Abstract
Using the twin pairs sample from the National Longitudinal Study of Adolescent Health, we estimate bivariate Cholesky models for the influence of stressful life events (SLEs) on depressive symptoms. We show that depressive symptoms ( ) and dependent SLEs (events influenced by an individual’s behavior) are both moderately heritable ( ). We find no evidence for the heritability of independent SLEs. Results from the bivariate Cholesky model suggest that roughly one-half of the correlation between depression and dependent SLEs is due to common genetic factors. Our findings suggest that attempts to characterize the causal effect of SLEs on mental health should limit their list of SLEs to those that are outside of the control of the individual.
Introduction
Stressful life events (SLE) are regularly linked to the mental health of adults. Those who have experienced more SLEs are consistently more likely to report worse mental health than those who have experienced few or no stressful events (Kessler 1997). SLEs are generally treated as exogenous shocks, and stress is characterized as something that happens to people. However, it is also possible that stress exposure and poor mental health are both derived in part from the same unobserved source; the genetic factors implicated in depression may also increase exposure to SLEs (Silberg et al. 1999).
The goals of this current study are as follows. Our first goal is to characterize the relative influence of genetic vis-à-vis environmental factors on both stress exposure and depression among adolescents. Second, we describe the extent to which common genetic influences affect both stress exposure and depression. Importantly, we differentiate between SLEs in which individuals have some control (dependent SLEs) and those that are exogenous (independent SLEs). Though at least five studies have examined the role of genetic factors on the relationship between SLEs and depression, (Rice, Harold, and Thapar 2003; Eley and Stevenson 2000; Silberg et al. 1999; Thapar, Harold, and McGuffin 1998; Billig Hershberger, Iacono, and McGue 1996), the bulk of this research has focused on adults (e.g., Plomin et al. 1990; Kendler, Karkowski, and Prescott 1999; Stein et al. 2002). Further, most accounts of SLEs in the previous studies emphasize recent events (within the past 12 months), and we examine stress exposure across adolescence and into young adulthood.
Genetic Influences on Depression and Stressful Life Events
A review by Sullivan, Neal, and Kendler (2000) suggests that 37 percent of the variation in major depression among adolescents and young adults is due to genetic factors. This estimate is derived from five twin studies using different designs and different samples, and similar estimates are obtained for depressive symptoms among adults (Kendler et al. 1994). There is also a large body of work examining genetic influences on stress exposure (Kendler and Baker 2007; Silberg et al. 1999; Stein et al. 2002; Wierzbicki 1989). In a recent review, Kendler and Baker (2007) report heritability estimates for SLEs comparable to those for depression, with an average heritability of 28 percent from six studies. These results provide some evidence of a gene-environment correlation (rGE) that occurs when environmental exposure is conditioned by genotype. In active rGE, persons with genetic tendencies to exhibit depressive symptoms actively select into the most stressful social environments. Therefore, an individual’s exposure to SLEs under active rGE is not an independent or exogenous shock. Accounting for this dependence and clarifying stressful life events that are the most susceptible to this endogeneity will make important contributions to the stress-mental health literature.
Although some evidence that SLEs are heritable is necessary to demonstrate rGE, it is not sufficient. The second requirement is evidence that the covariation between the two phenotypes—in this case stress exposure and depression—is due in part to common genetic influences. Using twin studies, researchers can estimate the genetic correlation coefficient describing the degree to which the genetic influences on one trait correlate with the genetic influences on another. This information, in conjunction with the heritability of each trait and the overall phenotypic correlation, allows researchers to estimate the proportion of the association between the two traits that is due to genetic influences. Using these techniques (described in detail further), Kendler et al. (1999) suggest that one-third of the association between stress and depression is due to common genetic factors, and comparable results have been reported using different samples (Billig et al. 1996; Kendler and Karkowski-Shuman 1997; Stein et al. 2002). Schnittker (2010) estimates a heritability of .29 for depressive symptoms among adult twins and shows some evidence that common stressors such as chronic health conditions, financial strain, marital strain, and discrimination are also heritable. He demonstrates that the association between depressive symptoms and exogenous environmental stressors (such as childhood stressors, current neighborhood conditions, and perceived discrimination) is independent of genetic influence. However, in cases in which individual characteristics are the most relevant (e.g., marital strain, financial strain, and health limitations), he finds evidence for gene-environment correlation.
Schnittker’s (2010) findings are in line with the SLE literature, which has differentiated between SLEs that are outside the control of the individual (independent SLEs) and those that may be influenced by the behaviors of the individual (dependent SLEs; see Brown and Harris 1978). It follows that the heritability of dependent SLEs should be substantially higher than the corresponding estimate for independent SLEs. This is exactly what Kendler and Baker (2007) report in their review. They report an average heritability of independent SLEs that is only 17 percent compared to an average heritability of 31percent for dependent SLEs. In a recent study, Bemmels et al. (2008) report a heritability of 45 percent for dependent SLEs and only 7 percent for independent SLEs; these figures are quite similar to results reported by Billig et al. (1999). These estimates are also in line with a widely cited study in which Plomin et al. (1990) estimate the heritability of dependent SLEs and independent SLEs to be 43 percent and 18 percent, respectively.
This study contributes to this important body of work by examining a nationally representative sample of adolescent twin pairs. The design of Add Health allows us to generalize to the larger population of adolescents from this particular cohort. Because some research suggests that the effects of SLEs may be cumulative over time (Geronimus 2001), we measure dependent and independent life events over a larger span of time than previous work in this area.
Data
All data in these analyses are drawn from Waves I-III of the National Longitudinal Study of Adolescent Health, a nationally representative longitudinal survey of adolescents and young adults obtained from an initial in-school survey of middle- and high school students conducted from September 1994 to April 1995. In total, 90,118 adolescents who attended 80 high schools and 54 feeder schools (both public and private) took part in the initial interview. During the months of April through December 1995, a sample of the in-school respondents (stratified by gender and grade) were selected to participate in an in-home face-to-face interview (Wave 1). These respondents have been followed up three times over the past 15 years for a total of four waves of in-home data collection.
The Add Health study oversampled twin pairs identified in the in-school survey, and this sample design enables quantitative genetic analysis. Respondents who reported during Wave I that they had a full sibling or a twin were included in the pairs roster, and of the 3,139 pairs who were asked, 83 percent (n = 2,612) agreed to take part in the study. In this study, we use a total of 221 identical and 320 fraternal twin pairs for our analyses. The twins range in age from 11 to 21 during the Wave I collection, 12 to 23 during Wave II, and 18 to 27 during Wave III.1
Measures
This study focuses on two variables: SLEs and depressive symptoms. Identifying SLEs can be complicated by differential perceptions of similar events (e.g., divorce) as stressful or not stressful. Because previous research indicates that “undesirable” events are more likely to adversely affect health (Horesh, Ratner, Laor, and Toren 2008), we follow the work of Adkins et al. (2009) and include only events that would generally be considered as negative. The stress data were self-reported and included whether the event had occurred and at what age. For each member of the sample, we constructed two simple additive indices that represented the cumulative number of dependent or independent SLEs that had occurred up until his or her age at Wave 3. Thus, for some pairs, the SLEs were cumulative through their late teens and for others through their mid-20s.
Like Billig and colleagues (1996), we used researcher judgment to classify events as independent (i.e., events seemingly outside the control of the individual) or dependent (i.e., events over which individuals may reasonably be expected to exert agency). Our categorizations are similar to those used in previous research (Bemmels et al. 2008; Billlig et al. 1996), with one exception: We classified extreme financial difficulties (e.g., getting evicted from one’s home or having utilities in one’s home discontinued) as independent because we were unable to determine whether the respondent was solely responsible for the financial maintenance of the household.2 SLEs were recorded if they occurred on or after the respondent’s tenth birthday.
The independent items included the following: miscarriage; death of a romantic partner; death of a spouse; death of a baby; suicide of a friend; having a baby with medical problems; relationship abuse (threatening, insulting, swearing, throwing things, pushing); being jumped; seeing violence; being shot or stabbed; being threatened; being raped; being injured in a fight; being unable to get needed health care; being evicted from one’s home; having one’s utility service cut off; or being involuntarily cut from welfare.
The dependent SLEs included the following: having sex for money; running away from home; being expelled from school; unwanted pregnancy; abortion; giving a baby up for adoption; end of cohabitation; end of marriage; end of nonromantic sexual relationship; being diagnosed with an STD; attempted suicide; threatening someone; shooting or stabbing someone; injuring someone in a fight; being discharged from the military; entering the military; juvenile conviction or detention; adult conviction; adult jailtime; or end of romantic relationship.
We measured depressive symptoms at Wave 3, when the pairs ranged in age from 18 to 27. Therefore, all SLEs occurred before the self-reported depressive symptoms assessment. We used a modified version of the Center for Epidemiologic Studies Depression (CES-D) scale, a nine-item index that asks respondents about the frequency of the following events over the past 7 days: (1) You were bothered by things that usually don’t bother you; (2) you could not shake off the blues, even with help from your family and your friends; (3) you felt that you were just as good as other people (reversed); (4) you had trouble keeping your mind on what you were doing; (5) you were depressed; (6) you were too tired to do things; (7) you enjoyed life (reversed); (8) you were sad; (9) you felt that people disliked you. Response options were 0 = never, rarely; 1 = sometimes; 2 = a lot of the time; or 3 = most of the time or all of the time. These items were summed within individuals to create the overall measure of depressive symptoms (mean = 4.8, standard deviation (SD) = 4.0, min = 9, max = 20). The CES-D has been shown to be a valid indicator of depression in general and for young adults (Radloff 1977, 1991). The descriptive statistics and phenotypic correlations by zygosity are provided in Table 1. Initial support for genetic influences on depression and SLEs can be seen from the pairwise correlation coefficients by zygosity; except for independent stressful life events, the correlation between identical twins is significantly higher than the corresponding correlation among fraternal twins.
Table 1.
Descriptive Statistics and Correlations by Zygosity
| Twin 1
|
Twin 2
|
|||||
|---|---|---|---|---|---|---|
| DEP | SLEI | SLED | DEP | SLEI | SLED | |
| Identical Twins (221 pairs) | ||||||
| Twin 1 | ||||||
| Depression | 1.00 | |||||
| SLE Independent | 0.06 | 1.00 | ||||
| SLE Dependent | 0.13 | 0.47 | 1.00 | |||
| Twin 2 | ||||||
| Depression | 0.29 | 0.09 | 0.12 | 1.00 | ||
| SLE Independent | 0.07 | 0.39 | 0.34 | 0.09 | 1.00 | |
| SLE Dependent | −0.07 | 0.33 | 0.42 | 0.09 | 0.27 | 1.00 |
| Mean | 4.81 | 1.62 | 4.22 | 5.01 | 1.51 | 4.22 |
| SD | 4.23 | 2.53 | 3.51 | 4.01 | 2.29 | 3.79 |
| Fraternal twins (320 pairs) | ||||||
| Twin 1 | ||||||
| Depression | 1.00 | |||||
| SLE Independent | 0.15 | 1.00 | ||||
| SLE Dependent | 0.09 | 0.51 | 1.00 | |||
| Twin 2 | ||||||
| Depression | 0.15 | 0.03 | 0.03 | 1.00 | ||
| SLE Independent | 0.23 | 0.33 | 0.23 | 0.14 | 1.00 | |
| SLE Dependent | 0.17 | 0.19 | 0.20 | 0.06 | 0.41 | 1.00 |
| Mean | 4.78 | 1.80 | 4.37 | 4.35 | 1.45 | 4.42 |
| SD | 3.90 | 2.57 | 3.68 | 3.93 | 2.04 | 3.96 |
Methods
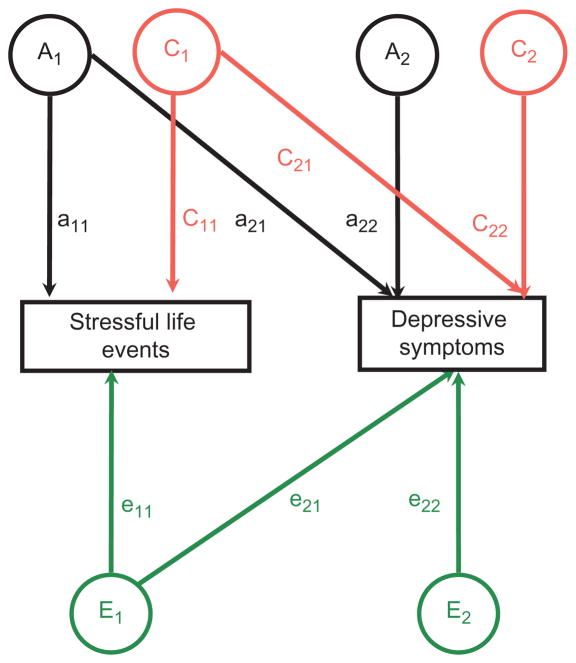
A series of bivariate twin models (Cholesky triangular decomposition models) were fit jointly to 4×4 covariance matrices (the four variables were depression and SLEs for twin-1 and depression and SLEs for twin-2) for MZ and DZ twins. Figure 1 shows the Cholesky decomposition for a simple additive genetic model. In Figure 1, the observed measures for one member of a twin pair are modeled as the sum of three underlying latent factors representing additive genetic influences (Ai), shared environmental influences (Ci), and nonshared environmental influences (Ei). Under this ACE model, the decomposition of the variance-covariance matrix among the observed measures can be obtained by regression of the observed measures on the latent factors according to the following equations:
where Depression and Stressful Life Events are the observed values, and Ai, Ci, and Ei are the latent factors representing additive genetic, shared environmental influences, and nonshared environmental influences on the observed measures.
Figure 1.
Schematic of bivariate Cholesky model. (color figure available online)
Notice that Figure 1 models the phenotypic measures for one member of a twin pair only. The phenotypic measures of the cotwin would be modeled in an identical manner. The lower case aij and eij represent the standardized regression weights (factor loadings) of the respective factors on the phenotypic measurements. These loadings are constrained to be equal for monozygotic (MZ) and dizygotic (DZ) twins. All latent factors are assumed to have unit variance and are uncorrelated within individual twins. However, theoretical assumptions regarding the genetic and environmental relationships between twins are used to constrain the correlations of these latent factors across twin pairs. As MZ twins are genetically identical, the additive (Ai) genetic factors correlate 1.0 across members of a twin pair. For DZ twins, who share half their alleles on average, the correlations among the additive factors (Ai) are constrained to be 0.5 across members of a twin pair following standard genetic theory (Falconer and MacKay 1996). By definition, shared environmental influences affect cotwins equally, so the correlations among the Ci latent factors are constrained to 1.0 for both MZ and DZ twins. Nonshared environmental influences are those environmental effects unique to each member of a twin pair. Thus, for both MZ and DZ twin pairs, correlations among the nonshared environmental factors (Ei) are constrained to be zero. Thus, this model predicts a variance-covariance matrix that is described in Table 2.
Table 2.
Expected Covariance Matrix for Stressful Life Events and Depression for Identical and Fraternal Twins
| Twin 1
|
Twin 2
|
||||||
|---|---|---|---|---|---|---|---|
| SLE | DEP | SLE | DEP | ||||
| Twin 1 | SLE |
|
|||||
| DEP | a11a21 + c11c21 + e11e21 |
|
|||||
| Twin 2 | SLE |
|
γa11a21 + c11c21 |
|
|||
| DEP | 1/.5a11a21 + c11c21 |
|
a11a21 + c11c21 + e11e21 |
|
|||
Note: The covariance estimates are estimated with the constant γ, which is 1 for MZ pairs and .5 for DZ pairs, reflecting the percentage of shared alleles.
Factor loadings are estimated by maximum likelihood methods using Mx (Neale 1993). Essentially the modeling program provides estimates for the factor loadings by numerical search for the parameter values that minimize a function that is twice the difference between the likelihood of the data under the model to be tested and the likelihood for the perfectly fitting model. Under assumptions of multivariate normality, the function is asymptotically distributed as χ2, with degrees of freedom equal to the number of observed statistics minus the number of estimated parameters. Submodels of the full Cholesky decomposition model are tested by constraining certain coefficients to zero and recalculating the likelihood ratio for the fit of the reduced model. The difference in -2LogLikelihood between two nested comparison models is also distributed as χ2 under multivariate normality assumptions, with degrees of freedom equal to the difference in degrees of freedom between the two models. Likelihood ratio tests are used to determine whether reduced models resulted in a significant loss of fit to the observed data).3
Results
Table 3 provides the model fit statistics for the univariate analysis. According to these results, the AE model (a model in which there is no shared environmental influence) was the best-fitting model relating depressive symptoms and dependent SLEs; dropping the C parameter did not decrease the overall fit, so we considered the AE model appropriate to explain depression. According to these estimates, 28 percent of the variance of depressive symptoms was due to additive genetic influences, and the remaining 72 percent was due to unique environmental influences.
Table 3.
Univariate ACE Estimates: Stressful Life Events and Depressive Symptoms
| −2ll | X2 | d.f. | Pr. < | a2 | c2 | e2 | |
|---|---|---|---|---|---|---|---|
| Depressive symptoms | |||||||
| ACE | 6045.85 | — | .24 (.00, .38) | .03 (.00, .26) | .73 (.62, .84) | ||
| AE | 6045.91 | 0.06 | 1 | 0.805 | .28 (.18, .38) | — | .72 (.62, .82) |
| CE | 6048.12 | 2.27 | 1 | 0.131 | — | .21 (.13, .28) | .79 (.71, .87) |
| E | 6072.12 | 26.26 | 2 | 0.000 | — | — | 1.0 (1.0,1.0) |
| Independent Stressful Life Events | |||||||
| ACE | 4860.96 | — | .12 (.00, .40) | .26 (.03, .41) | .62 (.52, .72) | ||
| AE | 4865.92 | 4.96 | 1 | 0.026 | .42 (.33, .51) | — | .58 (.49, .67) |
| CE | 4861.68 | 0.71 | 1 | 0.398 | — | .34 (.27, .42) | .65 (.58, .73) |
| E | 4930.31 | 69.34 | 2 | 0.000 | — | — | 1.0 (1.0,1.0) |
| Dependent Stressful Life Events | |||||||
| ACE | 5871.55 | .43 (.18, .52) | .00 (.00, .18) | .57 (.48, .68) | |||
| AE | 5874.54 | 0.01 | 1 | 0.921 | .43 (.33, .52) | — | .57 (.48, .67) |
| CE | 5884.39 | 9.84 | 1 | 0.002 | — | .29 (.21, .36) | .71 (.64, .79) |
| E | 5931.01 | 56.46 | 2 | 0.000 | — | — | 1.0 (1.0,1.0) |
Note: All data come from Waves I-III of the National Longitudinal Study of Adolescent Health. Parameter estimates were obtained using Mx statistical software (Neale et al. 2003). The bold rows represent the best fitting model based on the likelihood-ratio tests.
Similarly, for dependent SLEs, dropping C did not significantly decrease the predictive value of the model, and the likelihood ratio tests reported in Table 3 also suggested an AE model. Importantly, we observed a relatively high additive genetic estimate (h2 = .43) for dependent stressful life events. However, for independent SLEs, dropping the estimate for additive genetic influences (A) did not significantly decrease model fit. The CE environmental model best fit these data, suggesting that independent SLEs were not significantly influenced by genetic factors. Roughly one-third of the variance in independent SLEs was due to shared environmental influences and the remaining two-thirds to unique environmental influences. Although the CE model was the best fitting, it is nevertheless important to highlight that the heritability estimate from the ACE model, .12, was in line with other heritability estimates for uncontrollable SLEs (Bemmels et al. 2008; Billig et al. 1996; Plomin et al. 1990).
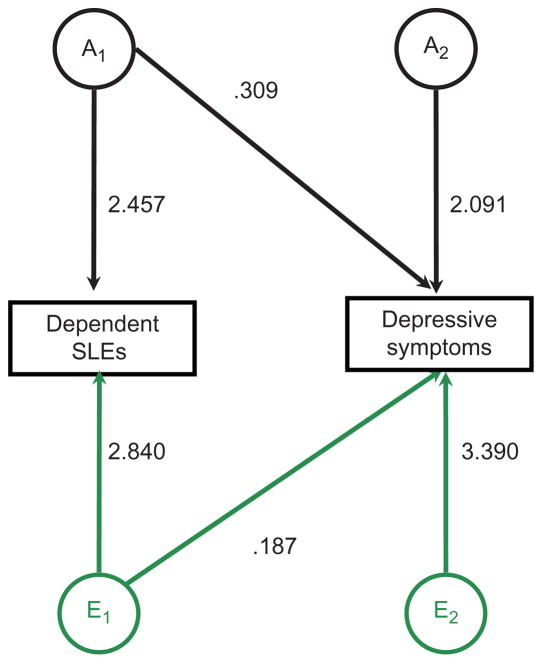
Our main question is how much of the observed association between stress and depression is due to a common and unobserved genetic source. Figure 2 summarizes the results from the bivariate Cholesky model. Because we failed to identify significant genetic variance for independent SLEs, we estimated this model only for dependent SLEs. Further, because the AE model was the best fitting model for both phenotypes, we estimated an AE bivariate model. The parameter estimates for this model are presented in Figure 2. In sum, these results presented evidence for genetic correlation between dependent SLEs and depression (rg = .15).
Figure 2.
Path coefficients from the best-fitting (AE) bivariate model. (color figure available online) Note: All data come from Waves I-III of the National Longitudinal Study of Adolescent Health. Parameter estimates were obtained using Mx statistical software (Neale et al. 2003) and denote the paths for the best-fitting model described in Table 2. Saturated model −2LL = 11910.32, reduced model (dropping C) −2LL = 11912.89, χ2 = 2.571, df = 3, p < .463.
When considered as a function of the heritabilities of the two traits x and y, the total amount of the correlation that was due to genetic factors was estimated to be . Taken as a proportion of the total phenotypic correlation in the overall sample (r = .09; weighted average), the standardized solution suggested that roughly 55 percent of the correlation between controllable life events and depression was due to genetic factors that influenced both traits, and the remaining 45 percent was due to common environmental influences that caused the two traits to correlate.4 That is, people who were more likely to be exposed to dependent SLEs were also more likely to have elevated symptoms of depression, but nearly two-thirds of this association was due to genetic factors that caused both of the outcomes rather than one’s causing the other.
Discussion
The aim of this study was to examine common genetic influences on the covariation between stress and symptoms of depression among adolescents and young adults. We distinguished between independent and dependent SLEs to examine the possibility that active gene-environment correlation is partially responsible for the observed association between stress and depression. We found evidence for this perspective by demonstrating sizable (and common) genetic influences on both dependent SLEs and depressive symptoms. In addition, our findings suggest that the association between independent SLEs and depressive symptoms is independent of genetic influences on both factors. That is, we do not demonstrate a significant genetic influence on life events that are outside of the control of individuals.
However, we found sizable genetic influences for dependent SLEs over which persons exert some form of control. Therefore, we argue that the association between SLEs and depression may be most meaningfully assessed when the SLEs considered are independent of the individual’s own behaviors. Independence may be difficult to measure at times. Indeed, even our measure of independent SLEs demonstrated a small additive genetic component (h2 = .12). This estimate is in line with similar work (h2 = .18) done by others (Plomin et al. 1990) and suggests that independent SLEs could be better defined to remove any measurable genetic influence. This would provide a more valid environmental measure to be used in epidemiologic studies of stress and well-being.
This perspective is echoed by Kendler and Baker (2007) in their review of standard measures of the environment. Not only do SLEs show signs of genetic influence, but nearly all self-reported environmental measures evidence additive genetic variation. For example, examining seven studies, Kendler and Baker report an average heritability for parental warmth of .34 when using child reports and .34 when using parent-based reports. Modest heritabilities are also shown for family factors including cohesion (h2 = .24), conflict (h2 = .30), and organization (h2 = .25) and social factors including social integration (h2 = .31) and the perception that friends have problems (h2 = .23). Twin and family studies provide an important perspective on the source of stressors that are generally believed to be exogenous environmental shocks. If the source of the environment partially overlaps with the source of the physical or mental morbidities, efforts should be made to remove components of environmental measures that may be confounded by codetermining genetic factors.
The evidence supporting rGE in the stress-depression association is also important because of the implications for gene-environment interaction (GxE) studies. As Jaffee and Price (2007: 437) point out, “rGE does not have to reach statistical significance to profoundly affect the interpretation of GxE estimates.” If the assumption of independence between the genotype and environment is violated, the GxE parameter estimate may be biased. Given our results in conjunction with the work of others (Bemmels et al. 2008; Billig et al. 1999; Kendler and Karkowski-Shuman 1997; Stein et al. 2002), it is important to consider the possible influence of rGE especially for dependent SLEs. Though it is difficult to assess the relative contribution of rGE influences as distinct from GxE effects (Rutter, Pickles, Murray, and Eaves 2001), an effort to refine life events to exogenous and nonheritable events that happen to people would improve the reliability and validity of these and other studies and would go a long way toward solidifying this important body of work.
Limitations
Several limitations are important to consider in interpreting the results of this study. First, all measures of SLEs are self-reported values, and there is no independent corroboration of the occurrence or timing of the event. This is important because it is possible that the probability of actually reporting an event is heritable rather than the probability of experiencing the event, per se. As such, stress will be characterized as heritable when in fact it is latent genetic factors that affect the framing and memory of particular events. This is most likely the case for stressors (e.g., being injured in a fight) in which interpretation plays a greater role, as is typically the case for the dependent SLEs. Thus, this measurement issue has the potential to bias our heritability estimates upward. Second, although we use measures of stress across the lifecourse, our measures of stress are a simple sum of scores. This procedure does not adequately capture the developmental perspective on stress exposure advocated by some (Adkins et al. 2009). The timing of the stressor vis-à-vis the age and gender of an adolescent may have important consequences for the etiology of mental health problems because of the social meaning of particular stressors (e.g., some are normative aspects of adolescent development). Further efforts should be made to more formally characterize the stress trajectory of “typical” U.S. adolescents and to examine how deviations from this norm affect individuals’ mental well-being. Finally, as described, the distinction between independent compared to dependent events was difficult for some of the items. In our assessment, in most cases this was most likely the case in which independent events could be characterized as dependent rather than the other way around. In this sense, our heritability estimate for independent SLEs may be biased slightly upward. Though this would not change our substantive conclusions, it is nevertheless important to consider when evaluating the results of our study.
Conclusion
Despite these limitations, this study makes important contributions to research on stress and depression, on gene-environment correlations and, most important, on the etiology of depression among adolescents. Rice and colleagues (2003) and Scourfield and colleagues (2003) have shown that adolescence is the period in which genetic influences on depression begin to emerge. Further, because adolescents are given increasing autonomy, genetic factors may play an important role in stress exposure as they select into specific environments (Shanahan and Boardman 2009). Rice and colleagues (2003) show that during childhood, genetic factors are important in determining life events (h2 = .24) but not depression (h2 = .09, n.s.). They show an increase in the genetic influences on life events (h2 = .28) and depression (h2 = .20) over the life course, and they also show that these two phenotypes are associated with one another because of emergent sources of genetic variation. Moreover, they show that this association is even more pronounced for dependent life events (h2 = .41). Similarly, Eley and Stevenson (2000) show that proper characterization of the source of the stress (as dependent or independent) is important for genetically informed studies of depression in persons as young as 12 years. The context in which adolescents are socialized sets a critical stage for changes in mental health throughout their lives. Therefore, it is important to correctly characterize not only the content, timing, and duration of stress exposure but also whether the stressors are exogenous or in part dependent on their own behavior.
Acknowledgments
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill and funded by grant P01HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health Web site (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis. Additional funding was provided by the following three NICHD grants: K01HD50336, R01HD060726, and R21HD051146.
Footnotes
Detailed information about the Add Health study design can be found in Harris et al. (2009), and readers are encouraged to visit the following website: http://www.cpc.unc.edu/projects/addhealth.
We are aware that using researcher judgment to classify SLEs is not a perfect method. The personal beliefs of any researcher may influence whether they think specific life events are exogenous or brought upon oneself by the respondent. For this reason, we relied heavily on past research with one adjustment.
The parameter tests from these models provide the following information: (1) the best-fitting model indicates whether genetic or environmental influences or both are important sources of variation for each trait; (2) the models decompose environmental influences into shared and nonshared sources and indicate whether both sources are needed to fully characterize environmental influences; and (3) most important, they describe the extent to which the observed covariation between the two traits is due to environmental or genetic covariation. Specifically, the path coefficients described in Figure 1, in combination with information about the variance of each trait and their covariance, can be used to identify the source of the variation that is unique to each trait and that which is shared. The equation describes this relatively simple element-by-element conversion. The solution to this equation provides standardized estimates for the heritability of SLEs ( ), the heritability of depressive symptoms ( ), and the proportion of the covariance between depression and SLEs that is due to common additive genetic influences (rA). A common summary statistic of the genetic covariation is the genetic correlation coefficient (rg), which is given as . Taken as a function of the univariate heritabilties of the two traits , the standardized genetic correlation summarizes the proportion of the phenotypic correlation that is due to shared genetic influences.
Because of the relatively low phenotypic correlation, the confidence interval for the standardized solution contains the value of zero. Simulations were used to obtain the following empirical 95 percent confidence interval for the genetic correlation coefficient [rg = .15(−.06, .38)]. Though this estimate is statistically significant at the p < .10 level, we nevertheless suggests caution when interpreting the parameter estimate for the proportion of shared genetic covariance between dependent stressful life events and depressive symptoms.
References
- Adkins DE, V, Wang W, Dupre ME, Van den Oord JCG, Elder GH., Jr Structure and stress: Trajectories of depressive symptoms across adolescence and young adulthood. Soc Forces. 2009;88:31–60. doi: 10.1353/sof.0.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemmels HR, Burt SA, Legrand LN, Iacono WG, McGue M. The heritability of life events: An adolescent twin and adoption study. Twin Res Hum Genet. 2008;11:257–265. doi: 10.1375/twin.11.3.257. [DOI] [PubMed] [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: Genetic and environmental relations. Behav Genet. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. London: Tavistock; 1978. [Google Scholar]
- Eley TC, Stevenson J. Specific life events and chronic experiences differentially associated with depression and anxiety in young twins. J Abnorm Child Psychol. 2000;28:383–394. doi: 10.1023/a:1005173127117. [DOI] [PubMed] [Google Scholar]
- Falconer DS, MacKay TFC. Introduction to quantitative genetics. Burnt Mill, England: Longman; 1996. [Google Scholar]
- Geronimus AT. Understanding and eliminating racial inequalities in women’s health in the United States: The role of the weathering conceptual framework. J Am Med Woman’s Assoc. 2001;56:133–136. [PubMed] [Google Scholar]
- Harris KM, Halpern CT, Whitsel E, Hussey J, Tabor J, Entzel P, Udry JR. [accessed September 15, 2010];The National Longitudinal Study of Adolescent Health: Research design. 2009 http://www.cpc.unc.edu/projects/addhealth/design.
- Horesh N, Ratner S, Laor N, Toren P. A comparison of life events in adolescents with major depression, borderline personality disorder and matched controls: A pilot study. Psychopathology. 2008;41:300–306. doi: 10.1159/000141925. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: A review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychol Med. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski-Shuman L. Stressful life events and genetic liability to major depression: Genetic control of exposure to the environment? Psychol Med. 1997;27:539–547. doi: 10.1017/s0033291797004716. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neal MC, Kessler RC, Health AC, Eaves LJ. The clinical characteristics of major depression as indices of the familial risk to illness. Br J Psychiatry. 1994;165:66–72. doi: 10.1192/bjp.165.1.66. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Ann Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. Richmond, VA: Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University; 2003. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. Richmond, VA: Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Department of Psychiatry; 2003. Available online at: http://www.vipbg.vcu.edu/~vipbg/software/mxmanual.pdf. [Google Scholar]
- Plomin R, Lichtenstein P, Pedersen NL, McClearn GE, Nesselroade JR. Genetic influence on life events during the last half of the life span. Psychol Aging. 1990;5:25–30. doi: 10.1037//0882-7974.5.1.25. [DOI] [PubMed] [Google Scholar]
- Radloff L. The Center for Epidemiological Studies depression scale: A self report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- Radloff L. The use of the Center for Epidemiologic Studies depression scale in adolescents and young adults. J Youth Adolesc. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. Negative life events as an account of age-related differences in the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2003;44:977–987. doi: 10.1111/1469-7610.00182. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on specific environmental causal effects on behavior. Psychol Bull. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Schnittker Jason. Gene-environment correlations in the stress-depression relationship. J Health Soc Behav. 2010;51(3):229–243. doi: 10.1177/0022146510378240. [DOI] [PubMed] [Google Scholar]
- Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: Changing aetiological influences with development. J Child Psychol Psychiatry. 2003;44(7):968–976. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Stein MB, Lang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neal MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Boardman JD. Genetics and behavior in the life course: A promising frontier. In: Elder Glen H, Jr, Giele Janet Z., editors. The craft of life course research. London: Guilford; 2009. pp. 215–235. [Google Scholar]
- Thapar A, Harold G, McGuffin P. Life events and depressive symptoms in childhood—shared genes or shared adversity? A research note. J Child Psychol Psychiatry. 1998;39(8):1153–1158. [PubMed] [Google Scholar]
- Wierzbicki M. Twins’ responses to pleasant, unpleasant, and life events. J Genet Psychol. 1989;150:135–145. doi: 10.1080/00221325.1989.9914585. [DOI] [PubMed] [Google Scholar]