Abstract
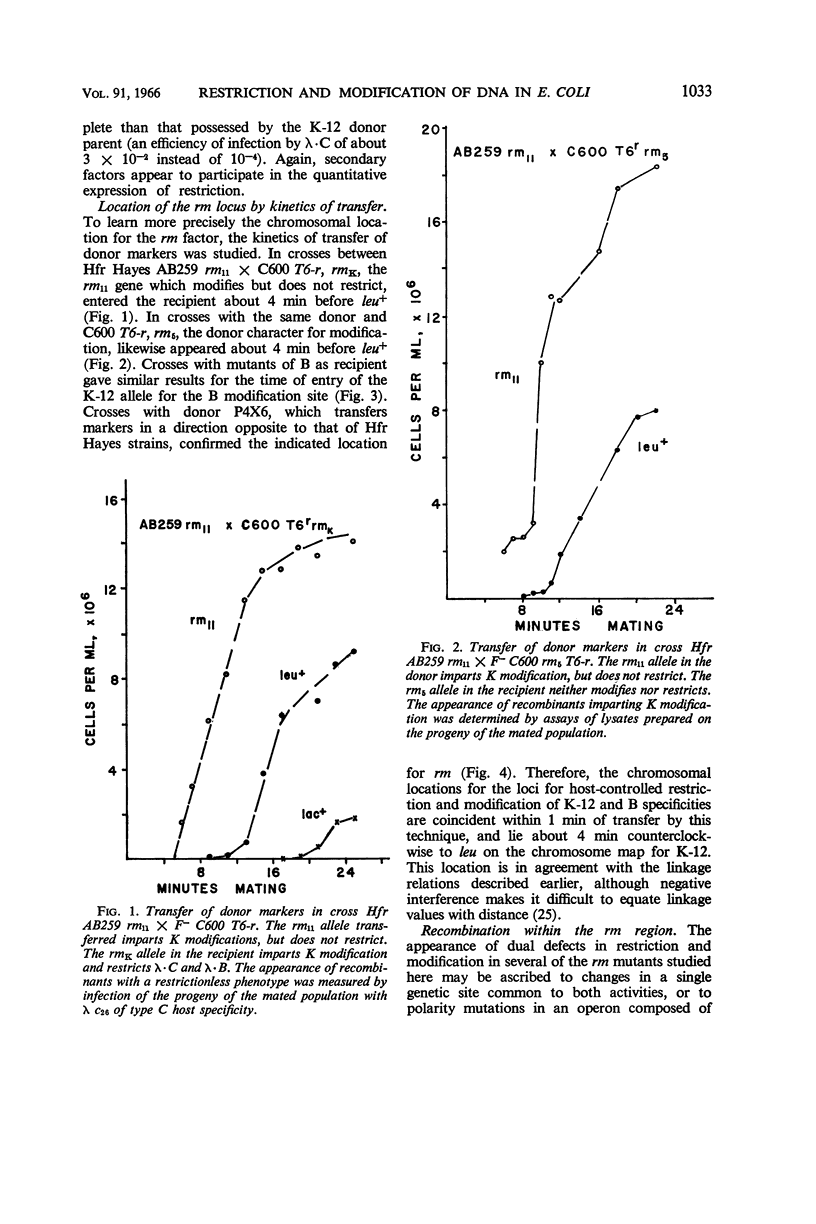
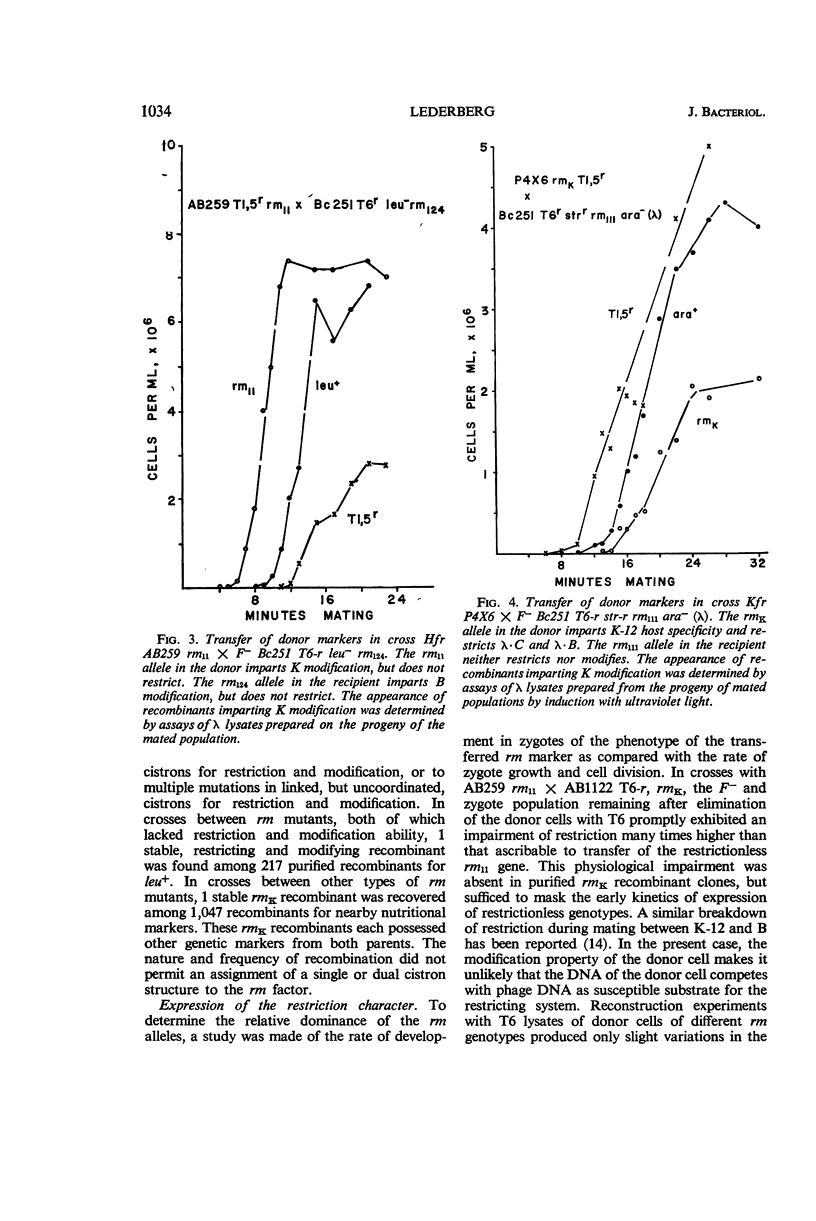
Lederberg, Seymour (Brown University, Providence, R.I.). Genetics of host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. J. Bacteriol. 91:1029–1036. 1966.—The locus for the host specific restriction and modification of deoxyribonucleic acid in Escherichia coli has been mapped by matings between mutants for these characters in strains K-12, C600, and B. Linkage analysis and kinetics of marker transfer indicate that a single or closely linked multiple chromosomal site located about 4 min counterclockwise to leucine is responsible for these activities. Secondary factors which affect the quantitative level of restriction also were detected. Wild-type recombinants were isolated in crosses between rm− (restriction or modification, or both) mutants. The expression in zygotes of the restrictionless character of a rm− donor is masked by a separate, physiological impairment of restriction, which results from mating and is independent of the modification state of the donor. The relevance of the restriction character to mating incompatibilities in these and other bacterial strains is considered.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., HATTMAN S., DUSSOIX D. ON THE HOST-CONTROLLED MODIFICATION OF BACTERIOPHAGE LAMBDA. Virology. 1963 Sep;21:30–35. doi: 10.1016/0042-6822(63)90300-3. [DOI] [PubMed] [Google Scholar]
- ARBER W., LATASTE-DOROLLE C. [Enlargement of the host area of bacteriophage lambda for Escherichia coli B]. Pathol Microbiol (Basel) 1961;24:1012–1018. [PubMed] [Google Scholar]
- ARBER W., MORSE M. L. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. VI. EFFECTS ON BACTERIAL CONJUGATION. Genetics. 1965 Jan;51:137–148. doi: 10.1093/genetics/51.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOICE L. B., LURIA S. E. Behavior of prophage P1 in bacterial matings. I. Transfer of the defective prophage P1 dl. Virology. 1963 May;20:147–157. doi: 10.1016/0042-6822(63)90150-8. [DOI] [PubMed] [Google Scholar]
- BOYER H. GENETIC CONTROL OF RESTRICTION AND MODIFICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1652–1660. doi: 10.1128/jb.88.6.1652-1660.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN D. A variant of phage P2 originating in Escherichia coli, strain B. Virology. 1959 Jan;7(1):112–126. doi: 10.1016/0042-6822(59)90180-1. [DOI] [PubMed] [Google Scholar]
- COUSIN D. LOCALISATION G'EN'ETIQUE D'UN PROPHAGE D'EFECTIF DE LA SOUCHE B D'ESCHERICHIA COLI. Ann Inst Pasteur (Paris) 1964 Jun;106:847–866. [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- Eisenstark A. Mutagen-induced hybridization of Salmonella typhimurium LT2 X Escherichia coli K12 Hfr. Proc Natl Acad Sci U S A. 1965 Jul;54(1):117–120. doi: 10.1073/pnas.54.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOVER S. W., COLSON C. THE BREAKDOWN OF THE RESTRICTION MECHANISM IN ZYGOTES OF ESCHERICHIA COLI. Genet Res. 1965 Feb;6:153–155. doi: 10.1017/s001667230000402x. [DOI] [PubMed] [Google Scholar]
- HAYES W. The mechanism of genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1953;18:75–93. doi: 10.1101/sqb.1953.018.01.016. [DOI] [PubMed] [Google Scholar]
- HOEKSTRA W. P., DEHAAN P. G. HOST CONTROLLED MODIFICATION OF PHAGE LAMBDA IN ZYGOTIC INDUCTION IN ESCHERICHIA COLI B. Antonie Van Leeuwenhoek. 1963;29:292–296. doi: 10.1007/BF02046071. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. P., de Haan P. G. The location of the restriction locus for lambda-K in Escherichia coli. Mutat Res. 1965 Jun;2(3):204–212. doi: 10.1016/0027-5107(65)90029-1. [DOI] [PubMed] [Google Scholar]
- IHLER G., MESELSON M. GENETIC RECOMBINATION IN BACTERIOPHAGE LAMBDA BY BREAKAGE AND JOINING OF DNA MOLECULES. Virology. 1963 Sep;21:7–10. doi: 10.1016/0042-6822(63)90297-6. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. M., FALKOW S., BARON L. S. RECIPIENT ABILITY OF SALMONELLA TYPHOSA IN GENETIC CROSSES WITH ESCHERICHIA COLI. J Bacteriol. 1964 Jan;87:54–60. doi: 10.1128/jb.87.1.54-60.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S., MESELSON M. DEGRADATION OF NON-REPLICATING BACTERIOPHAGE DNA IN NON-ACCEPTING CELLS. J Mol Biol. 1964 May;8:623–628. doi: 10.1016/s0022-2836(64)80112-1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., GREENBERG J. A new chemical mutagen for bacteria, 1-methyl-3-nitro-1-nitrosoguanidine. Biochem Biophys Res Commun. 1960 Dec;3:575–577. doi: 10.1016/0006-291x(60)90064-4. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UETAKE H., TOYAMA S., HAGIWARA S. ON THE MECHANISM OF HOST-INDUCED MODIFICATION. MULTIPLICITY ACTIVATION AND THERMOLABILE FACTOR RESPONSIBLE FOR PHAGE GROWTH RESTRICTION. Virology. 1964 Feb;22:202–213. doi: 10.1016/0042-6822(64)90005-4. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Hybrids of Escherichia and Salmonella. Science. 1960 Mar 18;131(3403):813–815. doi: 10.1126/science.131.3403.813. [DOI] [PubMed] [Google Scholar]



