Abstract
Seizure is a foreseeable risk in patients with brain lesion. However, seizure during treating non-brain lesion is not a familiar situation to neurosurgeon. Posterior reversible encephalopathy syndrome (PRES) is a relatively common situation after systemic chemotherapy. The aim of this study is to make neurosurgeons aware of this potential medical problem. A 52-year-old woman with advanced gastric cancer, presented with low back pain due to spinal metastasis at the 4th lumbar vertebra. Ten cycles of chemotherapy with FOLFOX (5-Fluoruracil/Oxaliplatin) had been completed 23 days ago. Two days before the planned operation, a generalized tonic clonic seizure occurred. She did not have a history of hypertension or seizure. The seizure was stopped with lorazepam 4mg. The brain magnetic resonance (MR) imaging showed high signal changes in both parieto-occipital lobes on the T2-weighted images, and these were partially enhanced, suggesting PRES. The surgery was preceded by treatment with an antiepileptic drug. The MR images, taken 1.5 months after the seizure, showed that the lesion was no longer present. At 3 month follow-up, no additional seizure attack occurred without any seizure medication. The possibility of a seizure attack should be considered if the patient has a history of chemotherapy.
Keywords: Posterior reversible encephalopathy syndrome, Seizure, Metastasis, Spine, Oxaliplatin
INTRODUCTION
Pain and neurological deficits are common presenting symptoms in patients with spinal metastasis. For the most part, seizure is not a foreseeable risk if there is no evidence of brain metastasis. However, we observed an unexpected seizure attack in a patient with spinal metastasis. Although posterior reversible encephalopathy syndrome (PRES) is a well-known syndrome that is usually associated with hypertension or chemotherapy, it is not familiar with neurosurgeon due to limited experience and report1,3,4,17). With the development of chemotherapy, the possibility of PRES is increasing in cancer patients1,3,4,17). If physicians are aware of this possibility of PRES, a diagnosis could be easily made using imaging. Moreover, PRES is reversible and can be managed with conservative treatment4,15). The aim of this report is to make neurosurgeons aware of this potential medical problem after chemotherapy and to present management experience with the literature review.
CASE REPORT
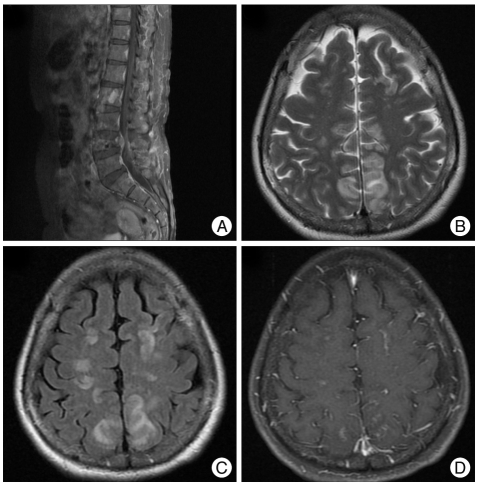
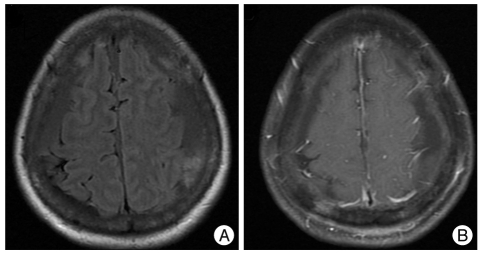
A 52-year-old woman with advanced gastric cancer, which was diagnosed 7 months prior to admission, presented with low back pain and radiculopathy in the left leg. The primary tumor was not resectable, and 10 cycles of chemotherapy with FOLFOX (5-Fluoruracil/Oxaliplatin) had been completed 23 days prior to admission. A metastatic tumor with an epidural extension was detected at the 4th lumbar vertebra (Fig. 1A). As a result, elective surgical decompression and stabilization were scheduled. Two days before the surgery, a generalized tonic clonic seizure occurred. She did not have a history of hypertension and had normal blood pressure at admission, but her systolic blood pressure was 166 mmHg at the time of the seizure. No electrolyte abnormality was observed. After emergency treatment for seizure (iv. lorazepam 4 mg), a brain computed tomography was conducted. However, no definite abnormality was observed. An antiepileptic drug was administered (valproate sodium 1,200 mg/day). After subsidence of the seizure, brain magnetic resonance (MR) imaging was conducted one day after the onset of seizure. A signal change was observed in both parieto-occipital lobes in the T2-weighted and fluid attenuated inversion recovery (FLAIR) axial MR imagings, and it was partially enhanced in the gadolinium enhanced T1-weighed axial MR imaging (Fig. 1B, C, D). A diffusion MR image showed no restriction of diffusivity, and no vascular abnormality was observed in the MR angiography. As a result of typical imaging features, PRES was diagnosed. The patient's blood pressure was within the normal range after the subsidence of the seizure without the use of anti-hypertensive drugs. Surgical decompression and stabilization for spinal metastasis was conducted as planned. The operation was completed without complication. No seizure or change of mental status was observed after the operation. However, 2 days after the operation, she became delirious. Treatment with an anti-epileptic drug had been maintained since the time of the seizure attack. Postoperative delirium was suspected, and as a result, we closely observed her status. The delirious status persisted for 4 days, and a steroid was administered (a dexamethasone 10 mg bolus and 16 mg per day). Two days after treatment with a steroid, she regained an alert mental status, and she was tapered off of the steroids. She was discharged 12 days after the operation without a neurological sequel, and she was tapered off of the anti-epileptic drug. The chemotherapy with the same regimen that the patient received before admission was resumed. A brain MR imaging was obtained 1.5 months later due to a mild headache and a history of head trauma. A bilateral subdural hemorrhage was detected. We decided not to operate on the subdural hemorrhage. Brain MR imaging revealed that the lesion in both parieto-occipital lobes was no longer present (Fig. 2). At follow-up (she expired 3 months after operation due to respiratory failure), no further seizure attacks or changes in mental status had occurred.
Fig. 1.
Computed tomography and magnetic resonance (MR) imaging at the time of the seizure attack. A metastatic tumor with an epidural extension is detected at the 4th lumbar vertebra in a gadolinium enhanced T1-weighted sagittal image (A). At the time of the seizure attack, there was no abnormality in the computed tomography. Magnetic resonance imaging was taken one day later. An increased signal intensity is detected in the cortex and the subcortical white matter of the parieto-occipital lobe in the T2-weighted axial image (B) and the fluid-attenuated inversion recovery MR imaging (C). This lesion shows a focal enhancement in the gadolinium enhanced T1-weighted axial image (D). There waiss no restriction of diffusion in the diffusion-weighted MR imaging. The MR angiography reveals no vascular abnormalities.
Fig. 2.
Magnetic resonance (MR) imaging 1.5 month after the seizure attack. A : A bilateral multi-staged subdural hemorrhage is detected, whereas the lesion in the parieto-occipital lobe is no longer present in the T2-weighted and FLAIR axial MR imaging. B : There is no enhancement in the gadolinium enhanced T1-weighted axial MR imaging. FLAIR : fluid attenuated inversion recovery.
DISCUSSION
PRES includes a group of disorders that present with headaches, seizures, and focal neurological signs that are associated with the location of the lesion6). This syndrome is also termed hypertensive encephalopathy or posterior reversible leukoencephalopathy6).
The pathogenesis of PRES is not yet clear. The known etiologies include hypertension, preeclampsia, systemic lupus erythematosus, immunosuppressive medications (e.g., Cyclosporine), anti-neoplastic agents, severe hypercalcemia, thrombocytopenia, hemolytic uremic syndrome, amyloid angiopathy, renal failure, infection/sepsis, or hallucinogens11,14). Vascular endothelial dysfunction is likely caused by the dilatation of the cerebral arterioles, the breakdown of endothelial tight junctions, or the deregulation of the cerebral blood flow. Following vasogenic edema, radiologic features may occur1,2,5). Hypertension is the most common cause of PRES17,19). However, PRES is not associated with hypertension in 20 to 30% of patients, and a lack of hypertension does not rule out the possibility of PRES.
During chemotherapy with cytotoxic drug (e.g., Methotrexate, Cisplatin, Oxaliplatin et al.), PRES could be expected and should be considered as one of the differential diagnoses of seizure6,7,9,12). However, for patients with non-brain lesion, PRES could not be expected easily, because clinical interest is not focused on the possibility of seizure. Moreover, lack of report on PRES in neurosurgical paper make neurosurgeon be less aware of PRES.
Radiological features
Typical MR imaging features of PRES are the reversible cortical and subcortical changes, which consist of high-intensity lesions on T2-weighted and FLAIR sequences, and focal enhancement on a T1-weighted MR image6). This change is usually bilaterally and symmetrically located in the cortical and subcortical regions of the parietal and occipital lobes. In addition to these locations, involvement of the frontal and temporal lobes, and the basal ganglia, the brain stem, and the cerebellum have also been described in patients with PRES15,18).
Usually, diffusion is not restricted in diffusion-weighted imaging (DWI), and this finding is helpful for differentiating PRES from ischemic change or infarction6,10). The radiological change is usually reversible7).
In the present case, initial MR images showed symmetric T2 high-signal and patch enhancement in both cerebral hemispheres, predominantly in the posterior parietal and occipital lobe, similar to previous descriptions. The DWI showed no restriction of diffusion. The follow-up MR images showed that the lesions were no longer present, and this result confirmed the diagnosis of PRES.
Oxaliplatin-related PRES
In the present case, the cause of PRES may not have been associated with hypertension. Because there was no history of hypertension, elevated blood pressureduring the seizure attack was likely a transient episode. Our case may be related to an anti-neoplastic agent. Chemotherapy with a FOLFOX regimen was performed for 10 cycles (from 8 months prior to admission to 23 days prior to admission). Oxaliplatin is a well-known cytotoxic drug9). Cytotoxic drug could damage blood-brain-barrier and cause vasogenic edema6). Oxaliplatin in combination with the FOLFOX (5-fluorouracil, oxaliplatin) regimen may be the causative factor of the seizure because neurological toxicity is one of side effects of Oxaliplatin9). The occurrence of PRES after use of Oxalipaltin observed between 10 days to 3 months after administration of drug7,9,13,16). In the present case, PRES occurred 23 days after finishing chemotherapy. Due to limited experience, the occurrence of PRES after use of Oxaliplain could not be expected exactly and could be delayed up to 3 months10,12,15,16).
Two similar reports have been published. A nineteen-year-old woman with rectal adenocarcinoma received FOLFOX chemotherapy. Ten days after the fourth cycle, she developed seizures and an altered mental status. In this case, the FLAIR MR images demonstrated signal abnormalities within the posterior parietal cortex at a midline location and within the splenium of the corpus callosum7). The other case was a 62-year-old man with urothelial bladder cancer. Six weeks after finishing his sixth chemotherapy cycle with FOLFOX, a generalized tonic-clonic seizure occurred. The T2-weighted and FLAIR MR imaging showed a symmetrical, high-intensity signal lesion in the cortex and the subcortical white matter in the bilateral occipital and parietal lobes9). These two cases were managed with conservative care7,9). When a patient has a history of chemotherapy, especially with oxaliplatin, PRES should be considered as one of causes of seizure or altered mentality. Treatment is usually symptomatic.
Treatment
PRES is usually managed with supportive care such as the control of hypertension, correction of electrolyte imbalance, and management of seizures15). If hypertension is present, the mean arterial pressure should only be reduced by 20 to 25% within the first 1 to 2 hrs, or the diastolic blood pressure should be reduced to 100 mmHg to reduce hypoperfusion17,19). If there is no hypertension, treatment is usually symptomatic. Seizures can be controlled with transient antiepileptic drugs during acute episodes of PRES20). However, there are no definite treatment guidelines for the use of steroids, even though we used steroids for symptomatic relief and observed recovery. PRES is known to be associated with vasogenic edema, and the use of steroids for this purpose may be relevant1,8,17). With appropriate treatment, PRES is usually reversible1,8,17). However, permanent neurologic disability and death from progressive cerebral edema and intracranial hemorrhages have been reported, and the possibility of irreversibility should be considered17).
CONCLUSION
With the development of chemotherapy, the possibility of PRES is increasing in cancer patients. The occurrence could be delayed until 3 months. If physicians are aware of this possibility of PRES, a diagnosis could be easily made. Moreover, PRES is reversible and can be managed with conservative treatment.
Acknowledgements
This research was jointly supported by grants from the Brain Research Center of the 21st Century Frontier Research Program (2009K001280) funded by the Ministry of Education, Science and Technology, the Republic of Korea.
References
- 1.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1 : fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2 : controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang UK, Youn SM, Park SQ, Rhee CH. Clinical results of cyberknife(r) radiosurgery for spinal metastases. J Korean Neurosurg Soc. 2009;46:538–544. doi: 10.3340/jkns.2009.46.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SM, Lee SH, Yang YS, Kim BC, Kim MK, Cho KH. 5-fluorouracil-induced leukoencephalopathy in patients with breast cancer. J Korean Med Sci. 2001;16:328–334. doi: 10.3346/jkms.2001.16.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Pontual L, Zaghloul NA, Thomas S, Davis EE, McGaughey DM, Dollfus H, et al. Epistasis between RET and BBS mutations modulates enteric innervation and causes syndromic Hirschsprung disease. Proc Natl Acad Sci U S A. 2009;106:13921–13926. doi: 10.1073/pnas.0901219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicuonzo F, Salvati A, Palma M, Lefons V, Lasalandra G, De Leonardis F, et al. conventional magnetic resonance and diffusion-weighted imaging findings. J Child Neurol. 2009;24:1013–1018. doi: 10.1177/0883073809332705. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BM, Main SH, Cantone KL, Smith SD, Warford A, Vaughan TJ. Isolation and tissue profiles of a large panel of phage antibodies binding to the human adipocyte cell surface. J Immunol Methods. 2000;245:67–78. doi: 10.1016/s0022-1759(00)00275-1. [DOI] [PubMed] [Google Scholar]
- 8.Gerretsen P, Kern RZ. Reversible cerebral vasoconstriction syndrome : a thunderclap headache-associated condition. Curr Neurol Neurosci Rep. 2009;9:108–114. doi: 10.1007/s11910-009-0018-5. [DOI] [PubMed] [Google Scholar]
- 9.Irigoyen MM, Findley S, Earle B, Stambaugh K, Vaughan R. Impact of appointment reminders on vaccination coverage at an urban clinic. Pediatrics. 2000;106:919–923. [PubMed] [Google Scholar]
- 10.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–210. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- 11.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 12.Merhemic Z, Milić-Pokrajac D, Bajramovic A, Sulejmanpasic G. Posterior reversible encephalopathy syndrome (PRES) Med Arh. 2009;63:55–56. [PubMed] [Google Scholar]
- 13.Rangi PS, Partridge WJ, Newlands ES, Waldman AD. Posterior reversible encephalopathy syndrome: a possible late interaction between cytotoxic agents and general anaesthesia. Neuroradiology. 2005;47:586–590. doi: 10.1007/s00234-005-1376-6. [DOI] [PubMed] [Google Scholar]
- 14.Servillo G, Apicella E, Striano P. Posterior reversible encephalopathy syndrome (PRES) in the parturient with preeclampsia after inadvertent dural puncture. Int J Obstet Anesth. 2008;17:88–89. doi: 10.1016/j.ijoa.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Sharief U, Perry DJ. Delayed reversible posterior encephalopathy syndrome following chemotherapy with oxaliplatin. Clin Colorectal Cancer. 2009;8:163–165. doi: 10.3816/CCC.2009.n.026. [DOI] [PubMed] [Google Scholar]
- 16.Steeghs N, de Jongh FE, Sillevis Smitt PA, van den Bent MJ. Cisplatin-induced encephalopathy and seizures. Anticancer Drugs. 2003;14:443–446. doi: 10.1097/00001813-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome : a misnomer reviewed. Intern Med J. 2005;35:83–90. doi: 10.1111/j.1445-5994.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsukimori K, Ochi H, Yumoto Y, Iwasaki S, Hojo S, Noguchi T, et al. Reversible posterior encephalopathy syndrome followed by MR angiography-documented cerebral vasospasm in preeclampsia-eclampsia : report of 2 cases. Cerebrovasc Dis. 2008;25:377–380. doi: 10.1159/000120689. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356:411–417. doi: 10.1016/S0140-6736(00)02539-3. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan M. Slavery, smallpox, and revolution : 1792 in Ile de France (Mauritius) Soc Hist Med. 2000;13:411–428. doi: 10.1093/shm/13.3.411. [DOI] [PubMed] [Google Scholar]