Angiotensinogen (AGT) is the only known substrate for renin, which is the rate-limiting enzyme of the renin–angiotensin (Ang) system. Because the level of AGT is close to the Michaelis–Menten constant for renin, not only renin levels but also AGT levels can control the activity of the renin–Ang system, and upregulation of AGT levels may lead to elevated Ang peptide levels.1 Recent studies on transgenic mice have documented the involvement of AGT in the activation of the renin–Ang system.2 Enhanced intrarenal AGT mRNA and/or protein levels have been observed in kidney diseases including diabetic nephropathy3 and experimental nephritis.4,5 Thus, intrarenal AGT has an important role in the development and progression of kidney diseases.6
We recently reported that Rho-kinase (ROCK) and nuclear factor-kappa beta (NF-κβ) have crucial roles in the development of AngII-induced renal injury.7 However, the involvement of intrarenal AGT in this mechanism was not elucidated yet. Therefore, this study was performed to test the hypothesis that the ROCK/NF-κβ/AGT axis has an important role in AngII-induced renal injury.
The experimental protocol was approved by the Animal Care and Use Committees of Tulane University. Male Sprague–Dawley rats (225–250 g, Charles River), maintained on a normal diet, received either a sham operation (N=8) or continuous AngII infusion (120 ng min−1) subcutaneously through minipumps (Alzet, Cupertino, CA, USA; N=24). The AngII-infused rats were further subdivided into three subgroups (N=8 each) to receive one of the following treatments during the entire period: vehicle, ROCK inhibitor (fasudil, Asahi Kasei, Tokyo, Japan, 3 mg kg−1 day−1, intraperitoneously),7 or NF-κβ inhibitor (parthenolide, Biomol, Plymouth Meeting, PA, USA, 1 mg kg−1 day−1, intraperitoneously).7 All rats were monitored for up to 12 days of AngII infusion with free access to a regular diet and water. Systolic blood pressure (SBP) was measured by tail-cuff plethysmography as described previously. 7,8 Twenty-four hour urine samples were collected the day before the tissue harvesting and the protein and albumin concentration in urine samples were measured as described previously.8
Kidney samples were harvested by decapitation after 12 days of AngII infusion. Immediately after removal, one kidney was homogenized in cold methanol and renal AngII was measured as described previously.8 The contralateral kidneys were separated into four pieces. The first piece was immersed in RNAlater (Ambion, Austin, TX, USA) for total RNA extraction. The second piece was immersed in zinc-saturated formalin (Anatech, Richmond, BC, Canada) for tissue fixation. The third and the last piece were immersed in liquid nitrogen in Cryotubes (Nalgene, Rochester, NY, USA) for protein extraction and nuclear protein extraction, respectively.
Total RNA extraction from rat kidneys and quantitative real-time polymerase chain reaction for RelA and AGT mRNA were performed as described previously.3,9 Data pertaining to quantitative real-time polymerase chain reaction were normalized by glyceraldehyde 3-phosphate dehydrogenase mRNA expression.
Protein extraction, sample purification and ROCK activity assay were performed with rat kidneys using a commercially available kit (CycLex, Ina, Nagano, Japan) as described previously.7
Nuclear protein extraction and electromobility shift assay for NF-κβ were performed with rat kidneys using a commercially available kit (Panomics, Santa Clara, CA, USA) as described previously.7
Using zinc-saturated formalin-fixed paraffin-embedded renal sections, the averaged intensity of immunoreactivity of AGT was examined by immunohistochemistry with a commercially available antibody against AGT (IBL, Fujioka, Gunma, Japan) using Image-Pro plus software (Media Cybernetics, Bethesda, MD, USA) as described previously.8,9
Using zinc-saturated formalin-fixed paraffin-embedded renal sections, the magnitude of arterial proliferation of afferent arteriolar walls was evaluated by immunohistochemistry with a commercially available antibody against the α-smooth muscle isoform of actin (Sigma) using the Image-Pro plus software (Media Cybernetics) as described previously.8,9
Using zinc-saturated formalin-fixed paraffin-embedded renal sections, periodic acid-Schiff (PAS) stain-positive areas were evaluated by the Image-Pro plus software (Media Cybernetics) as a marker of glomerular damage as described previously.8,9
Statistical analysis was performed using a one-way factorial analysis of variance with post hoc Scheffe’s F-test. All data are presented as mean ± s.e.m. P < 0.05 was considered significant.
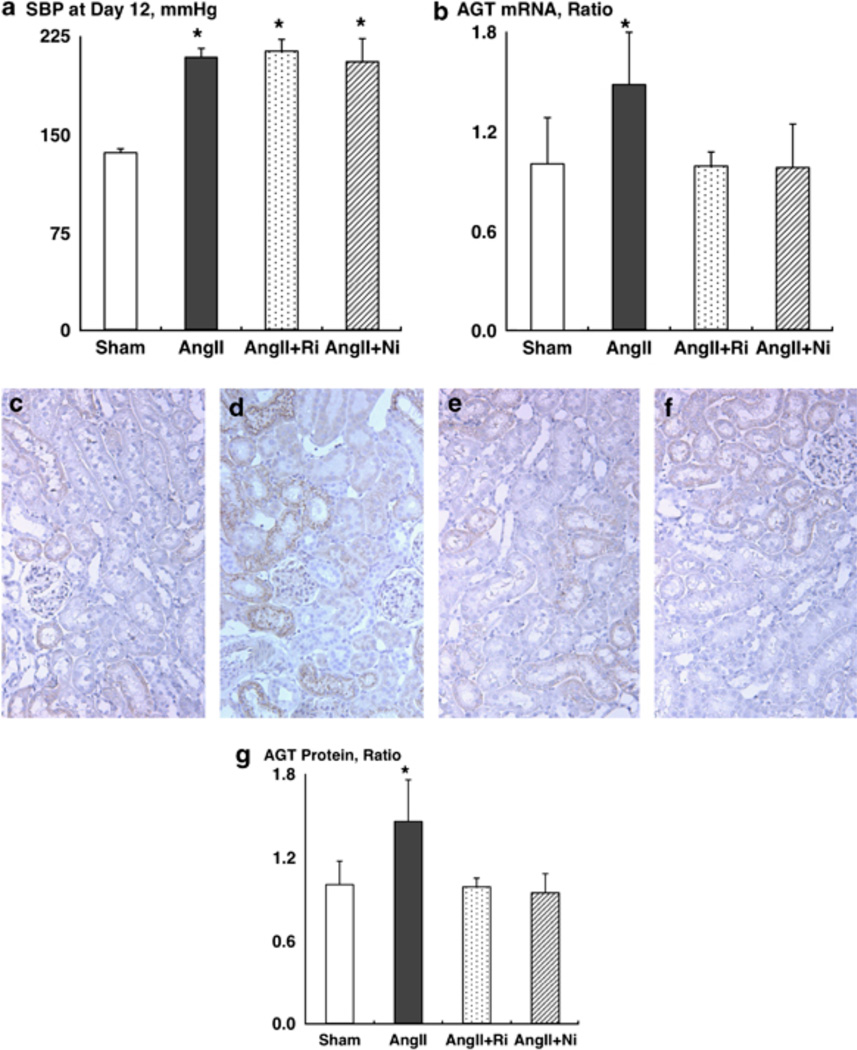
SBPs were similar between the groups before the treatment (data not shown). However, chronic AngII infusion significantly increased SBP (Figure 1a, 208 ± 7 mm Hg for AngII vs. 136 ± 3 mm Hg for sham). Treatment with fasudil or with parthenolide did not affect the SBP (213 ± 9 and 205 ± 18 mm Hg, respectively), which is consistent with a previous report.7
Figure 1.
(a) Systolic blood pressure (SBP) at day 12. (b) Angiotensinogen (AGT) mRNA levels. (c–g) AGT protein levels. Representative photographs of AGT immunohistochemistry from sham (c), AngII (d), AngII+fasudil (e) and AngII+parthenolide (f). Ni, nuclear factor-kappa beta inhibitor, parthenolide; Ri, Rho-kinase inhibitor, fasudil. *P < 0.05 compared with the sham group.
Chronic AngII infusion significantly increased ROCK activity (2.21 ± 0.16 arbitrary units for AngII vs. 1.00 ± 0.09 arbitrary units for sham). Importantly, while fasudil abolished AngII-induced ROCK activation, parthenolide did not alter AngII-induced ROCK activation (1.01 ± 0.17 arbitrary units for AngII+fasudil and 2.09 ± 0.19 arbitrary units for AngII+parthenolide, respectively).
For the evaluation of NF-κβ expression, mRNA levels of RelA (p65), a part of the NF-κβ complex, were measured by real-time polymerase chain reaction. Chronic AngII infusion significantly increased RelA mRNA levels (1.62 ± 0.17 arbitrary units for AngII vs. 1.00 ± 0.10 arbitrary units for sham). Both treatments completely blocked AngII-induced enhancement of RelA mRNA levels (0.95 ± 0.10 arbitrary units for AngII+fasudil and 0.69 ± 0.06 arbitrary units for AngII+parthenolide, respectively).
NF-κβ activity was evaluated by electromobility shift assay. Chronic AngII infusion significantly increased NF-κβ activity (2.28 ± 0.14 arbitrary units for AngII vs. 1.00 ± 0.15 arbitrary units for sham). Both treatments completely blocked AngII-induced enhancement of NF-κβ activity (0.98 ± 0.10 arbitrary units for AngII+fasudil and 0.95 ± 0.08 arbitrary units for AngII+parthenolide, respectively).
Chronic AngII infusion significantly increased kidney AGT mRNA levels (Figure 1b, 1.48 ± 0.31 arbitrary units for AngII vs. 1.00 ± 0.28 arbitrary units for sham). Both treatments completely blocked AngII-induced enhancement of kidney AGT mRNA levels (0.99 ± 0.08 arbitrary units for AngII+fasudil and 0.98 ± 0.26 arbitrary units for AngII+parthenolide, respectively).
Chronic AngII infusion significantly increased kidney AGT protein levels (Figures 1c–g, 1.45 ± 0.30 arbitrary units for AngII (Figure 1d) vs. 1.00 ± 0.17 arbitrary units for sham (Figure 1c)). Both treatments completely blocked AngII-induced enhancement of kidney AGT protein levels (0.98 ± 0.06 arbitrary units for AngII+fasudil (Figure 1e) and 0.94 ± 0.14 arbitrary units for AngII+parthenolide (Figure 1f), respectively).
Chronic AngII infusion significantly increased kidney AngII levels (164 ± 23 pg g−1 for AngII vs. 84 ± 13 pg g−1 for sham). Both treatments completely blocked AngII-induced enhancement of kidney AngII levels (103 ± 9 pg g−1 for AngII+fasudil and 117 ± 18 pg g−1 for AngII+parthenolide, respectively).
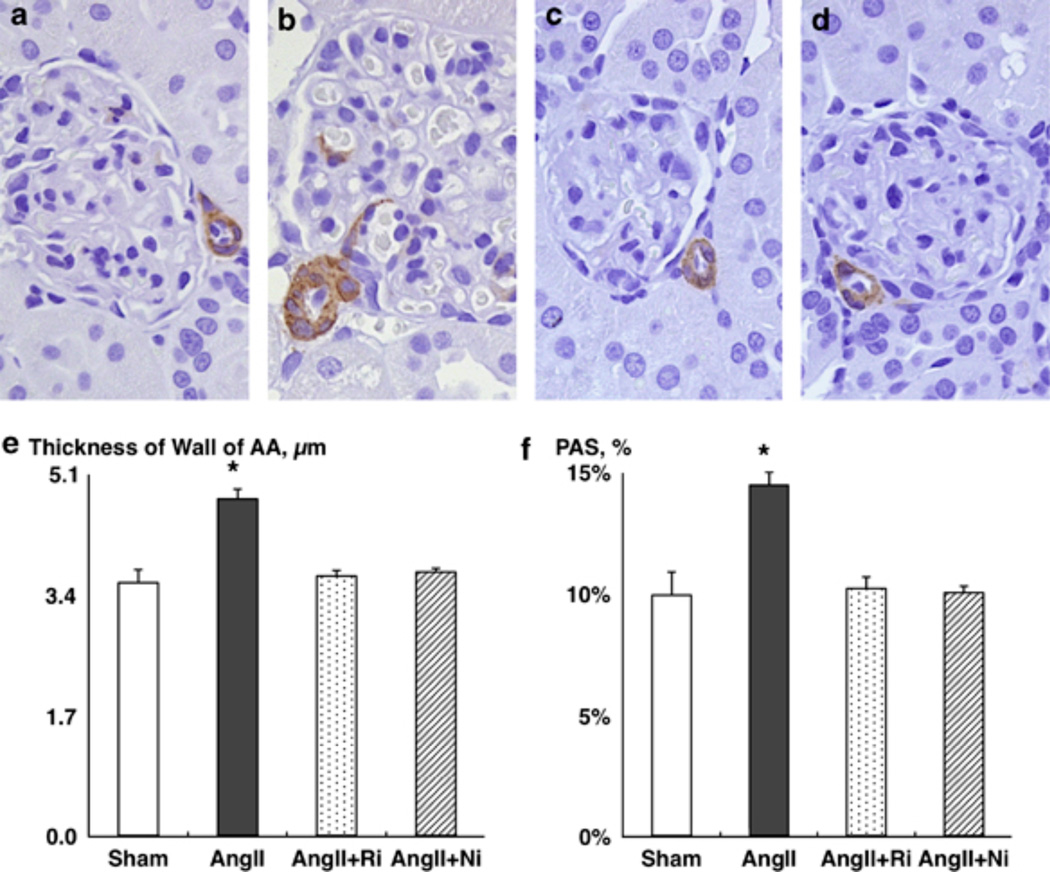
Chronic AngII infusion significantly enhanced the wall thickness of afferent arterioles (AAs) (Figures 2a–e, 4.74 ± 0.14 µm for AngII (Figure 2b) vs. 3.56 ± 0.18 µm for sham (Figure 2a)). Both treatments completely blocked AngII-induced enhancement of the wall thickness of AAs (3.65 ± 0.08 µm for AngII+fasudil (Figure 2c) and 3.71 ± 0.05 µm for AngII+parthenolide, respectively (Figure 2d)).
Figure 2.
(a–e) Wall thickness of afferent arterioles (AAs). Representative photographs of the α-smooth muscle isoform of actin immunohistochemistry from (a) sham, (b) AngII, (c) AngII+fasudil and (d) AngII+parthenolide. (f) Periodic acid-Schiff (PAS)-positive area as a marker of glomerular damage. Ni, nuclear factor-kappa beta inhibitor, parthenolide; Ri, Rho-kinase inhibitor, fasudil. *P < 0.05 compared with the sham group.
Chronic AngII infusion significantly increased urinary protein excretion (43 ± 6 mg day−1 for AngII vs. 11 ± 2 mg day−1 for sham). Both treatments completely blocked AngII-induced enhancement of urinary protein excretion (28 ± 5 mg day−1 for AngII+fasudil and 22 ± 3 mg day−1 for AngII+parthenolide, respectively).
Chronic AngII infusion significantly increased urinary albumin excretion (6.9 ± 1.7 mg day−1 for AngII vs. 2.7 ± 0.5mg day−1 for sham). Both treatments completely blocked AngII-induced enhancement of urinary albumin excretion (2.3 ± 0.3 mg day−1 for AngII+ fasudil and 3.8 ± 1.3 mg day−1 for AngII+parthenolide, respectively).
Chronic AngII infusion significantly increased PAS-positive area (Figure 2f, 14.4 ± 0.5% for AngII vs. 9.9 ± 0.9% for sham). Both treatments completely blocked AngII-induced enhancement of PAS-positive area (10.2 ± 0.5% for AngII+fasudil and 10.0 ± 0.3% for AngII+parthenolide, respectively).
We selected 3 mg kg−1 day−1 as the dose of fasudil treatment in the present study, because we previously reported that this dose of fasudil treatment had no effect on SBP,7 and a higher dose (10 mg kg−1 day−1) decreased SBP in rats.10 Moreover, we selected 1 mg kg−1 day−1 as the dose of parthenolide treatment in this study, because we previously reported that this dose of parthenolide treatment had no effect on SBP.7 In this study, treatment with a ROCK inhibitor, fasudil, or with an NF-κβ inhibitor, parthenolide, did not alter SBP but markedly attenuated the enhancement of wall thickness of AAs in AngII-infused rats, suggesting a potential contribution of AngII-induced ROCK activation and NF-κβ activation to renal injury independently of blood pressure changes. It is well known that AngII is one of the most potent vasoconstrictor in the body and it is well established that AngII-mediated vascular tone constitutes an important determinant of glomerular hemodynamics.11 However, AngII has multiple effects, and the AngII-induced vasoconstriction and high blood pressure are only a small part of the roles of AngII. For example, AngII causes aldosterone secretion,12 cell infiltration and migration,13 thrombosis14 and superoxide production.15–17 AngII also modulates transporters18,19 and channels20,21 in proximal as well as distal tubules. All of these factors are involved in AngII-induced renal injury independently of the hypertension-induced renal injury. The variety of roles of AngII may account for the blood pressure-independent renoprotective effect of ROCK inhibitor and NF-κβ inhibitor in this study.
In summary, this study was performed to test the hypothesis that ROCK/NF-κβ/AGT axis has an important role in AngII-induced renal injury. After 12 days of AngII infusion, SBP (Figure 1a), ROCK activity, NF-κβ activity, renal AGT levels (Figures 1b–g), renal AngII contents, wall thickness of AAs (Figures 2a–e), urinary protein excretion, urinary albumin excretion and PAS-positive area (Figure 2f) were significantly enhanced compared with the sham group. Treatments with both fasudil and parthenolide completely blocked AngII-induced enhancement of NF-κβ activity, renal AGT levels, renal AngII contents, wall thickness of AAs, urinary protein excretion, urinary albumin excretion and PAS-positive area. Importantly, parthenolide did not alter AngII-induced ROCK activation although fasudil abolished AngII-induced ROCK activation. It was reported previously that NF-κβ is located downstream of the ROCK.7 In addition, it is well established that NF-κβ is an important regulatory factor for AGT expression.22 Taken together, these data indicate that the ROCK/NF-κβ/AGT axis has an important role in AngII-induced renal injury.
ACKNOWLEDGEMENTS
We acknowledge the excellent technical assistance from My-Linh Rauv, Duy V Tran, Dale M Seth and Mark A Cabrera (Tulane University). We also thank the National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. R01DK072408) and National Center for Research Resources (Grant No. P20RR017659).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 2.Falcao S, Stoyanova E, Cloutier G, Maurice RL, Gutkowska J, Lavoie JL. Mice overexpressing both human angiotensinogen and human renin as a model of superimposed preeclampsia on chronic hypertension. Hypertension. 2009;54:1401–1407. doi: 10.1161/HYPERTENSIONAHA.109.137356. [DOI] [PubMed] [Google Scholar]
- 3.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urushihara M, Takamatsu M, Shimizu M, Kondo S, Kinoshita Y, Suga K, Kitamura A, Matsuura S, Yoshizumi M, Tamaki T, Kawachi H, Kagami S. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F167–F176. doi: 10.1152/ajprenal.00124.2009. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, Togawa A, Suzuki S, Fujigaki Y, Nakagawa T, Nakamura Y, Suzuki F, Kitagawa M, Hishida A. Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Renal Physiol. 2008;295:F1512–F1518. doi: 10.1152/ajprenal.00058.2008. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappab axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–2019. doi: 10.1046/j.1523-1755.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin Angiotensin Aldosterone Syst. 2001;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannken T, Schroeder R, Stahl RA, Wolf G. Angiotensin II-mediated expression of p27kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int. 1998;54:1923–1933. doi: 10.1046/j.1523-1755.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 16.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 17.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int. 2003;63:202–208. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 18.Berk BC, Vallega G, Muslin AJ, Gordon HM, Canessa M, Alexander RW. Spontaneously hypertensive rat vascular smooth muscle cells in culture exhibit increased growth and Na+/H+ exchange. J Clin Invest. 1989;83:822–829. doi: 10.1172/JCI113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 20.Carmines PK, Navar LG. Disparate effects of Ca channel blockade on afferent and efferent arteriolar responses to Ang II. Am J Physiol. 1989;256:F1015–F1020. doi: 10.1152/ajprenal.1989.256.6.F1015. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 22.Acres OW, Satou R, Navar LG, Kobori H. Contribution of a nuclear factor-{kappa}b binding site to human angiotensinogen promoter activity i. Hypertension. 2011;57:608–613. doi: 10.1161/HYPERTENSIONAHA.110.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]