depth is typically within a range of 2 to 4 cm depending on body habitus and needle angulation with the surface.
Using a 25-gauge, 1-inch-long needle, 8 to 10 mL of 0.125% bupivacaine (Marcaine®, 100 mg/20 mL Jeil pharmacy, Korea) and 1 mL of triamcinolone (Rheudenolone®, 40 mg/mL Kukje pharmacy, Korea) were injected into the ASM and MSM, while applying gentle pressure to the muscle with the index finger. Needle depth was 2-4 cm depending on body habitus and needle angulation (Fig. 2). We injected 1-2 mL at every tenderness point, for a maximum dose of 8 mL. The injection was preferably applied to different levels of the muscle. Aspiration just prior to injection avoided intravascular injection.
We considered the injection to have a positive result if the patient reported decreased symptoms (pain, numbness, and discomfort) around the injection area compared to those experienced before the procedure. Injections were repeated if tenderness remained after the procedure. Injections were also repeated if there was tenderness in the ipsilateral shoulder near the neck after the procedure. After completion of the procedure, patients remained under observation for approximately 30 minutes to monitor for acute complications such as seizure, dyspnea, or altered mental status.
RESULTS
Patients were comprised of 74 men and 68 women with a mean age of 47 years (range 18-80) and a mean follow-up period of 38 months (range 12-64). Their TOS etiologies included 71 cases of trauma-related injury and 71 cases of work-related repetitive stress. The trauma-related injuries included 38 cases of whiplash caused by traffic accidents, 18 cases of upper limb abduction-compression caused by breaking one's fall on outstretched arms, 15 cases of direct trauma to the shoulders and upper arms, and 8 cases of traction injury of the upper limbs (Table 1).
Table 1.
Summary of injury mechanisms
In this study, we experienced no complications or instances of inadvertent somatic or sympathetic ganglionic injury. The ASM and MSM were identified in all cases. The entire procedure, including testing and preparation, was completed within 0.5 hours in all cases.
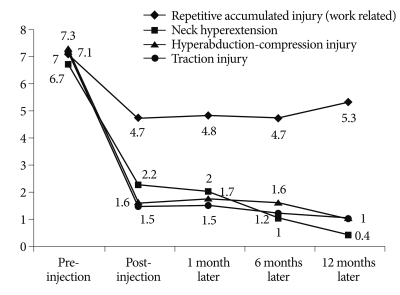
Overall, 111 (78.2%) of these patients had a positive response to the first scalene injection. The mean VAS pain score at the time of diagnosis was 7.1 (range 5-10); immediately after the scalene injection, 3.1 (range 1-9); 1 month after injection, 3 (range 1-7); 6 months after the injection, 2.4 (range 1-8); and 1 year after injection, 2.2 (range 1-7). In patients with work-related repetitive injuries, the mean VAS pain score was 6.7 before the procedure and less than 1 point after 1 year. Changed in VAS pain score classified by etiology of TOS are summarized in Fig. 3. We found significant differences between the 3 types of traumatic injuries and repetitive injury (p<0.01); each type of injury experienced significant improvement in symptoms (p<0.01).
Fig. 3.
VAS (visual analogue scale) after scalene injection according to injury mechanism.
Among the remaining 34 except 111 patients who had a positive response to the first scalene injection, 16 improved after conservative treatment such as additional medication, 16 received additional scalene injection, and 2 underwent anterior and middle scalenectomy. Of the 16 patients who received additional scalene injections, 14 showed little improvement of symptoms. Of the 2 patients who underwent surgery, 70% showed improvement of symptoms. Of all 142 patients in the study, 128 cases (88.2%) were improved after scalene injection and conservative treatment.
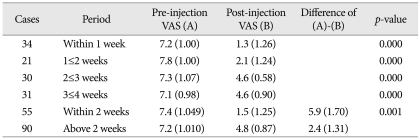
Of the 68 patients who returned to work during the study period, 54 returned within 1 week and 62 within 2 weeks. Of those who returned to work, 61 reported nearly full functional capacity. The mean time between symptom onset and scalene injection was 1 month (range, 6 days-12 months). There was no apparent correlation between this time interval and the effect of the scalene injection. However, for TOS caused by trauma-related injuries, we did observe a correlation between the time to performing a scalene injection and its effect; patients who received injection within 2 weeks of symptom onset showed significantly greater improvement than those who received injection later (Table 2).
Table 2.
Comparing the effect of scalene injection by the period from the onset of the symptoms to the time of performing scalene injection on cases by trauma-related injury
VAS : visual analogue scale
DISCUSSION
Most clinicians agree that conservative management is the best initial strategy for TOS, as 50-60% patients improve with this approach. Cooke reported the improvement in 60% of patients, and Zatocil et al. reported 50%2,12). In our study, we found that 86.4% of patients improved after scalene injection in addition to conservative treatment such as medication. In addition, Landry et al. reported that 78% of the patients treated conservatively returned to work within 4.8 years, while Lindgren et al. reported that 73% returned to work within 2 years. In our study, 91.8% of patients returned to work within 1 year, and 97.1% of these returned within 1 month. Furthermore, most patients regained full functional ability at work after treatment. The earlier return to work compared to other reports may be due to early symptomatic improvement from the scalene injection performed soon after diagnosis.
Sheldon reported a therapeutic effect of 68% after successful scalene injection9). In studies of the surgical treatment of TOS, Shenkin reported that 50% of patients had improvements in pain and post-operative function after anterior scalenectomy17) similar to the 52% reported by Bruin7), Takagi20) reported that 80% of patients had a positive response. A more recent study by Cikrit6) reported that 93% of patients experienced a positive response, with only experiencing pain at the operative site and post-operative functional disorders. In a report including all surgical methods, Sanders reported positive results in 57-86% of patients4,8,15-17). In our study, 111 patients (78.1%) had a positive response to scalene injection. Another 17 patients achieved full recovery with short-term medication in addition to scalene injections. Our study suggests that scalene injection is an effective initial treatment of TOS, and that additional short-term medication can be value in some patients.
The literature contains few reports comparing the effects of scalene injection by the mechanism of TOS injury5). In our study, we found that scalene injection was significantly more effective in patients with TOS caused by whiplash, hyperabduction-compression injury, or traction injury of the upper limbs than in patients with TOS caused by work-related repetitive injuries. We observed no significant relationship between the therapeutic effect and the proximity of scalene injection to symptom onset. Also, there was no apparent correlation between this time interval and the effect of the scalene injection. However, for TOS caused by trauma-related injuries, we did observe a correlation between the time to performing a scalene injection and its effect; patients who received injection within 2 weeks of symptom onset showed significantly greater improvement than those who received injection later.
The scientific literature contains very few reports regarding the efficacy of further TOS treatments after failed scalene injection. Sheldon et al. recommended that a negative response to scalene injection should not exclude surgical treatment if the overall weight of clinical data supports proceeding with surgery10). In patients who respond minimally to the first set of scalene injection, there is controversy over providing additional injections. The authors of this report performed additional scalene injection in 16 of 34 patients who did not respond initially, but there was little improvement in symptoms. From our experience, we suggest that clinicians consider alternative treatment options if patients with TOS do not initially respond to scalene injection.
CONCLUSION
Scalene injection confirms the clinical diagnosis of TOS in patients presenting with typical patterns of pain and paresthesia, and helps to identify the relative contribution of TOS when symptoms overlap with another disease. In patients with TOS, earlier return to everyday life and work is possible by combining scalene injection with conservative treatments such as medication.
Acknowledgements
This study was supported by a grant from Kosin University College of Medicine (2009).
References
- 1.Abe M, Ichinohe K, Nishida J. Diagnosis, treatment, and complication of thoracic outlet syndrome. J Orthop Sci. 1999;4:66–69. doi: 10.1007/s007760050075. [DOI] [PubMed] [Google Scholar]
- 2.Atasoy E. Combined surgical treatment of thoracic outlet syndrome : transaxillary first rib resection and transcervical scalenectomy. Hand Clin. 2004;20:71–82. vii. doi: 10.1016/s0749-0712(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 3.Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20:15–16. v. doi: 10.1016/s0749-0712(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 4.Atasoy E. Thoracic outlet Compression syndrome. Orthop Clin North Am. 1996;27:265–303. [PubMed] [Google Scholar]
- 5.Brantigan CO, Roos DB. Etiology of neurogenic thoracic outlet syndrome. Hand Clin. 2004;20:17–22. doi: 10.1016/s0749-0712(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 6.Cikrit DF, Haefner R, Nichols WK, Silver D. Transaxillary or supraclavicular decompression for the thoracic outlet syndrome. A comparison of the risks and benefits. Am Surg. 1989;55:347–352. [PubMed] [Google Scholar]
- 7.De Bruin TR. Costoclavicular space enlargement. Eight methods of relief of neurovascular compression. Int Surg. 1966;46:340–360. [PubMed] [Google Scholar]
- 8.Gage M. Scalenus anticus syndrome. A diagnostic and confirmatory test. Surgery. 1939;5:599–601. [Google Scholar]
- 9.Jordan SE, Ahn SS, Gelabert HA. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes : development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441–452. [PubMed] [Google Scholar]
- 10.Jordan SE, Machleder HI. Diagnosis of thoracic outlet syndrome using electrophysiologically guided anterior scalene blocks. Ann Vasc Surg. 1998;12:260–264. doi: 10.1007/s100169900150. [DOI] [PubMed] [Google Scholar]
- 11.Lee JP, Chang JC, Cho SJ, Park HK, Choi SK, Bae HG. A morphometric aspect of the brachial plexus in the periclavicular region. J Korean Neurosurg Soc. 2009;46:130–135. doi: 10.3340/jkns.2009.46.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascarelli EF, Hsu YP. Understanding work-related upper extremity disorders : clinical findings in 485 computer users, musicians, and others. J Occup Rehabil. 2001;11:1–21. doi: 10.1023/a:1016647923501. [DOI] [PubMed] [Google Scholar]
- 13.RAAF J. Surgery for cervical rib and scalenus anticus syndrome. J Am Med Assoc. 1955;157:219–233. doi: 10.1001/jama.1955.02950200017005. [DOI] [PubMed] [Google Scholar]
- 14.Sanders RJ. Thoracic outlet syndrome : common sequela of neck injruies. ed 1. Pennsylvania: J.B. Lippincott Company; 1991. pp. 71–84. [Google Scholar]
- 15.Sanders RJ. Thoracic outlet syndrome : Results of Treatment and Comments. Hand Clin. 2004:171–191. [Google Scholar]
- 16.Sanders RJ, Hammond SL. Etiology and pathology. Hand Clin. 2004;20:23–26. doi: 10.1016/s0749-0712(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 17.Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601–604. doi: 10.1016/j.jvs.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 18.Sanders RJ, Hammond SL, Rao NM. Thoracic outlet syndrome : a review. Neurologist. 2008;14:365–373. doi: 10.1097/NRL.0b013e318176b98d. [DOI] [PubMed] [Google Scholar]
- 19.Shenkin HA, Somach FM. Scalenotomy in patients with and without cervical ribs; analysis of surgical results. Arch Surg. 1963;87:892–896. doi: 10.1001/archsurg.1963.01310180008003. [DOI] [PubMed] [Google Scholar]
- 20.Takagi K, Yamaga M, Morisawa K, Kitagawa T. Management of thoracic outlet syndrome. Arch Orthop Trauma Surg. 1987;106:78–81. doi: 10.1007/BF00435418. [DOI] [PubMed] [Google Scholar]