Abstract
The hypoxia-inducible factors (HIFs; isoforms HIF-1α, HIF-2α, HIF-3α) mediate many responses to hypoxia. Their regulation is principally by oxygen-dependent degradation, which is initiated by hydroxylation of specific proline residues followed by binding of von Hippel-Lindau (VHL) protein. Chuvash polycythemia is a disorder with elevated HIF. It arises through germline homozygosity for hypomorphic VHL alleles and has a phenotype of hematological, cardiopulmonary, and metabolic abnormalities. This study explores the phenotype of two other HIF pathway diseases: classic VHL disease and HIF-2α gain-of-function mutation. No cardiopulmonary abnormalities were detected in classic VHL disease. HIF-2α gain-of-function mutations were associated with pulmonary hypertension, increased cardiac output, increased heart rate, and increased pulmonary ventilation relative to metabolism. Comparison of the HIF-2α gain-of-function responses with data from studies of Chuvash polycythemia suggested that other aspects of the Chuvash phenotype were diminished or absent. In classic VHL disease, patients are germline heterozygous for mutations in VHL, and the present results suggest that a single wild-type allele for VHL is sufficient to maintain normal cardiopulmonary function. The HIF-2α gain-of-function phenotype may be more limited than the Chuvash phenotype either because HIF-1α is not elevated in the former condition, or because other HIF-independent functions of VHL are perturbed in Chuvash polycythemia.—Formenti, F., Beer, P. A., Croft, Q. P. P., Dorrington, K. L., Gale, D. P., Lappin, T. R. J., Lucas, G. S., Maher, E. R., Maxwell, P. H., McMullin, M. F., O'Connor, D. F., Percy, M. J., Pugh, C. W., Ratcliffe, P. J., Smith, T. G., Talbot, N. P., Robbins, P. A. Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2α gain-of-function mutation.
Keywords: pulmonary hypertension, ventilation, metabolism, polycythemia
The hypoxia-inducible factor (HIF) family of transcription factors orchestrates a coordinated response to variations in oxygen availability by directly or indirectly altering the expression of hundreds of genes (1–4). These factors are composed of two subunits: the oxygen-regulated HIFα (isoforms are HIF-1α, HIF-2α, or HIF-3α) and the constitutively expressed HIFβ (5). In the presence of oxygen at physiological levels, HIFα undergoes rapid proteasomal degradation (6), triggered by prolyl hydroxylation (7–11), and subsequent binding to the von Hippel-Lindau (VHL) tumor suppressor protein (12, 13). When oxygen falls below physiological levels, the rate of hydroxylation slows, and HIFα accumulates within the cell.
Chuvash polycythemia (CP) is a rare disorder caused by germline homozygosity for a hypomorphic allele of VHL, which increases the levels of HIF within cells (14, 15). It is associated with a specific phenotype of excessive erythrocytosis, increased pulmonary ventilation and arterial hypocapnia, pulmonary hypertension, excessive pulmonary vascular and ventilatory responses to hypoxia, early and marked accumulation of lactate during exercise, limited exercise capacity, and shortened life expectancy (16–19). This study explores the phenotype of two other genetic disorders of the HIF-signaling pathway.
Classic VHL disease arises through a heterozygous mutation in VHL that has a severe effect on function of the associated protein (20). In this hereditary cancer syndrome, tumors form following a second (somatic) mutation, so that the function of both alleles of VHL in these cells has been disrupted (21–23). In this disease, a systemic phenotype would not be predicted without some degree of haploinsufficiency in relation to VHL. As there is now some evidence for this (ref. 24; see Discussion), our first aim (VHL study) was to determine whether any aspects of the CP phenotype are detectable in classic VHL disease.
Germline, heterozygous gain-of-function mutations in HIF-2α form a further type of disorder of the HIF pathway (25–27). These mutations in HIF-2α are associated with excessive erythrocytosis, but it is unknown whether other aspects of the CP phenotype are reproduced in these patients. As there is no evidence that these gain-of-function mutations in HIF-2α affect HIF-1α protein levels, the associated phenotype could be smaller than in CP. Our second aim (HIF2 study) was to determine what other aspects of the CP phenotype are also present in patients with HIF-2α gain-of-function mutations.
MATERIALS AND METHODS
Participants
VHL study
Six patients with classic VHL disease (VHL patients) and 6 healthy control participants took part in the study. The patients were free from any associated neoplasia at the time of the study and had no other medical disorders. VHL patients were recruited through their respective physicians. Control participants were recruited through advertisement and were chosen to match VHL patients for sex, age, height, weight, and body mass index.
HIF2 study
Three patients with gain-of-function mutations in HIF-2α (HIF2 patients) and 6 healthy control participants took part in the study. The patients had not been venesected within several weeks of the experiment and had no other medical disorders. They were identified from previous studies (25, 28) and recruited through their respective physicians. The number of patients recruited was limited by the rarity of the condition. Control participants were recruited through advertisement and were chosen to match HIF2 patients for sex, age, height, weight, and body mass index.
In both studies, all participants were informed about the aims, procedure, and details of the study and signed a consent form before taking part in the experiments. The studies conformed to the Declaration of Helsinki and had been approved by the Oxfordshire Research Ethics Committee.
Experimental protocol
Experiments were performed during the course of a single day. Participants were asked to avoid vigorous exercise and alcohol and caffeine consumption for 48 h prior to the experiments; they were also asked to eat a light breakfast on the morning of the experimental day. Room temperature was kept at 21°C throughout. Air-breathing end-tidal partial pressures of oxygen (PETo2) and carbon dioxide (PETco2) were measured before venous and arterial blood samples were taken. Participants were then asked to undertake four 20-min hypoxia response protocols, separated by three 20-min intervals. Each protocol started with 5 min of isocapnic euoxia (PETo2 100 mmHg), followed by 10 min of isocapnic hypoxia and ended with a final 5 min of isocapnic euoxia. The first two protocols examined the responses to mild hypoxia (PETo2 70 mmHg), similar to those experienced on a commercial flight. The last two protocols examined the responses to moderate hypoxia (PETo2 50 mmHg), similar to those associated with acute exposure to an altitude of ∼3500 m. PETco2 was maintained close to each participant's baseline level throughout each protocol. For the HIF2 study only, participants undertook an incremental exercise test to exhaustion after the hypoxia response protocols had been completed. For one HIF2 patient, the experiments were repeated on a second occasion after the patient had undergone a program of venesection to reduce his hematocrit to within the normal range.
Experimental technique
Respiratory measurements and control of end-tidal gases
During the hypoxia response protocols, participants had their nostrils occluded and breathed through a mouthpiece connected to a turbine used to measure ventilation (29). Respired gases were sampled through a catheter and analyzed continuously by mass spectrometry. The control of gases was managed through the dynamic end-tidal forcing technique: end-tidal measured values were used as input for a computer that controlled a fast gas-mixing system, which, in turn, allowed a rapid and accurate manipulation of PETo2 and PETco2 in the subsequent breaths (30). Breath-by-breath PETo2, PETco2, and ventilation values were recorded on a computer in real time. Throughout each protocol, participants were asked to recline in the left lateral position on a suitably modified couch.
Echocardiographic measurements
Pulmonary arterial systolic pressure and cardiac output were estimated through a continuous recording of Doppler echocardiography (Vivid-i; GE Healthcare, Little Chalfont, UK) with a 2-dimensional S3 probe (1.5–3.6 MHz). In the first and third protocols, pulmonary arterial systolic pressure was estimated by measurement of the maximum gradient of blood pressure across the tricuspid valve. This technique has been extensively validated (31–36). Right atrial pressure is not affected by hypoxia (37), so variations in the maximum gradient of blood pressure across the tricuspid valve mirror variations in pulmonary arterial systolic pressure. In the second and fourth protocols, cardiac output was estimated by measuring the velocity of blood flow through the aortic valve (38–40). An automatic monitor (Omron M5-I; Omron Healthcare Co., Kyoto, Japan) was used to measure blood pressure and heart rate every minute.
Incremental exercise test to exhaustion for HIF2 study
In the HIF2 study, exercise capacity was measured through an incremental exercise test to exhaustion on a modified, electrically braked cycle ergometer (Mijnhardt KEM3; Cardiokinetics, Salford, UK). Participants were given 30 s to achieve the required pedaling frequency (∼65 rpm) before the workload was increased by 20 W/min until exhaustion. Exhaustion was defined as the point at which the participants could no longer maintain the required pedaling frequency. Venous blood was sampled at the end of each work load (i.e., every minute) in order to measure venous blood lactate concentration. During the course of the protocol, participants breathed through a mouthpiece, with their nostrils occluded, and respiratory measurements were made as described for the hypoxia response protocols.
Data analysis
Measurements of PETo2, PETco2, ventilation, the maximum gradient of blood pressure across the tricuspid valve, and cardiac output were averaged over 1-min periods prior to further analysis. Differences between patients and control participants were assessed statistically using ANOVA (SPSS 12.0; SPSS, Chicago, IL, USA). Statistical significance was assumed at values of P < 0.05. Variables are presented as means ± sd, unless otherwise stated.
RESULTS
VHL study
Details of the specific mutation for each VHL patient, together with the physical characteristics of patients and control participants, are given in Supplemental Table S1. The physical characteristics of patients and control participants were well matched. VHL patients did not differ significantly from control participants in arterial Pco2, arterial pH, hemoglobin, hematocrit, mean corpuscular volume, serum iron, ferritin, and transferrin (Supplemental Table S2). In 3 VHL patients and in 1 control participant, the arterial Po2 was lower than is predicted for their age, but average arterial Po2 did not differ significantly between VHL patients and control participants.
Pulmonary measurements
VHL patients did not differ significantly from control participants in either baseline ventilation or in the increment in ventilation with mild or moderate hypoxia. The responses are illustrated in Supplemental Fig. S1.
An element of tricuspid regurgitation was detectable echocardiographically in 5 of the 6 VHL patients, but in only 2 of the control participants. VHL patients did not differ significantly from control participants in either baseline pulmonary arterial systolic pressure or in the increments in that pressure with mild or moderate hypoxia. The responses are illustrated in Supplemental Fig. S1. These responses were also similar to those of the control participants for the HIF2 study.
Systemic vascular measurements
The systemic vascular responses to hypoxia are illustrated in Supplemental Fig. S2. VHL patients did not differ significantly from control participants in either baseline values for heart rate, blood pressure, and cardiac output or in the increments in these variables with mild or moderate hypoxia.
Summary of VHL study
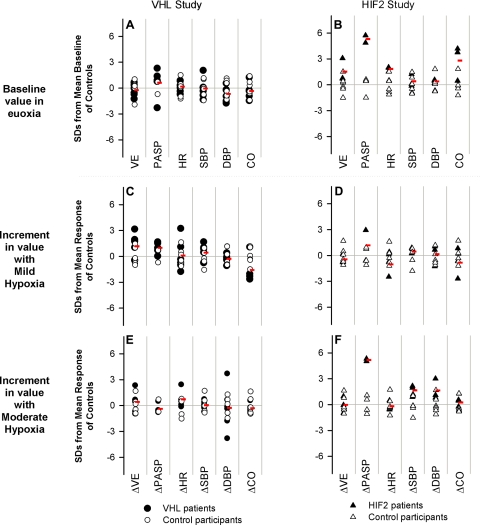
Figure 1 illustrates the deviation of each participant's baseline value (or incremental response) from the mean value for the control group, expressed in units of sd of the control group. There was considerable overlap between VHL patients and control participants for all variables.
Figure 1.
Cardiopulmonary variables in both the VHL and HIF2 studies: baseline measurements (A, B) and incremental responses with mild hypoxia (C, D) and moderate hypoxia (E, F). Results are shown in terms of number of (control group) sd by which each participant's baseline and response differed from the mean baseline and mean response, respectively, of the control participants. Red symbols show average results for patient groups. A, C, E) VHL patients were not significantly different from the control participants for any variable. B, D, F) Under baseline conditions, HIF2 patients had significantly higher pulmonary arterial pressure, heart rate, and cardiac output than control participants. Compared with control participants, HIF2 patients also had a significantly greater rise in pulmonary arterial pressure with moderate hypoxia. VE, ventilation; PASP, pulmonary arterial systolic pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; CO, cardiac output; Δ, increment.
HIF2 study
Details of the specific mutations for each HIF2 patient, together with the physical characteristics of patients and control participants, are given in Supplemental Table S3. The physical characteristics of the patients and control participants were well matched. The results obtained from the analysis of the venous and arterial blood samples are given in Table 1. Compared with the control participants, HIF2 patients had lower arterial Pco2 (−10.6±5.3 mmHg, P<0.01), higher hemoglobin concentration (+2.4±0.9 g/dl, P<0.03), higher hematocrit (+0.08±0.03 L/L, P<0.03), and higher value for transferrin (+0.8±0.3 g/L, P<0.003). HIF2 patients did not differ significantly from control participants in arterial pH, mean corpuscular volume, serum iron, and ferritin.
Table 1.
Arterial and venous blood analyses
| Analysis | Normal range | HIF2, n = 3 | Control, n = 6 | P value |
|---|---|---|---|---|
| Arterial Po2 (mmHg) | 83–105 | 105.0 ± 13.0 | 99.8 ± 9.5 | 0.52 |
| Arterial Pco2 (mmHg) | 35–45 | 32.1 ± 6.5 | 42.7 ± 3.7 | 0.01 |
| Arterial pH | 7.35–7.45 | 7.43 ± 0.04 | 7.38 ± 0.04 | 0.10 |
| Hemoglobin (g/dl) | 13–17 | 18.5 ± 1.8 | 16.1 ± 1.0 | 0.03 |
| Hematocrit (L/L) | 0.40–0.50 | 0.57 ± 0.05 | 0.49 ± 0.03 | 0.03 |
| Mean corpuscular volume (fl) | 83–105 | 84.6 ± 21.2 | 89.6 ± 2.8 | 0.56 |
| Serum iron (μM) | 14–31 | 17.0 ± 10.3 | 18.7 ± 4.6 | 0.75 |
| Ferritin (μg/L) | 20–300 | 75.8 ± 66.3 | 162.9 ± 93.2 | 0.27 |
| Transferrin (g/L) | 1.8–3.6 | 3.5 ± 0.2 | 2.7 ± 0.2 | 0.003 |
Values are means ± sd. P values compare the HIF2 patient group with the respective matched control participant group.
Pulmonary measurements
HIF2 patients did not differ significantly from control participants in either baseline ventilation or increment in ventilation with mild or moderate hypoxia. The responses are illustrated in Fig. 2.
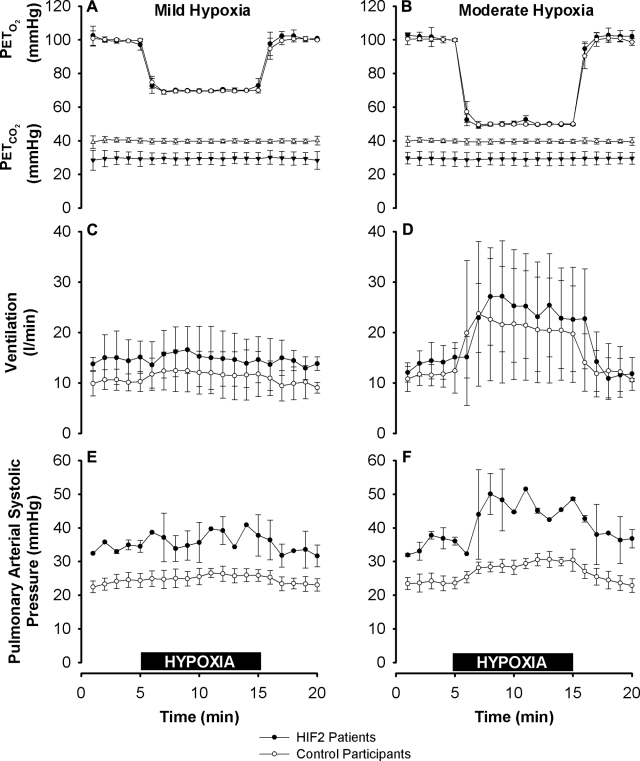
Figure 2.
End-tidal gas control, ventilation, and pulmonary arterial systolic pressure during mild and moderate hypoxia. A, B) PETo2 and PETco2 in mild hypoxia (A) and moderate hypoxia (B). PETo2 was well matched between groups. PETco2 was lower for the HIF2 patients, reflecting their lower baseline air-breathing PETco2. C, D) Ventilation, given at body temperature and pressure, saturated with water vapor, in mild (C) and moderate hypoxia (D). Baseline ventilation and the increase in ventilation provoked by hypoxia were not significantly different between the two groups. E, F) Pulmonary arterial systolic pressure. Compared with control participants, HIF2 patients had elevated pulmonary arterial systolic pressures at baseline (24±2 vs. 35±1 mmHg, P<0.003), and increased sensitivity to moderate (F) but not mild (E) hypoxia (5.5±1.3 vs. 11.5±0.7 mmHg, P<0.004). Values are expressed as means ± sd. Horizontal black bars indicate 10-min periods of mild (A, C, E) and moderate (B, D, F) exposures to hypoxia.
Echocardiographic measurements of pulmonary arterial systolic pressure were possible in 2 HIF2 patients and 4 control participants. Compared with the control participants, the HIF2 patients had significantly higher pulmonary arterial systolic pressures at baseline (35±2 vs. 24±1 mmHg, HIF2 patients vs. control participants, P<0.003), and a significantly greater increase in pulmonary arterial systolic pressure in response to moderate hypoxia (11±1 vs. 5±1 mmHg, P<0.004). HIF2 patients did not differ significantly from control participants in the increase in pulmonary arterial systolic pressure with mild hypoxia. The responses are illustrated in Fig. 2.
Systemic vascular measurements
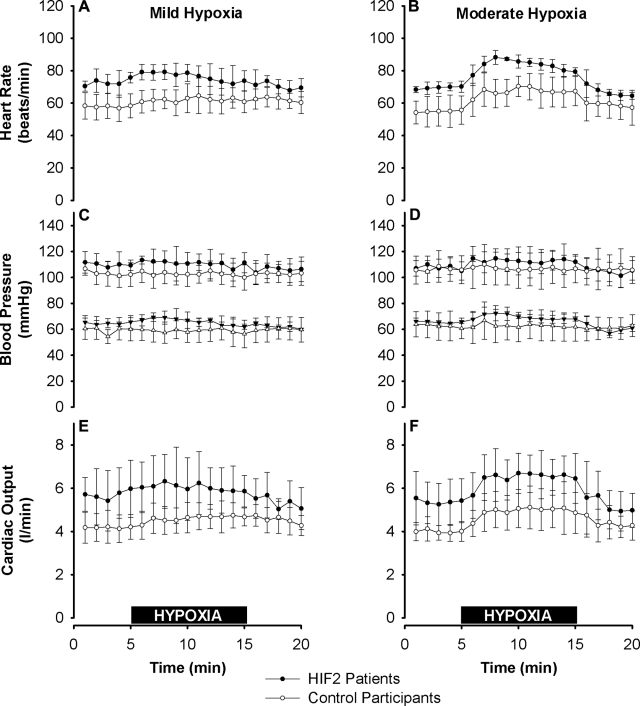
Figure 3 illustrates the systemic vascular responses to hypoxia. At baseline, compared with the control participants, the HIF2 patients had significantly higher heart rate (71±2 vs. 56±8 beats/min, P<0.02) and significantly greater cardiac output (5.5±1.0 vs. 4.1±0.5 L/min, P<0.02), while they did not differ significantly in blood pressure. HIF2 patients did not differ significantly from control participants in the increments in heart rate, blood pressure, and cardiac output with mild or moderate hypoxia.
Figure 3.
Systemic vascular responses in HIF2 patients and control participants to mild and moderate hypoxia. A, B) Heart rate in mild hypoxia (A) and moderate hypoxia (B). At baseline, heart rate was significantly higher in the HIF2 patients than in the control participants (P<0.02). C, D) Systolic (top) and diastolic (bottom) blood pressure in mild (C) and moderate hypoxia (D). Blood pressure did not differ significantly between the two groups. E, F) Cardiac output, assessed noninvasively using Doppler echocardiography, in mild (E) and moderate hypoxia (F). At baseline, cardiac output was significantly greater in the HIF2 patients than in the control participants (P<0.02). Increments in heart rate, blood pressure, and cardiac output with hypoxia did not differ significantly between the two groups. Values are expressed as means ± sd. Horizontal black bars indicate 10-min periods of mild (A, C, E) and moderate (B, D, F) exposures to hypoxia.
Summary of cardiopulmonary responses for HIF2 study
Figure 1 presents a summary of the variations in both baseline values and in the incremental responses to hypoxia for the various cardiopulmonary measurements. For baseline pulmonary arterial systolic pressures and for the increments in pulmonary arterial systolic pressure with moderate hypoxia, no overlap was observed in responses between the control participants and the HIF2 patients. For other variables, there was some overlap between the two groups, although the cardiac output of 2 of the 3 HIF2 patients was >3 SD from the mean of the control participants.
Incremental exercise test to exhaustion
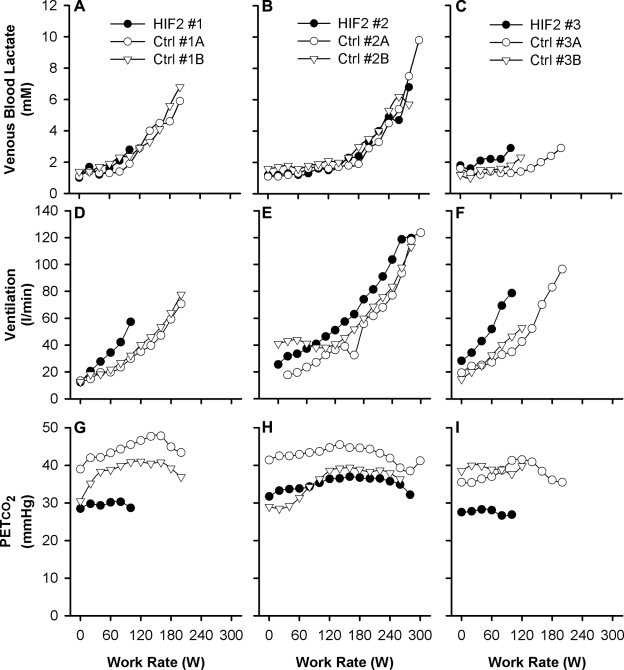
Figure 4 illustrates the responses obtained during the incremental exercise test to exhaustion. The rise in venous lactate with work rate appeared to be similar between HIF2 patients and control participants. Two of the 3 HIF2 patients appeared somewhat limited in their maximum exercise capacity, but the third patient exercised very comparably with the control participants. Overall, there were no significant differences between HIF2 patients and control participants for the maximum work rate achieved and the associated lactate level attained (2.0±0.9 vs. 2.9±0.9 W/kg, NS; and 4.3±2.2 vs. 5.4±2.7 mM, NS; respectively). The hyperventilation and associated hypocapnia at rest persisted throughout the period of incremental exercise to exhaustion.
Figure 4.
Individual responses for HIF2 patients and control participants during the incremental exercise test to exhaustion. A–C) Venous blood lactate concentration as a function of work rate. D–F) Ventilation as a function of work rate. G–I) PETco2 as a function of work rate. Results are for HIF2 patient 1 (A, D, G), HIF2 patient 2 (B, E, H), and HIF2 patient 3 (C, F, I) and their associated control participants. Data are minute averages.
Effects of venesection on one of the HIF2 patients
One of the HIF2 patients (patient 2) was studied both before and after venesection, which reduced the hemoglobin and hematocrit to values within the normally accepted range, serum iron to 7.8 μM, ferritin to 8.9 μg/L, and increased transferrin concentration to 4.24 g/L (Supplemental Table S4).
Venesection in this patient had no clear effect on ventilation and pulmonary arterial systolic pressure (Supplemental Fig. S3); ventilation appeared normal, while pulmonary arterial systolic pressure remained above normal. Venesection had no clear effect on heart rate, blood pressure, and cardiac output (Supplemental Fig. S4) at baseline and with hypoxia. Also, venesection had no clear effect on the accumulation of lactate in venous blood, ventilation, and end-tidal pressure of carbon dioxide (Supplemental Fig. S5), and on the maximum work rate and maximum venous blood lactate concentration achieved during the incremental exercise test to exhaustion; all these parameters remained within the normally accepted range (Supplemental Table S4).
DISCUSSION
In patients with classic VHL disease, the tumors that occur always have a somatic mutation of the wild-type allele for VHL, and, for this reason, it has generally been assumed that a single normal allele for VHL is sufficient to maintain normal cellular function. Recently, however, it has been shown that freshly isolated neutrophils from VHL patients displayed delayed apoptosis and enhanced bacterial phagocytosis, consistent with a pseudohypoxic phenotype (24). Haploinsufficiency resulting in HIF activation was further supported by the presence of elevated levels of PHD3 transcript in these cells, which is a known HIF target gene (41, 42). From these observations, the researchers concluded that, in humans, “heterozygous VHL defects are sufficient to perturb normal responses” (24), at least in some cells.
Despite this conclusion, erythrocytosis is not a feature of VHL disease, except in the context of cerebellar or renal tumors. In these cases, erythrocytosis is due to unregulated erythropoietin production from the tumor tissue, where both copies of VHL are mutated. Removal of the tumor tissue corrects the erythrocytosis, confirming that the underlying regulation is normal in the context of a single wild-type allele for VHL (43). In a relatively small number of VHL patients, the present study did not detect any of the major cardiopulmonary abnormalities present in the CP phenotype. Thus, a single functional allele for VHL appears to be sufficient to ensure either normality, or at least near normality, for these components of the CP phenotype.
In common with CP, gain-of-function mutations in HIF-2α were first identified through excessive erythrocytosis (14, 28). This similarity between the two conditions suggests that, in CP, elevation of HIF-2α alone may be sufficient to generate the erythrocytosis. Consistent with this, erythrocytosis in a mouse model of CP can be reversed by loss of the HIF-2α allele in VHLR/RHIF2-α+/− mice (where R indicates the Chuvash allele), but not by loss of the HIF-1α allele in VHLR/RHIF1-α+/− mice (46).
Components of the CP phenotype (18) identified in the HIF2 patients in this study include the pulmonary hypertension present under air breathing conditions and the elevation of pulmonary ventilation in relation to metabolism, as indicated by arterial and end-tidal hypocapnia. Indeed, clinically severe pulmonary hypertension has already been noted in two HIF2 patients from one family (25). Again, these findings suggest that elevation of HIF-2α alone in CP may be sufficient to generate these components of the phenotype. Consistent with this notion, pulmonary hypertension, which is also present in the mouse model of CP, was reduced in severity in VHLR/RHIF2-α+/− mice, but not in VHLR/RHIF1-α+/− mice (46). Overall, these findings suggest that HIF-1α may have little physiological role in the regulation of basal ventilation, pulmonary blood pressure, and red blood cell production.
In the present study, two novel phenotypic components were identified in the HIF2 patients, namely elevations in basal heart rate and cardiac output, which have not been demonstrated in CP. This raises the possibility that the HIF2 phenotype is not simply a subset of the CP phenotype. However, a previous study of the CP phenotype (18) noted trends toward higher cardiac outputs and heart rates in CP that did not quite reach statistical significance. As such, it would seem unlikely that these features are indeed unique to the HIF2 phenotype.
Other aspects of the CP phenotype appeared to be either reduced in magnitude or absent in the HIF2 patients. However, a serious limitation of the present study is the very small number of HIF2 patients that was available for investigation. Thus, negative findings may simply arise as a type II statistical error through lack of power, and, therefore, cannot be used to differentiate HIF2 patients from CP patients. However, as previous studies of CP patients from our laboratory have used very similar protocols, and as some aspects of the CP phenotype are quite extreme, it is possible that significant differences exist between the CP and HIF2 patients, despite the very small numbers in both groups. These comparisons are made below.
For the ventilatory responses to hypoxia, CP patients (18) could not be distinguished from HIF2 patients (CP vs. HIF2: mild hypoxia at 70 Torr, 4.5±2.3 vs. −0.1±1.7 L/min, P<0.054; moderate hypoxia at 50 Torr, 21±11 vs. 10±8 L/min, P<0.29). For the pulmonary vascular responses to hypoxia, CP patients could be distinguished from HIF2 patients (CP vs. HIF2: mild hypoxia at 70 Torr, 12±2 vs. 4±4 mmHg, P<0.053; moderate hypoxia at 50 Torr, 33±1 vs. 11±0 mmHg, P<0.001). Apart from the cardiopulmonary phenotype, CP patients exhibit an early and above-normal accumulation of lactate in the venous blood during incremental exercise, and a reduced maximum work rate capacity (19). In that study of CP patients, 3 of 5 patients and 3 of 5 control participants exercised hard enough to generate a significant metabolic stress, as evidenced by a venous lactate concentration >5 mM (47), and this occurred at 157 ± 12 W for CP patients and 257 ± 12 W for controls (P<0.001). Only one HIF2 patient exercised sufficiently hard to generate a venous lactate concentration >5 mM, but this occurred at 270 W—a value that differs significantly (P<0.02) from the distribution of values from the CP patients. The absence of effect of the HIF-2α mutation on metabolic function is consistent with studies undertaken in cell culture, which suggest that HIF-2α plays no role in regulating the expression of genes that affect glycolytic function (48). While metabolic abnormalities have been observed in HIF-2α-null mice, these changes are only part of a very severe and extensive overall phenotype associated with complete HIF-2α inactivation (49).
In summary, the HIF2 phenotype appeared to recapitulate those aspects of the CP phenotype that are most closely related to the regulation of absolute (or basal) values for variables within the hematological, cardiovascular, and respiratory systems. These include the hematocrit, the (euoxic) pulmonary vascular resistance (as reflected by the pulmonary hypertension), the ratio of ventilation to metabolic rate (as reflected by arterial/end-tidal Pco2), the cardiac output, and possibly cardiac sympathetic tone (as reflected by an increased heart rate). Aspects of the CP phenotype that are not so well replicated by the HIF2 phenotype are the increased sensitivity of the pulmonary vasculature to acute hypoxia and the excessive production of lactic acid during exercise. The limited number of available patients has made it impossible to draw definitive conclusions with respect to the ventilatory sensitivity to hypoxia. While it may be tempting to ascribe those aspects of the CP phenotype that were not observed in the HIF2 patients to an elevation of HIF-1α, it has been reported that VHL possesses a number of HIF-independent effects (44, 45) and so this is not necessarily correct.
Hemoglobin concentration, hematocrit, serum iron, and ferritin were all significantly reduced in one HIF2 patient after venesection. Despite the decrease in blood viscosity that would have occurred, no associated reduction in pulmonary arterial systolic pressure was detected. It is possible that the elevated HIF-2α levels in this patient contributed to maintaining a high pulmonary blood pressure, despite a marked decrease in hematocrit. However, in a study of venesection in patients with excessive erythrocytosis from chronic mountain sickness, pulmonary arterial blood pressure increased rather than decreased, suggesting that iron depletion can exacerbate hypoxic pulmonary hypertension (50). This underlines that the effects of venesection are not yet clear (51), and an important question is the extent to which patients with CP or HIF-2α gain of function erythrocytosis should be venesected.
Supplementary Material
Acknowledgments
The authors thank the patients and volunteers who took part in the studies. F.F. was funded by a University College Oxford postdoctoral research scholarship and by the Fell Fund from the Department of Physiology, Anatomy, and Genetics, University of Oxford, Oxford, UK.
This work was supported by the Wellcome Trust (grant 075876 to P.A.R.), by the Dunhill Medical Trust (to K.L.D.), by the Royal Society (grant TG092416 to F.F.), and by the NIHR Cambridge Biomedical Research Centre, Cambridge, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. F.F., P.A.B., Q.P.P.C., K.L.D., D.P.G., T.R.J.L., G.S.L., E.R.M., P.H.M., M.F.M., D.F.O., M.J.P., C.W.P., P.J.R., T.G.S., N.P.T., and P.A.R. agree with the manuscript's results and conclusions; F.F., C.W.P., and P.A.R. designed the experiments and the study; F.F. analyzed the data; F.F., Q.P.P.C., K.L.D., D.F.O., T.G.S., and N.P.T. collected data and conducted experiments for the study; F.F., P.A.B., D.P.G., T.R.J.L., G.S.L., E.R.M., P.H.M., M.F.M., M.J.P., T.G.S., and P.A.R. enrolled patients; F.F., P.J.R., and P.A.R. wrote the manuscript; K.L.D. and P.A.R. supervised and obtained funding. P.J.R., P.H.M., and C.W.P. are scientific cofounders of, and hold equity in, ReOx Ltd, a university spinoff company that is seeking to make HIF hydroxylase inhibitors. The other authors declare no competing financial interests.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Schofield C. J., Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell. Biol. 5, 343–354 [DOI] [PubMed] [Google Scholar]
- 2. Semenza G. L. (2004) Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19, 176–182 [DOI] [PubMed] [Google Scholar]
- 3. Semenza G. L. (2007) Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 405, 1–9 [DOI] [PubMed] [Google Scholar]
- 4. Wenger R. H. (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 [DOI] [PubMed] [Google Scholar]
- 5. Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salceda S., Caro J. (1997) Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272, 22642–22647 [DOI] [PubMed] [Google Scholar]
- 7. Bruick R. K., McKnight S. L. (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 [DOI] [PubMed] [Google Scholar]
- 8. Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 [DOI] [PubMed] [Google Scholar]
- 9. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 10. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 11. Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 13. Ohh M., Park C. W., Ivan M., Hoffman M. A., Kim T. Y., Huang L. E., Pavletich N., Chau V., Kaelin W. G. (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell. Biol. 2, 423–427 [DOI] [PubMed] [Google Scholar]
- 14. Sergeyeva A., Gordeuk V. R., Tokarev Y. N., Sokol L., Prchal J. F., Prchal J. T. (1997) Congenital polycythemia in Chuvashia. Blood 89, 2148–2154 [PubMed] [Google Scholar]
- 15. Ang S. O., Chen H., Hirota K., Gordeuk V. R., Jelinek J., Guan Y. L., Liu E. L., Sergueeva A. I., Miasnikova G. Y., Mole D., Maxwell P. H., Stockton D. W., Semenza G. L., Prchal J. T. (2002) Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat. Genet. 32, 614–621 [DOI] [PubMed] [Google Scholar]
- 16. Bushuev V. I., Miasnikova G. Y., Sergueeva A. I., Polyakova L. A., Okhotin D., Gaskin P. R., Debebe Z., Nekhai S., Castro O. L., Prchal J. T., Gordeuk V. R. (2006) Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica 91, 744–749 [PubMed] [Google Scholar]
- 17. Gordeuk V. R., Prchal J. T. (2006) Vascular complications in Chuvash polycythemia. Sem. Thromb. Hemostasis 32, 289–294 [DOI] [PubMed] [Google Scholar]
- 18. Smith T. G., Brooks J. T., Balanos G. M., Lappin T. R., Layton D. M., Leedham D. L., Liu C., Maxwell P. H., McMullin M. F., McNamara C. J., Percy M. J., Pugh C. W., Ratcliffe P. J., Talbot N. P., Treacy M., Robbins P. A. (2006) Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 3, e290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Formenti F., Constantin-Teodosiu D., Emmanuel Y., Cheeseman J., Dorrington K. L., Edwards L. M., Humphreys S. M., Lappin T. R., McMullin M. F., McNamara C. J., Mills W., Murphy J. A., O'Connor D. F., Percy M. J., Ratcliffe P. J., Smith T. G., Treacy M., Frayn K. N., Greenhaff P. L., Karpe F., Clarke K., Robbins P. A. (2010) Regulation of human metabolism by hypoxia-inducible factor. Proc. Natl. Acad. Sci. U. S. A. 107, 12722–12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L., Schmidt L., Zhou F., Li H., Hui Wei M., Chen F., Glenn G., Choyke P., Walther M. M., Weng Y., Duan D. S. R., Dean M., Glavač D., Richards F. M., Crossey P. A., Ferguson-Smith M. A., Le Paslier D., Chumakov I., Cohen D., Chinault A. C., Maher E. R., Linehan W. M., Zbar B., Lerman M. L. (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260, 1317–1320 [DOI] [PubMed] [Google Scholar]
- 21. Knudson A. G., Jr. (1986) Genetics of human cancer. Annu. Rev. Genet. 20, 231–251 [DOI] [PubMed] [Google Scholar]
- 22. Prowse A. H., Webster A. R., Richards F. M., Richard S., Olschwang S., Resche F., Affara N. A., Maher E. R. (1997) Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am. J. Human Genet. 60, 765–771 [PMC free article] [PubMed] [Google Scholar]
- 23. Knudson A. G., Jr., Strong L. C. (1972) Mutation and cancer: neuroblastoma and pheochromocytoma. Am. J. Human Genet. 24, 514–532 [PMC free article] [PubMed] [Google Scholar]
- 24. Walmsley S. R., Cowburn A. S., Clatworthy M. R., Morrell N. W., Roper E. C., Singleton V., Maxwell P., Whyte M. K., Chilvers E. R. (2006) Neutrophils from patients with heterozygous germline mutations in the von Hippel Lindau protein (pVHL) display delayed apoptosis and enhanced bacterial phagocytosis. Blood 108, 3176–3178 [DOI] [PubMed] [Google Scholar]
- 25. Gale D. P., Harten S. K., Reid C. D., Tuddenham E. G., Maxwell P. H. (2008) Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood 112, 919–921 [DOI] [PubMed] [Google Scholar]
- 26. Percy M. J., Beer P. A., Campbell G., Dekker A. W., Green A. R., Oscier D., Rainey M. G., van Wijk R., Wood M., Lappin T. R., McMullin M. F., Lee F. S. (2008) Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood 111, 5400–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martini M., Teofili L., Cenci T., Giona F., Torti L., Rea M., Foa R., Leone G., Larocca L. M. (2008) A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica 93, 1068–1071 [DOI] [PubMed] [Google Scholar]
- 28. Percy M. J., Furlow P. W., Lucas G. S., Li X., Lappin T. R., McMullin M. F., Lee F. S. (2008) A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N. Engl. J. Med. 358, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Howson M., Khamnei S., O'Connor D., Robbins P. A. (1986) The properties of a turbine device for measuring respiratory volumes in man. J. Physiol. 382, 12P [Google Scholar]
- 30. Robbins P. A., Swanson G. D., Howson M. G. (1982) A prediction-correction scheme for forcing alveolar gases along certain time courses. J. Appl. Physiol. 52, 1353–1357 [DOI] [PubMed] [Google Scholar]
- 31. Yock P. G., Popp R. L. (1984) Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70, 657–662 [DOI] [PubMed] [Google Scholar]
- 32. Skjaerpe T., Hatle L. (1986) Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur. Heart. J. 7, 704–710 [DOI] [PubMed] [Google Scholar]
- 33. Peacock A. J., Challenor V., Sutherland G. (1990) Estimation of pulmonary artery pressure by Doppler echocardiography in normal subjects made hypoxic. Respir. Med. 84, 335–337 [DOI] [PubMed] [Google Scholar]
- 34. Grunig E., Mereles D., Hildebrandt W., Swenson E. R., Kubler W., Kuecherer H., Bartsch P. (2000) Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J. Am. Coll. Cardiol. 35, 980–987 [DOI] [PubMed] [Google Scholar]
- 35. Chemla D., Castelain V., Humbert M., Hebert J. L., Simonneau G., Lecarpentier Y., Herve P. (2004) New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 126, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 36. Allemann Y., Sartori C., Lepori M., Pierre S., Melot C., Naeije R., Scherrer U., Maggiorini M. (2000) Echocardiographic and invasive measurements of pulmonary artery pressure correlate closely at high altitude. Am. J. Physiol. 279, H2013–H2016 [DOI] [PubMed] [Google Scholar]
- 37. Groves B. M., Reeves J. T., Sutton J. R., Wagner P. D., Cymerman A., Malconian M. K., Rock P. B., Young P. M., Houston C. S. (1987) Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J. Appl. Physiol. 63, 521–530 [DOI] [PubMed] [Google Scholar]
- 38. Sugawara J., Tanabe T., Miyachi M., Yamamoto K., Takahashi K., Iemitsu M., Otsuki T., Homma S., Maeda S., Ajisaka R., Matsuda M. (2003) Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol. Scan. 179, 361–366 [DOI] [PubMed] [Google Scholar]
- 39. Eriksen M., Walloe L. (1990) Improved method for cardiac output determination in man using ultrasound Doppler technique. Med. Biol. Eng. Comput. 28, 555–560 [DOI] [PubMed] [Google Scholar]
- 40. Christie J., Sheldahl L. M., Tristani F. E., Sagar K. B., Ptacin M. J., Wann S. (1987) Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oximetry, and thermodilution. Circulation 76, 539–547 [DOI] [PubMed] [Google Scholar]
- 41. Appelhoff R. J., Tian Y. M., Raval R. R., Turley H., Harris A. L., Pugh C. W., Ratcliffe P. J., Gleadle J. M. (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 [DOI] [PubMed] [Google Scholar]
- 42. Aprelikova O., Chandramouli G. V., Wood M., Vasselli J. R., Riss J., Maranchie J. K., Linehan W. M., Barrett J. C. (2004) Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J. Cell. Biochem. 92, 491–501 [DOI] [PubMed] [Google Scholar]
- 43. Lonser R. R., Glenn G. M., Walther M., Chew E. Y., Libutti S. K., Linehan W. M., Oldfield E. H. (2003) von Hippel-Lindau disease. Lancet 361, 2059–2067 [DOI] [PubMed] [Google Scholar]
- 44. Frew I. J., Krek W. (2007) Multitasking by pVHL in tumour suppression. Curr. Opin. Cell. Biol. 19, 685–690 [DOI] [PubMed] [Google Scholar]
- 45. Kaelin W. G., Jr. (2008) The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 [DOI] [PubMed] [Google Scholar]
- 46. Hickey M. M., Richardson T., Wang T., Mosqueira M., Arguiri E., Yu H., Yu Q. C., Solomides C. C., Morrisey E. E., Khurana T. S., Christofidou-Solomidou M., Simon M. C. (2010) The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J. Clin. Invest. 120, 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wasserman K., Hansen J. E., Sue D. Y., Stringer W. W., Whipp B. J. (2005) Principles of Exercise Testing and Interpretation, Lippincott, Williams & Wilkins, Philadelphia [Google Scholar]
- 48. Hu C. J., Wang L. Y., Chodosh L. A., Keith B., Simon M. C. (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 23, 9361–9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scortegagna M., Ding K., Oktay Y., Gaur A., Thurmond F., Yan L. J., Marck B. T., Matsumoto A. M., Shelton J. M., Richardson J. A., Bennett M. J., Garcia J. A. (2003) Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat. Genet. 35, 331–340 [DOI] [PubMed] [Google Scholar]
- 50. Smith T. G., Talbot N. P., Privat C., Rivera-Ch M., Nickol A. H., Ratcliffe P. J., Dorrington K. L., Leon-Velarde F., Robbins P. A. (2009) Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 302, 1444–1450 [DOI] [PubMed] [Google Scholar]
- 51. Gordeuk V. R., Sergueeva A. I., Miasnikova G. Y., Okhotin D., Voloshin Y., Choyke P. L., Butman J. A., Jedlickova K., Prchal J. T., Polyakova L. A. (2004) Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103, 3924–3932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.